Antioxidant bisabolane-type sesquiterpenoids from algalderived fungus Aspergillus sydowii EN-434*
HU Xueyi , LI Xiaoming , MENG Linghong , , WANG Bingui ,
1 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, and Laboratory of Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China 2 College of Earth Science, University of Chinese Academy of Sciences, Beijing 100049, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract A new aromatic bisabolene-type sesquiterpenoid (7 S,8 S)-8-hydroxysydowic acid ( 1), together with a pair of new bisabolene derivative (±)-(7 R*,10 R*)-10-hydroxysydowic acid ( 2), as well as two known analogues hydroxysydonic acid ( 3) and sydowic acid ( 4) were isolated and identifi ed from the endophytic fungal strain Aspergillus sydowii EN-434 that was obtained from the marine red alga Symphyocladia latiuscula. Their structures were elucidated by nuclear magnetic resonance (NMR), high resolution electrospray ionization mass spectroscopy (HRESIMS), and quantum chemical electronic circular dichroism (ECD) calculations as well as comparison of the data with literature reports. Compound 1 exhibited potent DPPH free radical scavenging activity with IC 50 value of 113.5 μmol/L.
Keyword: Aspergillus sydowii; algal-derived fungus; bisabolene-type sesquiterpenoid; antioxidant activity
1 INTRODUCTION
Marine-derived fungi are important part of marine microbiological environments and can be found in all habitats (Tasdemir, 2017). Following the discovery of penicillin, fungi hold great potential to discover novel bioactive agents for drug development (Schinke et al., 2017; Barzkar et al., 2019). Since the mid of 1990s, the popularity of the Ascomycota, especially species from the genus Aspergillus, displayed a very rapid growth (Blunt et al., 2015). Aspergillus sydowii has attracted much attention due to the discovery of various bioactive secondary metabolites, such as cyclopentanoids (Teuscher et al., 2006), trispyrogallol ethers (Liu et al., 2013), diphenyl ethers (Wang et al., 2018), sesquiterpenes (Trisuwan et al., 2011; Xu et al., 2017), xanthones (Trisuwan et al., 2011; Wang et al., 2018), diketopiperazine dimer (Cho et al., 2018), and alkaloids (Li et al., 2018). Some of them showed signifi cant antibiotic (Li et al., 2018) and antioxidant (Trisuwan et al., 2011) activities as well as mild cytotoxicity (Liu et al., 2017).
Our study started with the purpose of fi nding new bioactive metabolites from A. sydowii and four compounds (Fig.1), including a new aromatic bisabolene-type sesquiterpenoid (7 S,8 S)-8-hydroxysydowic acid (1), a pair of new bisabolene derivative (±)-(7 R*,10 R*)-10-hydroxysydowic acid (2), and two known bisabolene analogues (3and4) (Hamasaki et al., 1975; Hamasaki et al., 1978) , were obtained from the culture extract of A. sydowii EN-434, which was obtained from the marine red alga Symphyocladia latiuscula. Their structures were determined by NMR and HRESIMS spectra data. The absolute confi guration of compound1was identifi ed by quantum chemical ECD calculations. All new compounds were examined for antioxidant activities. Details of isolation, structural elucidation, and bioactivity evaluation are discussed below.
2 MATERIAL AND METHOD
2.1 General experimental procedure
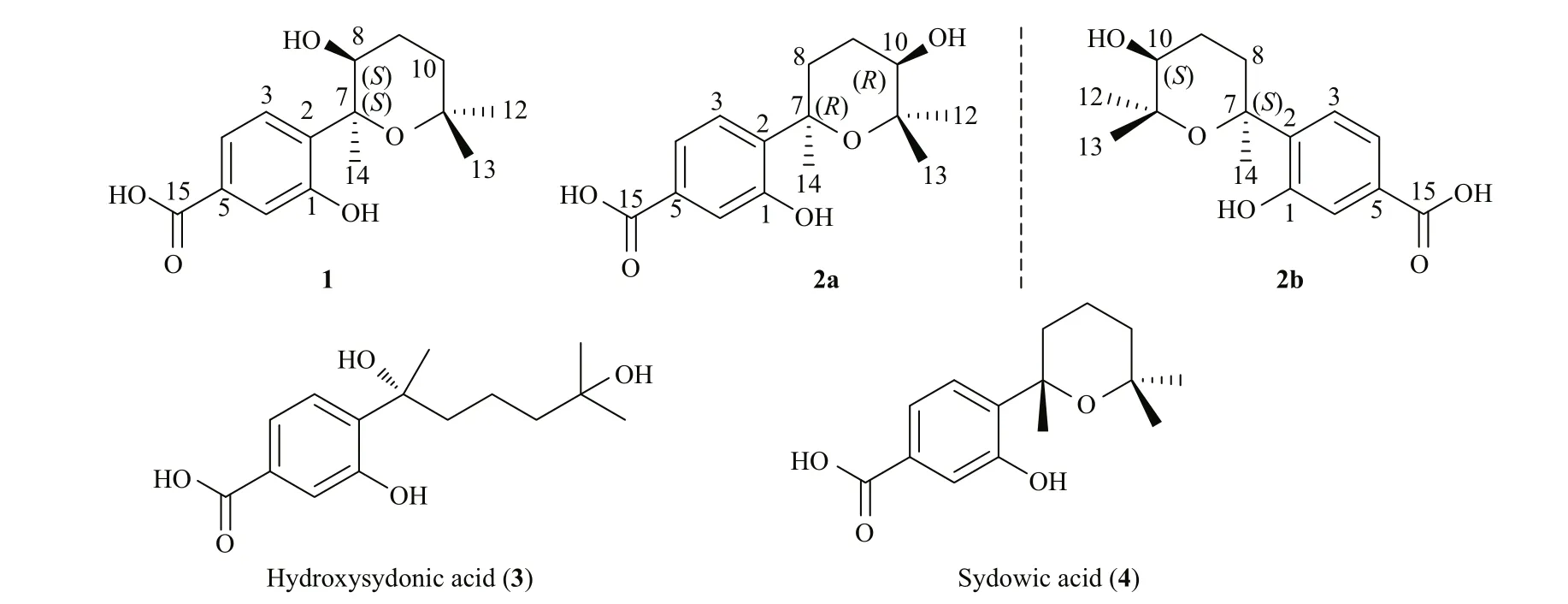
Fig.1 The structures of compounds 1-4 isolated from A. sydowii
Optical rotations were evaluated using an Optical Activity AA-55 polarimeter (Optical Activity Ltd., Cambridgeshire, UK). UV spectra were recorded using a Lengguang Gold S54 spectrophotometer (Shanghai Lengguang Technology Co. Ltd., Shanghai, China). ECD spectra were measured on a JASCO J-715 spectropolarimeter (JASCO, Tokyo, Japan). A Bruker Avance 500 spectrometer (Bruker Biospin Group, Karlsruhe, Germany) was used to acquire 1D and 2D NMR spectra. HRESIMS were measured with a VG Autospec 3000 or an API QSTAR Pulsar 1 mass spectrometer. Chiral HPLC separations were performed using a Dionex HPLC system on a Chiralpak AD-H column (5 μm, 250 mm, 4.6 mm) with hexane/isopropanol (80:20) as eluent. Column chromatography (CC) was carried out with silica gel (200-300 mesh, Qingdao Haiyang Chemical Factory, Qingdao, China), Sephadex LH-20 (18-110 μm, Merck), and Lobar LiChroprep RP-18 (40-60 μm, Merck, Darmstadt, Germany).
2.2 Fungal material
The fungal strain EN-434 was obtained from the fresh tissue of marine red alga Symphyocladia latiuscula, which was collected from Qingdao coastline (36°N, 120°E) in March 2014. The strain was identifi ed as Aspergillus sydowii based on sequence analysis of ITS region of its 18S rDNA as described previously (Wang et al., 2006). The sequenced data have been deposited in GenBank (accession no. MN809362). The strain was kept in store at the Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences (IOCAS).
2.3 Fermentation and extraction
The fresh mycelia of A. sydowii EN-434 (1 cm×1 cm agar plugs) were inoculated into 60×1-L Erlenmeyer fl asks with sterilized rice solid medium which consisted of 70 g of rice, 0.1 g of corn fl our, 0.3 g of peptone, 0.1 g of sodium glutamate, and 100 mL of naturally sourced and fi ltered seawater (obtained from the Huiquan Gulf of the Yellow Sea near the campus of the IOCAS, pH 6.5-7.0), and statically cultured for 30 days at room temperature. After incubation, the fermented cultures were exhaustively extracted with EtOAc for three times. The fi nal extract (69.1 g) was obtained after concentrating the combined EtOAc solution under reduced pressure.
2.4 Isolation
The organic extract was subjected to vacuum liquid chromatography (VLC) eluting with diff erent solvents of growing polarity from petroleum ether (PE) to MeOH to obtain 9 fractions (Frs. 1-9). Fr. 6 (8.8 g), eluted with PE-EtOAc (1:1), was disposed by reversephase column chromatography (CC) containing Lobar LiChroprep RP-18 (with a MeOH-H2O gradient from 10:90 to 100:0) to obtain three subfractions (Fr.6-1-Fr.6-3). Fr. 6-1 was further purifi ed by CC on silica gel (eluted with CH2Cl2-MeOH, 150:1 to 40:1) to get compound 1 (7.8 mg). Fr. 6-2 (eluted with MeOH-H2O (40:60)) was further purifi ed by prep. TLC (plate: 20 cm×20 cm, developing solvents: CH2Cl2-MeOH, 40:1) to obtain compounds (±)- 2 (11.4 mg) and 3 (23.6 mg). Fr. 6-3 (eluted with MeOH-H2O (60:40)) was further purifi ed by CC on silica gel (eluted with CH2Cl2-MeOH, 200:1 to 20:1) to get compound 4 (130 mg).
2.5 Radical scavenging assay
The radical scavenging activity of compounds 1 and (±)- 2, on the α, α-diphenyl- β-picrylhydrazyl (DPPH) free radical was measured. A reaction mixture containing 100 μL of DPPH (0.16 mmol/L in MeOH) and 100 μL samples in MeOH diluted to give fi nalconcentrations of 100, 50, 25, 12.5, 6.25 and 3.13 μg/mL were placed in 96 cell plates incubated in the dark for 30 min (Wang et al., 2007). After the reaction, absorbance was measured at 517 nm and percent inhibition was calculated. The mixture of 100-μL MeOH and 100-μL of DPPH was measured as a blank control group. IC50values denote the concentration of sample required to scavenge 50% of the DPPH free radical. Butylated hydroxy-toluene (BHT) was used as a positive control.

Table 1 1 H and 13 C NMR spectroscopic data of compounds 1 and (±)-2
2.6 ECD calculation
Conformational searches were carried out via molecular mechanics using the MMFF method in Macromodel software, and the geometries were reoptimized at B3LYP/6-31G(d) PCM/MeOH level by means of Gaussian 09 software (Frisch et al., 2013) to generare the energy-minimized conformers. After this, the optimized conformers were used to calculate of ECD spectra using TDDFT at BH and HLYP/TZVP; solvent eff ects of the MeOH solution were estimated at the same DFT level using the SCRF/PCM method.
2.7 Spectral data
(7 S,8 S)-8-hydroxysydowic acid ( 1): Colorless oily liquid; [α]D20=+6.67 ( c 0.1, MeOH); UV (MeOH) λmax(log ε) 213 (4.62), 245 (4.15), 300 (3.75) nm; ECD (1.0 mg/mL, MeOH) λmax(Δ ε) 212 (+0.99), 220 (-0.28) nm;1H and13C NMR data (Table 1); HRESIMS m/ z 281.1387 [M+H]+(calcd for C15H21O5, 281.138 4).
(±)-(7 R*,10 R*)-10-hydroxysydowic acid ( 2): Colorless oily liquid; [α]D20=0 ( c 0.1, MeOH); UV (MeOH) λmax(log ε) 225 (5.30), 276 (4.52) nm;1H and13C NMR data (Table 1); HRESIMS m/ z 281.139 1 [M+H]+(calcd for C15H21O5, 281.138 4).
3 RESULT AND DISCUSSION
Compound 1 was obtained as colorless oil and its molecular formula was decided as C15H20O5based on the positive HRESIMS data (Supplementary Fig.S1), indicating six degrees of unsaturation. The1H NMR spectrum (Supplementary Fig.S2) showed three aromatic proton signals at δH7.47 (H-3, 1H, d, J=8.2 Hz), 7.35 (H-4, 1H, dd, J=8.2, 1.6 Hz) and 7.23 (H-6, 1H, d, J=1.6 Hz), one oxygenated methine signal at δH3.91 (H-8, 1H, m), two methylenes at δH1.78 (Hα-9, 1H, m) and δH1.47 (Hβ-9, 1H, m) as well as δH1.78 (H-10, 2H, m); and three methyl signals at δH1.50 (H-14, 3H, s), 1.31 (H-12, 3H, s) and 1.12 (H-13, 3H, s). Its13C NMR and DEPT spectroscopic data (Supplementary Fig.S3) presented 15 carbon atoms, including three methyls, two methylenes, four methines (with one oxygenated and three aromatic), and six nonprotonated carbons (with one carbonyl, two oxygenated and three aromatic) (Table 1). The structure of compound 1 was determined to be similar to sydowic acid ( 4) by the detailed analysis of the 1D and 2D NMR spectra (Hamasaki et al., 1975), revealing that 1 belongs to the family of phenolic bisabolanes. The NMR shifts of C-8 in compound 1 was changed from a methylene signal at δH/C2.06, 1.64/30.8 in 4 to a methine signal at δH/C3.91/69.2, indicating the hydroxylation of C-8. Further COSY correlation (Supplementary Fig.S4) between H-8 and H-9, as well as HMBC correlation (Supplementary Fig.S5) from H-14 to C-8 also indicated that the hydroxylation linked at C-8 (Fig.2). Based on the above spectroscopic evidence, the planar structure of 1 was determined.

Fig.2 Key COSY (bold lines) and HMBC (red arrows) correlations for 1 and (±)-2
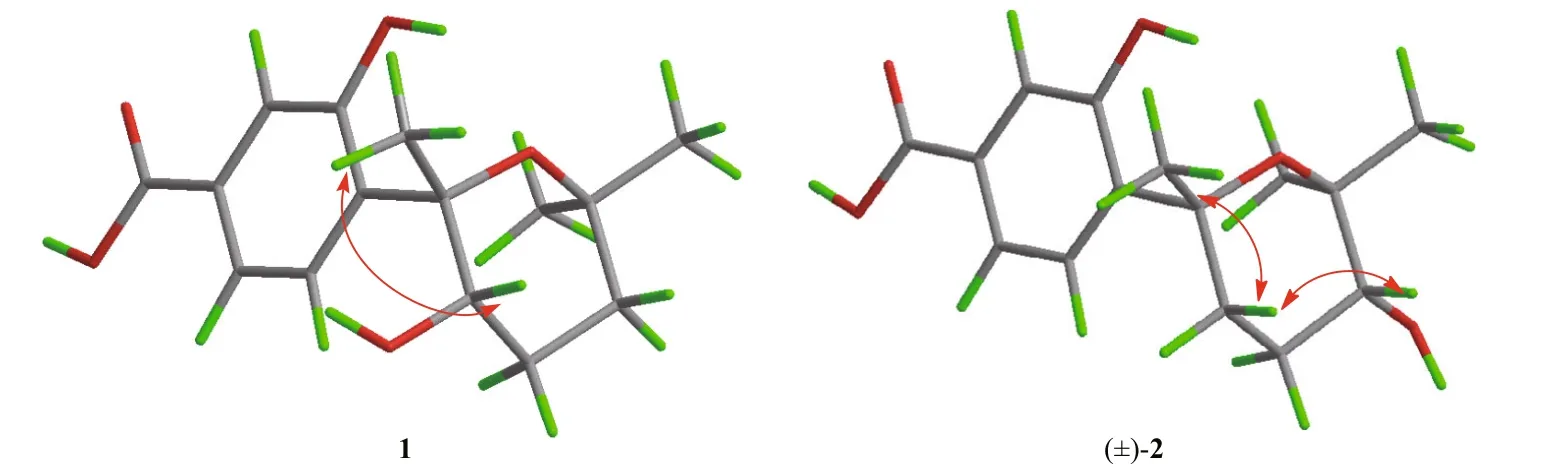
Fig.3 Key NOESY (blue arrows) correlations for 1 and (±)-2
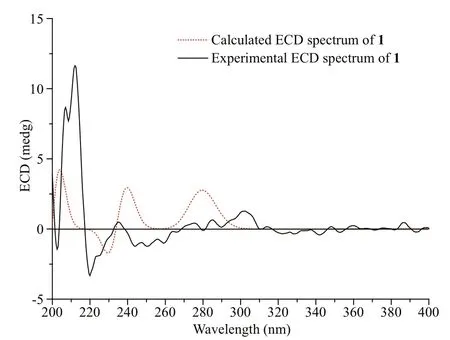
Fig.4 Experimental and calculated ECD for 1
Based on the analysis of NOESY data (Supplementary Fig.S6), the relative confi guration of 1 was assigned (Fig.3). The crucial NOE correlation between H3-14 and H-8 indicated that they were on the identical side of the molecule. The electronic circular dichroism (ECD) spectrum of 1 was measured and then calculated with the time-dependent density function theory (TDDFT) method at the B3LYP/6-31G (d) PCM/MeOH level to determine its absolute confi guration. The experimental ECD spectrum for 1 matched well with the calculated spectrum for (7 S,8 S)- 1 (Fig.4). Therefore, the 7 S, 8 S confi guration of 1 was established, and the structure of compound 1 was determined as (7 S,8 S)-8-hydroxysydowic acid.
Compound (±)- 2 was obtained as colorless oil. The molecular formula of (±)- 2 was also determined to be C15H20O5by HREIMS (Supplementary Fig.S7), showing that it is the isomer of compound 1. The diff erence in1H NMR spectrum (Supplementary Fig.S8-S9) between compounds 1 and (±)- 2 is that the oxygenated methine signal changed from δH3.91 in 1 to δH3.27 in (±)- 2 (Table 1). Further COSY correlation (Supplementary Fig.S10) between H2-9 and H-10, as well as HMBC correlations (Supplementary Fig.S11) from H3-12 and H3-13 to C-10 confi rmed the hydroxylation linked at C-10 (Fig.2).
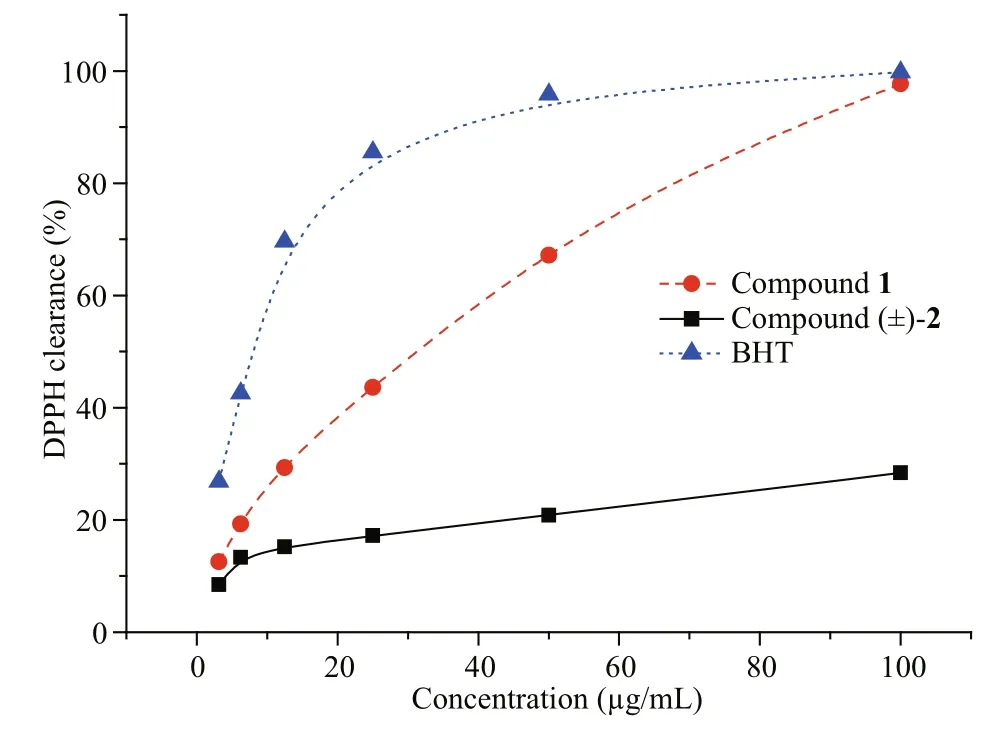
Fig.5 DPPH free radical scavenging activity for 1, (±)-2 and BHT (positive control)
The key NOE correlations (Supplementary Fig.S12) from H-8 α to H-10 and H3-14 indicated the relative confi guration of (±)- 2 is 7 R*, 10 R* (Fig.3). The baseline ECD curve and zero specifi c rotation value ([α]D20=0 (c 0.1, MeOH)) suggested that it was a racemic mixture of two enantiomers with identical NMR data. The chiral HPLC analysis of (±)- 2 showed the separation of the two enantiomers (Supplementary Fig.S13). However, we cannot obtain single enantiomer because both of them will change to each other in dynamic balance after separation.
The isolated new Compounds 1 and (±)- 2 were evaluated for DPPH free radical scavenging potency. Compound 1 showed signifi cant activity with IC50value of 113.5 μmol/L, while compound (±)- 2 had no activity (IC50value >300 μmol/L). BHT was used as a positive control with IC50value of 30.8 μmol/L (Fig.5). Racemization and the position of the hydroxyl group may aff ect the DPPH free radical scavenging activity.
4 CONCLUSION
Two new bisabolene sesquiterpenoids along with two known analogues had been isolated from the culture extract of A. sydowii EN-434, the marine red algal-derived fungus, indicating that this type of bisabolene sesquiterpenoids are the main metabolites of A. sydowii. The structures of these compounds were elucidated using NMR and HRESIMS as well as quantum chemical ECD calculations. Compound 1 showed a potent DPPH free radical scavenging activity.
5 DATA AVAILABILITY STATEMENT
All the data supporting the results of this study are available within the article and the supplementary material.
References
Barzkar N, Jahromi S T, Poorsaheli H B, Vianello F. 2019. Metabolites from marine microorganisms, micro, and macroalgae: immense scope for pharmacology. Mar. Drugs, 17(8): 464, https://doi.org/10.3390/md17080464. Blunt J W, Copp B R, Keyzers R A, Munro M H G, Prinsep M R. 2015. Marine natural products. Nat. Prod. Rep., 32(2): 116-211, https://doi.org/10.1039/C4NP00144C.
Cho K H, Sohn J H, Oh H. 2018. Isolation and structure determination of a new diketopiperazine dimer from marine-derived fungus Aspergillus sp. SF-5280. Nat. Prod. Res., 32(2): 214-221, https://doi.org/10.1080/14786 419.2017.1346642.
Frisch M J, Trucks G W, Schlegel H B et al. 2013. Gaussian 09, Revision D.01. Wallingford, CT: Gaussian, Inc.
Hamasaki T, Nagayama K, Hatsuda Y. 1978. Two new metabolites, sydonic acid and hydroxysydonic acid, from Aspergillus sydowi. Agric. Biol. Chem., 42(1): 37-40, https://doi.org/10.1271/bbb1961.42.37.
Hamasaki T, Sato Y, Hatsuda Y, Tanabe M, Cary L W. 1975. Sydowic acid, a new metabolite from Aspergillus sydowi. Tetrahedron Lett., 16(9): 659-660, https://doi.org/10.1016/S0040-4039(00)71947-2.
Li W T, Luo D, Huang J N, Wang L L, Zhang F G, Xi T, Liao J M, Lu Y Y. 2018. Antibacterial constituents from Antarctic fungus, Aspergillus sydowii SP-1. Nat. Prod. Res., 32(6): 662-667, https://doi.org/10.1080/14786419.2017.1335730.
Liu S, Wang H B, Su M Z, Hwang G J, Hong J K, Jung J H. 2017. New metabolites from the sponge-derived fungus Aspergillus sydowii J05B-7F-4. Nat. Prod. Res., 31(14): 1 682-1 686, https://doi.org/10.1080/14786419.2017.1289205.
Liu X R, Song F H, Ma L, Chen C X, Xiao X, Ren B, Liu X T, Dai H Q, Piggott A M, Av-Gay Y, Zhang L X, Capon R J. 2013. Sydowiols A-C: Mycobacterium tuberculosis protein tyrosine phosphatase inhibitors from an East China Sea marine-derived fungus, Aspergillus sydowii. Tetrahedron Lett., 54(45): 6 081-6 083, https://doi.org/10. 1016/j.tetlet.2013.08.137.
Schinke C, Martins T, Queiroz S C N, Melo I S, Reyes F G R. 2017. Antibacterial compounds from marine bacteria, 2010-2015. J. Nat. Prod., 80(4): 1 215-1 228, https://doi.org/10.1021/acs.jnatprod.6b00235.
Tasdemir D. 2017. Marine fungi in the spotlight: opportunities and challenges for marine fungal natural product discovery and biotechnology. Fungal Biol. Biotechnol., 4(1): 5, https://doi.org/10.1186/s40694-017-0034-1.
Teuscher F, Lin W H, Wray V, Edrada R, Padmakumar K, Proksch P, Ebel R. 2006. Two new cyclopentanoids from the endophytic fungus Aspergillus sydowii associated with the marine alga Acanthophora spicifera. Nat. Prod. Comm., 1(11): 927-933, https://doi.org/10.1177/193457 8X0600101103.
Trisuwan K, Rukachaisirikul V, Kaewpet M, Phongpaichit S, Hutadilok-Towatana N, Preedanon S, Sakayaroj J. 2011. Sesquiterpene and xanthone derivatives from the sea fanderived fungus Aspergillus sydowii PSU-F154. J. Nat. Prod., 74(7): 1 663-1 667, https://doi.org/10.1021/np200374j.
Wang S, Li X M, Teuscher F, Li D L, Diesel A, Ebel R, Proksch P, Wang B G. 2006. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod., 69(11): 1 622-1 625, https://doi.org/10.1021/np060248n.
Wang W L, Lu Z Y, Tao H W, Zhu T J, Fang Y C, Gu Q Q, Zhu W M. 2007. Isoechinulin-type alkaloids, Variecolorins A-L, from halotolerant Aspergillus variecolor. J. Nat. Prod., 70(10): 1 558-1 564, https://doi.org/10.1021/np070208z.
Wang Y N, Mou Y H, Dong Y, Wu Y, Liu B Y, Bai J, Yan D J, Zhang L, Feng D Q, Pei Y H, Hu Y C. 2018. Diphenyl ethers from a marine-derived Aspergillus sydowii. Mar. Drugs, 16(11): 451, https://doi.org/10.3390/md16110451.
Xu X L, Zhao S J, Yin L Y, Yu Y, Chen Z K, Shen H H, Zhou L. 2017. A new sydonic acid derivative from a marine derivedfungus Aspergillus sydowii. Chem. Nat. Compd., 53(6): 1 056-1 058, https://doi.org/10.1007/s10600-017-2200-3.
 Journal of Oceanology and Limnology2020年5期
Journal of Oceanology and Limnology2020年5期
- Journal of Oceanology and Limnology的其它文章
- Distribution, sources and burial fl ux of sedimentary organic matter in the East China Sea*
- Photoelectrochemical cathodic protection of Cu 2 O/TiO 2 p-n heterojunction under visible light*
- Calcium isotopic signatures of depleted mid-ocean ridge basalts from the northeastern Pacifi c*
- Application of confocal laser Raman spectroscopy on marine sediment microplastics*
- Corrosion behavior of Q235B carbon steel in simulated seawater pumped storage system under operational conditions*
- Inductive eff ect of bioactive substances on strobilation of jellyfi sh Aurelia coerulea*
