Sulcus and otolith outline analyses: complementary tools for stock discrimination in white croaker Pennahia argentata in northern Chinese coastal waters*
SONG Junjie , DOU Shuozeng , CAO Liang LIU Jinhu
1 Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract This study analyzed and compared variations of the sulcus and otolith outlines of three geographic stocks (Huanghe (Yellow) River estuary (HHE), Jiaozhou Bay (JZB), and Changjiang (Yangtze) River estuary (CJE)) of white croaker Pennahia argentata in northern Chinese coastal waters. The sulcus and otolith outline analyses via elliptical Fourier transform (EFT, i.e., outline analysis) achieved an overall classifi cation rate of 80.4% and 87.2%, respectively. Based on a combination of sulcus and otolith shape indices (SIs) and two derivative ratios, a moderate discriminatory effi ciency of 64.7% was obtained. The results indicate that sulcus outline analysis could be used alone to discriminate white croaker stocks, and that both sulcus and otolith outline analyses discriminated the fi sh stocks at a higher classifi cation rate than the shape indices. The sulcus outline analysis provided complementary information to the whole otolith outline analysis for stock discrimination. Both the sulcus and otolith outline analyses effi ciently discriminated between the most geographically separated CJE and HHE stocks, indicating that they could be considered discrete stocks for fi shery management.
Keyword: sulcus; otolith; elliptic Fourier coeffi cients; shape indices; stock discrimination
1 INTRODUCTION
Otoliths are calcifi ed structures in the inner ears of teleost fi sh that are metabolically inert and grow throughout the lifetime of the fi sh (Campana and Neilson, 1985). Otoliths are of interest in fi sh population studies because they can provide diff erent types of information (e.g., microstructure, microchemistry, and morphology) for investigating the life histories and population dynamics of fi sh (Popper et al., 2005). Otolith shape is intraspecifi c and interspecifi c due to environmental or genetic regulation (Campana and Casselman, 1993; Lombarte and Lleonart, 1993; Cardinale et al., 2004). Moreover, shape analysis is a relatively inexpensive and timeeffi cient method compared with other techniques such as genetic and microchemical methods. Therefore, otolith shape analysis has often been used for stock discrimination (Castonguay et al., 1991; Burke et al., 2008; Agüera and Brophy, 2011; Avigliano et al., 2015) and species identifi cation (Lombarte and Morales-Nin, 1995; Tuset et al., 2003a; Avigliano et al., 2016).
Facilitated by the development of image processing tools, otolith shape analysis has advanced from the measurement of morphometrics (e.g., length, width, area, and perimeter) and derivative shape indices (SIs; e.g., roundness, form-factor, circularity, rectangularity, and ellipticity) to the extraction of geometric parameters (i.e., outline analysis) to discriminate groups (Cadrin and Friedland, 1999; Tuset et al., 2003b; Libungan and Pálsson, 2015). Geometric methods can quantify boundary shapes so that intraspecies or interspecies variations in otolith outlines can be quantitatively evaluated (Stransky, 2014); in particular, geometric outline analysis has been increasingly used to discriminate groups in recent decades. This method commonly involves 1) capturing the otolith outline with imaging equipment; 2) fi tting a geometric model to extract coeffi cients that concisely describe the outline; and 3) using the coeffi cients as multivariate variables to detect patterns of variance, discriminate groups, and classify individuals into groups (Stransky, 2014). Coeffi cients of otolith outlines are commonly extracted by Fourier transform (e.g., fast Fourier transform (FFT), elliptical Fourier transform (EFT)) or wavelet transform, and a number of studies have demonstrated the effi ciency of this method for species identifi cation and stock discrimination (Torres et al., 2000a; Stransky et al., 2008; Libungan and Pálsson, 2015). Furthermore, the use of otolith outline analysis with complementary tools such as elemental composition and genetics can improve the effi ciency of group discrimination (Longmore et al., 2010; Ferguson et al., 2011; Avigliano et al., 2014).
Previous studies have commonly used whole otolith shape to discriminate groups, but the shapes of certain otolith structures have not been well evaluated. Analyzing the shapes of these structures might provide additional information about otoliths that is complementary to the whole otolith shape analysis. Additionally, during the process of extracting and preserving otoliths, problems such as broken edges often occur that can confound the application of whole otolith shape analysis, particularly when samples are rare. In these cases, certain structures that are usually present inside the otolith and are thus in good condition can be used as a substitute for the whole otolith in stock discrimination.
The sulcus, a longitudinal depression on the medial side of the otolith connecting with the sensory epithelium or macula, is such a structure that might be applicable to stock discrimination (Popper et al., 2005; Tuset et al., 2008). The sulcus is commonly divided into two parts, the ostium and the cauda, which are limited by a developed and evident rim (the crista) and frequently exhibit a raised fl oor (the colliculum; Tuset et al., 2008), and in some species, there is a clear morpho-anatomical relationship between the sulcus and the macula. Meanwhile, a fi sh with a larger relative space between the macula and the otolith would be more sensitive to a particular acoustic frequency. Thus, the morphological development of the sulcus can be related to the ecomorphological adaptions of the auditory system (Gauldie, 1988; Torres et al., 2000b; Jaramillo et al., 2014).
Certain morphological characteristics of the sulcus are important features for species identifi cation, such as the morphological relationship between the ostium and the cauda, the type of opening displayed by the sulcus, and the position of the sulcus on the otolith (Tuset et al., 2008). A number of studies have investigated the interspecifi c variation in sulcus morphology in diff erent fi sh genera or families (Lombarte and Morales-Nin, 1995; Torres et al., 2000b; Montanini et al., 2015), only a few have investigated the intraspecifi c variation in sulcus morphology, usually by combining sulcus morphometrics with those of the corresponding whole otolith to discriminate stocks (Avigliano et al., 2014, 2015). Overall, the use of shape analysis (particularly geometric outline analysis) of the sulcus for stock discrimination has not yet been well documented.
The white croaker Pennahia argentata is an important demersal sciaenid that is widely distributed along the Chinese coast. This species usually spawns in spring in the coastal waters, lives and forages from summer to autumn in the adjacent nursery areas, and migrates to deeper shelf waters in winter (Chen, 1991). Due to overfi shing and habitat destruction in recent decades, the catch of the white croaker fi shery has been declining. Meanwhile, like many fi shery species, the white croaker has shown a tendency toward miniaturization of biological traits such as size, age, and maturity (Ju et al., 2016). Although it is commonly speculated that diff erent white croaker stocks occur along the Chinese coast, they have not been consistently clarifi ed or well documented. For example, Chen (1991) assumed that there may be three major populations (i.e., the Bohai Sea stock, the Yellow Sea stock and the East China Sea stock) in northern Chinese coastal waters, while Chen and Xu (2011) considered the Bohai Sea population and the Yellow Sea population to be a single population. Data on stock discrimination and structures are essential to eff ectively manage fi shery stocks. Additionally, as the white croaker is a member of the Sciaenidae, its sagittae are characterized by a relatively large and visible sulcus, making this species an ideal target for testing the effi ciency of sulcus and otolith shape analysis for stock discrimination.
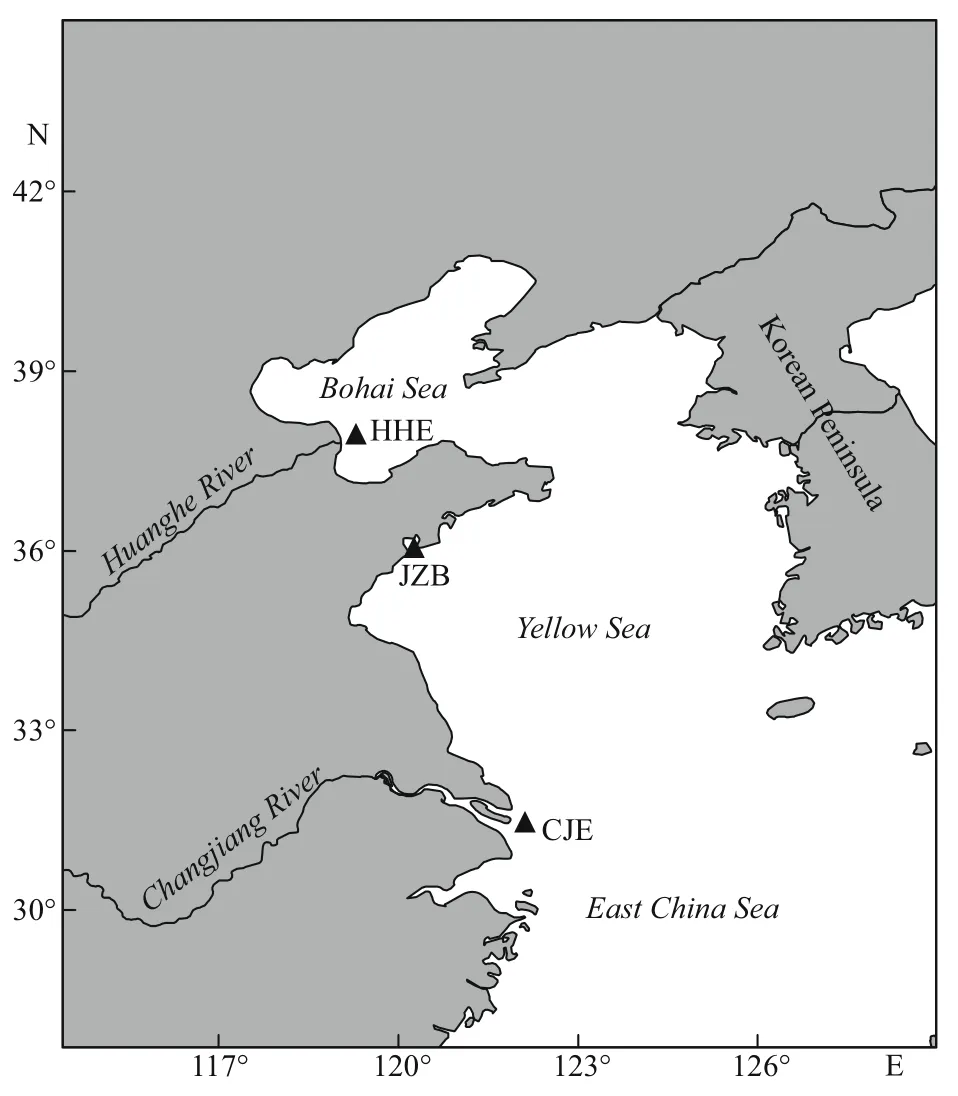
Fig.1 White croaker sampling sites
In this study, the variations in otolith and sulcus shape of three white croaker stocks in northern Chinese coastal waters were analyzed using both shape indices and geometric outline analysis by EFT. The main objective was to determine whether sulcus shape analysis can be as eff ective as otolith shape analysis in discriminating stocks, but the discriminatory effi ciencies of the shape indices and geometric outlines were also compared and assessed. Finally, this study sought to provide insights into white croaker stocks that could promote a better understanding for implementing stock-based fi shery management.
2 MATERIAL AND METHOD
2.1 Sample collection and preparation
Fish were collected by bottom trawling from April to July (the main spawning season of white croakers) during fi shery surveys of the northern Chinese coast. The sampling sites included the Huanghe River estuary (HHE) in the Bohai Sea, Jiaozhou Bay (JZB) in the Yellow Sea, and the Changjiang River estuary (CJE) in the East China Sea, all of which are traditional spawning areas for white croaker (Fig.1).
Immediately after capture, fi sh were sampled from the catch, labeled and frozen for subsequent biologicalanalysis; and the biological parameters of the samples were measured and recorded in the laboratory. The standard length of the fi sh was used in this study. The sagittae were removed from each fi sh, cleaned of adhering tissues in distilled water, air dried, and stored in sealed glass vials for subsequent analysis. Otoliths of 1+-year-old fi sh, as estimated by the fi sh length-age relationship and capture time, were used for this study (Table 1). Since otolith shape commonly changes with the ontogeny of the otolith throughout the life of fi sh (Waessle et al., 2003; Naya et al., 2012; de Carvalho et al., 2015), only adult fi sh were used for this study. All the samples were larger than the minimum standard length at maturity, which was around 130 mm (Lin et al., 2006).

Table 1 Basic information about the white croaker samples
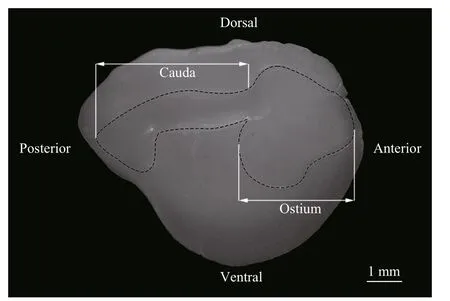
Fig.2 Left otolith and sulcus outlines of the white croaker
Prior to imaging to obtain morphological parameters, the otoliths were rinsed in an ultrasonic cleaner, dried in an oven at 35°C, and weighed to the nearest 0.01 mg.
2.2 Data acquisition
Imaging was performed by positioning the medial side (i.e., the side with the sulcus) of each otolith to face up under a binocular microscope (Nikon SMZ1000, Tokyo, Japan) connected to a digital video camera, and ACT-2 software was used to capture the images (Fig.2) at the same magnifi cation (10×). The lighting conditions were adjusted to capture the clearest image of each sulcus. Because the contrast between the sulcus outline and the other regions of the otolith was not as distinct as that between the otolith outline and the black background, the sulcus was identifi ed with the aid of Photoshop CS 5.0 (Adobe Systems Inc., San Jose, CA). The pen tool was used to outline the sulcus boundary. The sulcus area was then selected and painted white, while the other regions of the otolith were painted black. Thus, a clear image of the sulcus could be captured for subsequent information extraction and statistical analysis of morphological parameters.
To validate the determination of the sulcus outline with Photoshop CS 5.0, ten specimens were randomly selected to defi ne the sulcus outline, which was repeated six times per specimen. The coeffi cient of variation (CV) of the morphometrics (length, width, area, and perimeter) of these repetitions were calculated according to the following formula (Zar, 2010):

The images were imported into R (R Core Team; www.r-project.org) and evaluated using the shapeR package (Libungan and Pálsson, 2015) to generate the morphometrics and elliptic Fourier harmonics of both the sulcus and the otolith. The shape indices (i.e., form-factor, roundness, circularity, rectangularity, and ellipticity) and ratios (SA/OA, sulcus area/otolith area; SP/OP, sulcus perimeter/otolith perimeter) were calculated (Tuset et al., 2003b; Avigliano et al., 2014).
EFT was employed because it can be applied to complex curves (such as those exhibited by the sulcus of the white croaker). Each elliptic Fourier harmonic has four coeffi cients (EFc) that are automatically normalized based on the fi rst three coeffi cients of harmonic 1 so that the EFc are independent of the rotation, dilation, and translation of the outline (Kuhl and Giardina, 1982). These three coeffi cients are constants and are removed in the subsequent analyses. To determine the appropriate number of harmonics to adopt in the subsequent analyses, the Fourier power of each harmonic was calculated according to the following formula (Crampton, 1995):

where An- Dnare four coeffi cient of the nthharmonic. The Fourier harmonics were truncated at the value of n when the cumulative power exceeded 99.99% of the total power.
To demonstrate the Fourier power of the harmonics to describe the outline of the otolith or sulcus, a series of Fourier reconstructions of both structure were conducted using the harmonics adopted for the subsequent analyses.
2.3 Statistical analysis
The otolith and sulcus morphological data were subjected to the same analytical procedures. Data processing and analysis were performed in SPSS 20.0 (IBM Corp., Armonk, NY) and SAS 9.4 (SAS Institute, Cary, NC), and diff erences were considered signifi cant at P <0.05.
The Kruskal-Wallis test was adopted to examine the size distributions of fi sh among stocks, and no signifi cant diff erences were observed.
The diff erences in sulcus and otolith morphometrics among stocks were examined using multivariate analysis of covariance (MANCOVA) with sampling site as the main factor and fi sh length as the covariate. Before the MANCOVA was performed, the Shapiro-Wilk test and Levene’s test were performed to verify the normality and homogeneity of variance of the data, respectively. If any variable did not meet the assumptions, the data were logarithm, square-root, or arcsine transformed. At this stage, the variables that met the assumptions were kept for subsequent analyses. Multiple comparisons with the Student-Newman-Keuls (SNK) test were performed to compare the diff erences in morphometrics between stocks.
The combination of sulcus and otolith shape indices, the sulcus EFc, and the otolith EFc were used for stock discrimination, respectively. To be informative in discriminating among stocks, the variables should be independent of fi sh size; therefore, the eff ects of fi sh length on shape indices or EFc were tested by MANCOVA with sampling site as the factor and fi sh length as the covariate. Furthermore, if a signifi cant interaction was found, the corresponding coeffi cient was also excluded from further statistical analysis because it could not be accurately adjusted (Begg and Brown, 2000). The remaining variables that were signifi cantly infl uenced by fi sh length were adjusted (i.e., standardized to a mean fi sh length) according to the following formula (Campana and Casselman, 1993):

where Yi*is the adjusted parameter; Yiis the original variable; X0is the mean fi sh length (162 mm in this study); Xiis the length of a given fi sh specimen; and a is the regression coeffi cient between the variable and the fi sh length, which is common to all groups.
After adjusting for length, the variables (shape indices or EFc) were separately adopted for principal component analysis (PCA). Since signifi cant correlations might exist among the variables, which would confound the results of the discriminant analyses, the PCA generated a new series of orthogonal variables, the principal component scores (PCs), to remove this potential eff ect. Furthermore, a small number of PCs can usually explain most of the variance in parameters, thus reducing the number of variables adopted for the subsequent discriminant analyses. A scree plot was used to determine the number of PCs for each subsequent canonical discriminant analysis (CDA). The correlation matrices were selected to calculate the PCs of the shape indices, while the variance-covariance matrices were selected for EFc.
Based on the determined PCs, stepwise CDA was adopted to evaluate the accuracy of using sulcus and otolith shape analysis to discriminate the three white croaker stocks. Classifi cation success rates were generated based on leave-one-out cross-validations, and since the sample sizes diff ered among the stocks, the prior probabilities were computed from the sample sizes. In the CDA, the homogeneity of the withingroup covariance matrices was tested to determine the use of a linear model (homogenous matrices; using pooled matrices) or quadric model (heterogeneous matrices; using within-group matrices; Tuset et al., 2003b).
3 RESULT
3.1 Intraspecifi c variations in sulcus and otolith morphometrics
The sulcus morphometrics signifi cantly diff ered among stocks (MANCOVA, P <0.05; Table 2). The results of multiple comparisons indicate that the mean sulcus length of the CJE stock was signifi cantly greater than those of the HHE and JZB stocks, with the latter two showing no signifi cant diff erences. The mean sulcus widths of the three stocks diff ered from each other, with the greatest in the CJE stock followed by the HHE and JZB stocks. The mean sulcus perimeter, area and SA/OA did not diff er signifi cantlybetween the CJE and HHE stocks, but they were all signifi cantly larger than those of the JZB stock.
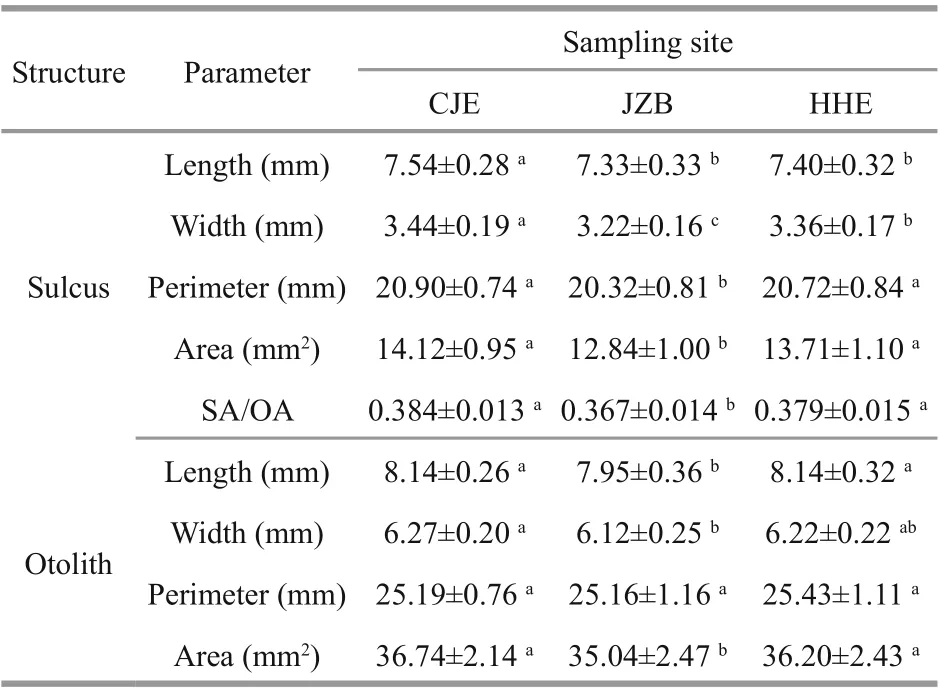
Table 2 Sulcus and otolith morphometrics of the white croaker samples
Similarly, the overall otolith morphometrics diff ered signifi cantly among the stocks (MANCOVA, P <0.05; Table 2). The results of multiple comparisons indicate that neither the mean otolith length nor the area diff ered signifi cantly between the CJE and HHE stocks, but they were both signifi cantly larger than in the JZB stock. The mean otolith width of the CJE stock was signifi cantly larger than that of the JZB stock, while that of the HHE stock was not signifi cantly diff erent from the other two. The mean otolith perimeter did not diff er signifi cantly among the three stocks.
3.2 Stock discrimination using sulcus and otolith shape indices
After adjustment for length, seven shape indices of the sulcus (form-factor, roundness, circularity, rectangularity, and ellipticity) and otolith (rectangularity and ellipticity), together with two derivative ratios (SA/OA and SP/OP), were found to meet the corresponding statistical assumptions and were thus adopted in the PCA. The correlation coeffi cients between the discriminant functions and the PCs adopted in the stock discrimination as well as the main components of each PC are summarized in Table 3. The prior probabilities of the CJE, JZB, and HHE stocks were 31.6%, 27.8%, and 40.6%, respectively. In the CDA, the within-group covariance matrices were homogenous ( P >0.05); therefore, the linear model was adopted.
Seven PCs were generated by the PCA, of whichsix (PC1, PC2, PC3, PC4, PC6, and PC7) were adopted in the CDA. These six PCs explained 39.9%, 20.4%, 15.3%, 10.5%, 4.0%, and 1.4% of the variance in the variables, respectively. The fi rst discriminant function ( F 1; eigenvalue, E=0.472) explained 65.5% of the variance and could basically discriminate the JZB stock from the other two; F 1 was mainly correlated with PC1 ( R=0.654). The second discriminant function (F2; E=0.249) explained 34.5% of the variance and could basically discriminate between the CJE and HHE stocks; F2 was mainly correlated with PC6 ( R=0.900). PC1 and PC2 were mainly correlated with the sulcus shape indices and the two ratios (SA/OA and SP/OP), whereas the other PCs were closely correlated with the otolith shape indices and the two ratios (Fig.3a, Table 3).

Table 3 Summary of the correlation coeffi cients between the discriminant functions and the PCs adopted for stock discrimination and the main components of each PC
The CDA produced an overall classifi cation success rate of 64.7%, with the highest rate obtained for the HHE stock (70.4%), followed by the CJE (64.3%) and JZB (56.8%) stocks (Table 4).
3.3 Stock discrimination using the sulcus outline via EFT
Fourteen Fourier harmonics for the sulcus were adopted in the PCA. The correlation coeffi cients between the discriminant functions and the PCs adopted in the stock discrimination as well as the main components of each PC are summarized in Table 3. The prior probabilities of the CJE, JZB, and HHE stocks were 31.6%, 27.8%, and 40.6%, respectively. In the CDA, the within-group covariance matrices were heterogeneous ( P <0.05); therefore, the quadric model was adopted.

Fig.3 Scatterplots of the two discriminant functions for the white croaker stocks using shape indices (a), sulcus outline (b), and otolith outline (c)
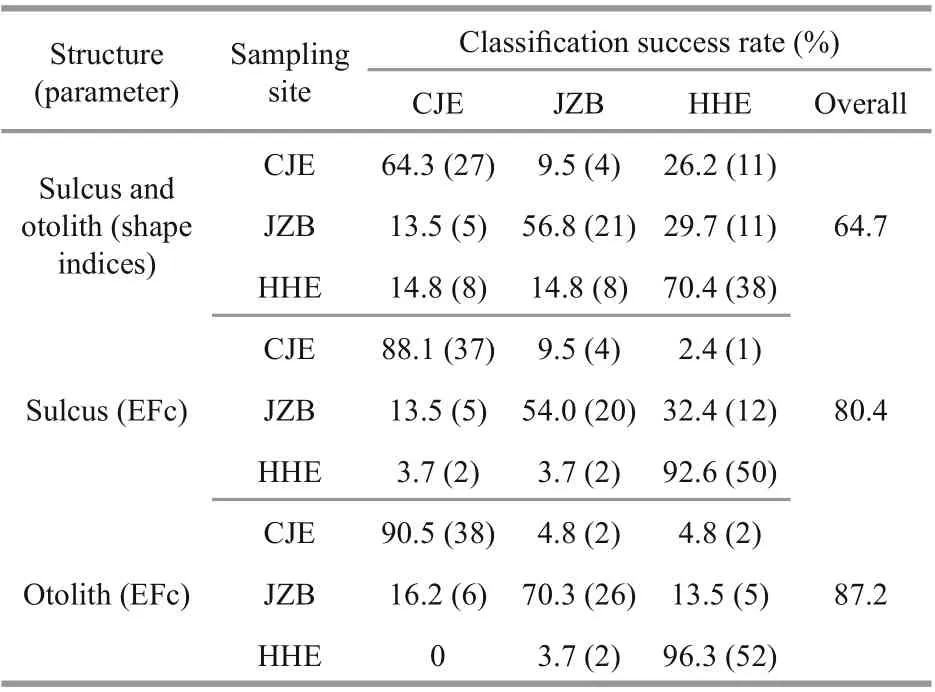
Table 4 Classifi cation success rates for the white croaker samples based on otolith and sulcus shape analysis
Seven PCs were generated by the PCA, of which fi ve (PC1, PC2, PC3, PC5, and PC7) were adopted in the CDA. These fi ve PCs explained 40.6%, 25.0%, 11.1%, 4.0%, and 1.7% of the variance in the variables, respectively. F 1 ( E=2.015) explained 85.3% of the variance and could discriminate well between the CJE and HHE stocks; F 1 was primarily correlated with PC1 ( R=0.847). F 2 ( E=0.346) explained 14.7% of the variance and could basically discriminate the JZB stock from the other two; F 2 was primarily correlated with PC5 ( R=0.744) (Fig.3b, Table 3).
The CDA produced an overall classifi cation success rate of 80.4%, with the highest rate obtained for the HHE stock (92.6%), followed by the CJE (88.1%) and JZB (54.0%) stocks (Table 4). Reconstructions of the mean sulcus outlines of the three stocks are shown in Fig.4a. The mean sulcus outlines of the three stocks diff ered most obviously approximately 270°, with several small diff erences observed approximately 10°, 80°, 170°, and 190°.
Reconstruction of the sulcus outline based on the fourteen Fourier harmonics demonstrated that they could precisely describe the sulcus outline (Fig.5a).
3.4 Stock discrimination using the otolith outline via EFT
Nine Fourier harmonics for the otolith were adopted in the PCA. The correlation coeffi cients between the discriminant functions and the PCs adopted in the stock discrimination as well as the main components of each PC are summarized in Table 3. The prior probabilities of the CJE, JZB, and HHE stocks were 31.6%, 27.8%, and 40.6%, respectively. In the CDA, the within-group covariance matrices were heterogeneous ( P <0.05); therefore, the quadric model was adopted.
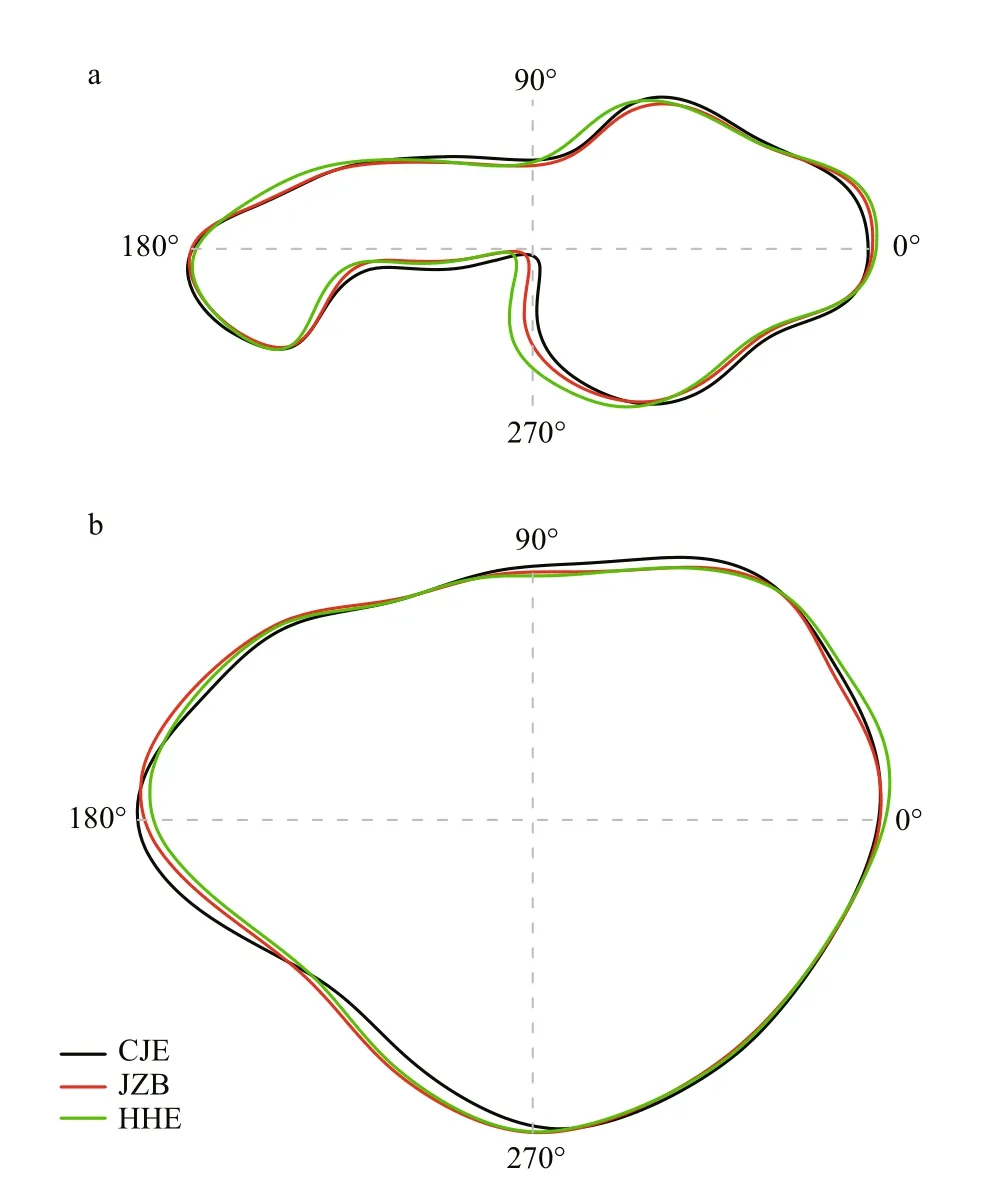
Fig.4 Mean sulcus (a) and otolith (b) outlines of the three white croaker stocks based on Fourier harmonics
Eight PCs were generated by the PCA, all of which were adopted in the CDA. These eight PCs explained 33.4%, 22.1%, 12.3%, 9.8%, 5.6%, 2.9%, 2.3%, and 1.9% of the variance in these variables. F 1 ( E=2.226) explained 75.5% of the variance and could discriminate well between the CJE and HHE stocks; F 1 was mainly correlated with PC1 ( R=-0.641). F 2 ( E=0.723) explained 24.5% of the variance and could well discriminate the JZB stock from the other two; F 2 was mainly correlated with PC3 ( R=0.699) (Fig.3c, Table 3).
The CDA produced an overall classifi cation success rate of 87.2%, with the highest rate obtained for the HHE stock (96.3%), followed by the CJE (90.5%) and JZB (70.3%) stocks (Table 4). Reconstructions of the mean otolith outlines of the three stocks are shown in Fig.4b. The mean otolith outlines of the three stocks diff ered most obviously approximately 0°-90° and 135°-270°.
Reconstructions of the otolith outline based on the nine Fourier harmonics demonstrated that they could precisely describe the otolith outline (Fig.5b).
4 DISCUSSION

Fig.5 Fourier reconstruction of a white croaker sulcus outline (a) and the corresponding otolith outline (b)
Otolith shape (e.g., shape indices and geometric outlines) analysis, either used alone or in combination with other parameters such as elemental fi ngerprints and genetic traits, has become an effi cient and powerful tool in stock discrimination (Longmore et al., 2010; Avigliano et al., 2014; Stransky, 2014). In contrast to the widely used otolith shape, sulcus shape alone has been used less often for discriminating stocks, so the lack of suffi cient knowledge does not allow us to reasonably assess the effi ciency of discriminating stocks by sulcus shape analysis alone. Nonetheless, sulcus and otolith morphometrics have been combined to discriminate the stocks of some fi sh species such as river croaker Plagioscion ternetzi (Avigliano et al., 2015), gurnard Aspitrigla cuculus and Eutrigla gurnardus (Montanini et al., 2015), comber Serranus cabrilla (Tuset et al., 2003b), and three Merluccius species (Torres et al., 2000a). Since the studied species and regions, as well as the adopted morphological parameters and analytical methods, diff ered among studies, the specifi c discriminatory effi ciencies diff ered. However, the results of these studies suggested that the sulcus, when combined with the corresponding whole otolith, was an appropriate method for stock discrimination with reasonable effi ciency. In the present study, the use of a combination of sulcus and otolith shape indices obtained a moderate discriminatory effi ciency for white croaker stocks. The sulcus shape indices and the two ratios (SA/OA and SP/OP) contributed more to the main discriminant functions than the otolith shape indices, but three commonly used otolith shape indices (i.e., form-factor, roundness, and circularity) were excluded because they failed to meet the assumptions of the corresponding statistical analyses. In addition, certain typical sulcus shape indices were not included in the shape analysis because the morphometrics of the corresponding specifi c structures (e.g., the ostium and cauda) could not be accurately defi ned and measured due to their vague boundaries. The absence of such morphological information could confound the discriminatory effi ciency of the shape indices in the present study.
Biological factors (e.g., growth, metamorphosis, and genetics) and environmental conditions (e.g., temperature, habitat, and food) have interactive eff ects on otolith and sulcus morphology (Lombarte and Lleonart, 1993; Cardinale et al., 2004; Berg et al., 2018), so the morphometrics and shape indices of these structures are commonly of biological or ecological relevance (Gauldie, 1988; Lombarte, 1992; Volpedo and Echeverría, 2003). For example, the relative size of the sulcus compared to the otolith (SA/OA) is closely correlated with fi sh hearing, suggesting that fi sh with larger SA/OA produce broader frequencies and are more sensitive to sound, and SA/OA can vary among species or groups (Gauldie, 1988; Arellano et al., 1995). As a sciaenid, the mean SA/OA (0.377) of the white croaker was relatively large compared with those of many other nonsciaenid species, corresponding to their high sensitivity to sound and the relatively broad sound frequencies (199 to 2 570 Hz) that they can produce (Tsai, 2009). The diff erence in SA/OA among the three stocks made this factor an important contributor to stock discrimination in the present study.
Compared with the shape indices, sulcus or otolith outline analysis with EFT alone could discriminate the white croaker stocks with relatively high classifi cation success rates (80.4% and 87.2%, respectively). Although Fourier coeffi cients are diffi cult to reasonably interpret from a biological or ecological perspective, this diffi culty does not limit the application of EFT as an eff ective tool for describing shapes and discriminating groups (Lohmann and Schweitzer, 1990; Stransky, 2014). This method has been advanced by the development of image processing and the application of statistical software to shape analysis, which can facilitate the depiction of fi ne structures and outlines to accurately acquire morphological parameters. Another advantage of EFT is that the appropriate number of harmonics required to accurately describe the outline of the structures can be determined statistically, which could hardly be achieved by the routine shape indices. Overall, the more complicated or irregular the shape, the more harmonics may be required to accurately describe the outlines for discriminating stocks. In this study, nine otolith harmonics and fourteen sulcus harmonics were required to reach a cumulative Fourier power exceeding 99.99% of the total power, the threshold for accurately describing the outlines, which may promote the discriminatory effi ciency of EFT. Moreover, this approach can potentially aid in the interpretation of the morphometric or biological relevance of intraspecifi c variations in the two structures (Stransky et al., 2008; Agüera and Brophy, 2011; Cañás et al., 2012). However, the sample size could also aff ect the results of EFT in discriminating stocks. Generally, the more samples are analyzed, the more credible results could be obtained. Compared to the number of Fourier harmonics used for sulcus shape description in the present study, the sample size of each site was relatively small. This could lead to the bias of statistical results in otolith and sulcus shape analysis.
The classifi cation success rates of the CJE and HHE stocks were similar when using the two structures separately, but the JZB stock showed relatively lower classifi cation rates. These classifi cation results were generally consistent with those of the otolith shape analysis. Compared to many other otolith shape analyses adopted for stock discrimination, the overall classifi cation success rate using sulcus shape analysis was relatively high in the present study. For example, the stock discrimination classifi cation success rates were greater than 80.0% for Atlantic herring Clupea harengus (Burke et al., 2008), horse mackerel Trachurus trachurus (Stransky et al., 2008), and mulloway Argyrosomus japonicus (Ferguson et al., 2011), but only approximately 50% for Icelandic cod Gadus morhua (Campana and Casselman, 1993; Petursdottir et al., 2006) and roundnose grenadier, Coryphaenoides rupestris (Longmore et al., 2010). These fi ndings suggested that the sulcus (especially the outline analyzed with EFT), like the otolith, is a potentially useful structure for stock discrimination in fi sh.
However, technical or biological problems can aff ect the discriminatory power of the sulcus outline analysis. For example, when depicting the sulcus outline during data acquisition, the contrast between the sulcus and the otolith was typically not as distinct as the contrast between the whole otolith and the black background. Additionally, since the outlines were depicted on the basis of two-dimensional measurements, it was diffi cult to extract the geometric information about some specifi c structures (e.g., the sulcus concavity, crista convexity, and the outline or boundary of the ostium and cauda), which is potentially useful for outline analysis. This lack of imaging contrast and the loss of fi ne morphological information during data acquisition could confound the accuracy of the outline description and consequently compromise the discriminatory effi ciency. Therefore, methods should be developed to extract fi ne sulcus morphological information and select appropriate parameters to improve the discriminatory power. For example, researchers can consider adopting 3D micro-scan techniques and develop appropriate software to extract fi ner morphological information about the sulcus so that the true outlines of the structures can be more accurately described for outline analysis. It is commonly believed that otolith shape changes during ontology throughout the life of fi sh, particularly in juvenile stage (Waessle et al., 2003; Naya et al., 2012; de Carvalho et al., 2015). So far, the changes of sulcus shape throughout the life of fi sh have not been adequately evaluated. However, considering that sulcus is a characteristic structure of otolith and could also vary considerably during ontology, it is safe to use the sulcus shape of the adults instead of the juveniles for stock discrimination at present.
Few studies investigated the stock structures of white croakers along the Chinese coasts, although it has traditionally been assumed that there are three major populations from the Bohai Sea (HHE stock in this study) south to the Yellow Sea (JZB stock) and the East China Sea (CJE stock; Chen, 1991). A recent study based on historical catch data suggested that only two major populations might exist, a single Bohai Sea-Yellow Sea population and the East China Sea population (Chen and Xu, 2011). Meanwhile, a genetic study suggested that white croakers along the Chinese coast could not be considered a single stock, but genetic diff erentiation was found to be low at the mitochondrial level (Han et al., 2008). Such inconsistent information makes it diffi cult to reach agreement about the stock structures of white croaker in these regions. Interestingly, using either shape indices or geometric outlines of the sulcus and otolith in the present study could more effi ciently discriminate between the CJE and HHE stocks, which are the most geographically separated, than between either of those stocks and the JZB stock. Furthermore, the assignment of misclassifi ed individuals between the CJE and HHE stocks was low. It is well known that genetics generally have a major eff ect on otolith shape, but phenotypic plasticity results in divergent phenotypes in response to environmental factors, such as revealed in Atlantic herring (Berg et al., 2018). In the case of low genetic diff erentiation in the white croaker stocks along the Chinese coast, environmental factors could strongly infl uence otolith growth and formation and could consequently contribute to the variations in otolith morphology. The HHE stock experiences quite diff erent environmental conditions (e.g., temperature, salinity, and food as well as overwintering grounds) from the CJE stock (Chen, 2006; Li et al., 2010; Sun and Sun, 2011), which could contribute to the variations in otolith shape between the two stocks and partly account for the relatively high classifi cation success rates between them. Therefore, it was reasonable to consider them as two discrete stocks, corresponding to the Bohai Sea population and the East China Sea population, respectively, in previous studies (Chen, 1991; Chen and Xu, 2011).
However, the JZB stock (corresponding to the Yellow Sea population) had a relatively low classifi cation success rate compared to the other two stocks. The majority of the misclassifi ed JZB individuals were more frequently assigned to the geographically close HHE stock than the CJE stock. T he HHE and JZB stocks were assumed to share overwintering grounds in the northern Yellow Sea and to have geographically close spawning areas (Chen, 1991). Thus, the two stocks experience similar environmental conditions during overwintering and spawning migration, which could potentially reduce the heterogeneity in their otolith shapes. On the other hand, misclassifi cation often originated from other cues besides genetic and abiotic factors, such as individual metabolism and growth. For example, otolith shape analysis still resulted in misclassifi cation, even though the origins of genetically diff erentiated stocks were known (Berg et al., 2018).
Generally, the fi ndings of the present study tended to support the discreteness of the CJE and HHE stocks, which should be considered diff erent units for fi shery management, while the JZB and HHE stocks could be considered a single unit for population management. However, further studies (e.g., genetics or otolith elemental fi ngerprinting) are needed to comprehensively understand the stock structure along the Chinese coast.
5 CONCLUSION
In conclusion, the sulcus outline analysis via elliptical Fourier transform (EFT) was used to discriminate among the white croaker stocks and achieved an overall high classifi cation rate comparable to that using the whole otolith outline analysis via EFT. A combination of sulcus outline and otolith shape indices (SIs yielded only a moderate discriminatory effi ciency. The EFc of both sulcus and otolith outlines discriminated the white croaker stocks more effi ciently than the SIs. The sulcus outline analysis could be a useful tool for stock discrimination of sciaenids, which provided complementary information to the whole otolith outline analysis.
6 DATA AVAILABILITY STATEMENT
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We thank the people who contributed to the sample collection and Dr. ZHAO B. and Dr. YU X. for their help in data analysis.
References
Agüera A, Brophy D. 2011. Use of saggital otolith shape analysis to discriminate Northeast Atlantic and Western Mediterranean stocks of Atlantic saury, Scomberesox saurus saurus (Walbaum). Fisheries Research, 110(3): 465-471, https://doi.org/10.1016/j.fi shres.2011.06.003.
Arellano R V, Hamerlynck O, Vincx M, Mees J, Hostens K, Gijselinck W. 1995. Changes in the ratio of the sulcus acusticus area to the sagitta area of Pomatoschistus minutus and P. lozanoi (Pisces, Gobiidae). Marine Biology, 122(3): 355-360, https://doi.org/10.1007/BF00350868.
Avigliano E, Comte G, Rosso J J, Mabragaña E, Rosa P D, Sanchez S, Volpedo A, del Rosso F, Schenone N F. 2015. Identifi cation of fi sh stocks of river crocker ( Plagioscion ternetzi) in Paraná and Paraguay rivers by using otolith morphometric analysis. Latin American Journal of Aquatic Research, 43(3): 718-725, https://doi.org/10. 3856/vol43-issue4-fulltext-10.
Avigliano E, Jawad L A, Volpedo A V. 2016. Assessment of the morphometry of saccular otoliths as a tool to identify triplefi n species (Tripterygiidae). Journal of the Marine Biological Association of the United Kingdom, 96(5): 1 167-1 180, https://doi.org/10.1017/s0025315415001101.
Avigliano E, Martinez C F R, Volpedo A V. 2014. Combined use of otolith microchemistry and morphometry as indicators of the habitat of the silverside ( Odontesthes bonariensis) in a freshwater-estuarine environment. Fisheries Research, 149: 55-60, https://doi.org/10.1016/j.fi shres.2013.09.013.
Begg G A, Brown R W. 2000. Stock identifi cation of haddock Melanogrammus aeglefi nus on Georges Bank based on otolith shape analysis. Transactions of the American Fisheries Society, 129(4): 935-945, https://doi.org/10. 1577/1548-8659(2000)129<0935:SIOHMA>2.3.CO;2.
Berg F, Almeland O W, Skadal J, Slotte A, Andersson L, Folkvord A. 2018. Genetic factors have a major eff ect on growth, number of vertebrae and otolith shape in Atlantic herring ( Clupea harengus). PLoS One, 13(1): e0190995, https://doi.org/10.1371/journal.pone.0190995.
Burke N, Brophy D, King P A. 2008. Otolith shape analysis: its application for discriminating between stocks of Irish Sea and Celtic Sea herring ( Clupea harengus) in the Irish Sea. ICES Journal of Marine Science, 65(9): 1 670-1 675, https://doi.org/10.1093/icesjms/fsn177.
Cadrin S X, Friedland K D. 1999. The utility of image processing techniques for morphometric analysis and stock identifi cation. Fisheries Research, 43(1-3): 129-139, https://doi.org/10.1016/S0165-7836(99)00070-3.
Campana S E, Casselman J M. 1993. Stock discrimination using otolith shape analysis. Canadian Journal of Fisheries and Aquatic Sciences, 50(5): 1 062-1 083, https://doi.org/10.1139/f93-123.
Campana S E, Neilson J D. 1985. Microstructure of fi sh otoliths. Canadian Journal of Fisheries and Aquatic Sciences, 42(5): 1 014-1 032, https://doi.org/10.1139/f85-127.
Cañás L, Stransky C, Schlickeisen J, Sampedro M P, Fariña A C. 2012. Use of the otolith shape analysis in stock identifi cation of anglerfi sh ( Lophius piscatorius) in the Northeast Atlantic. ICES Journal of Marine Science, 69(2): 250-256, https://doi.org/10.1093/icesjms/fss006.
Cardinale M, Doering-Arjes P, Kastowsky M, Mosegaard H. 2004. Eff ects of sex, stock, and environment on the shape of known-age Atlantic cod ( Gadus morhua) otoliths. Canadian Journal of Fisheries and Aquatic Sciences, 61(2): 158-167, https://doi.org/10.1139/f03-151.
Castonguay M, Simard P, Gagnon P. 1991. Usefulness of Fourier analysis of otolith shape for Atlantic mackerel ( Scomber scombrus) stock discrimination. Canadian Journal of Fisheries and Aquatic Sciences, 48(2): 296-302, https://doi.org/10.1139/f91-041.
Chen D G. 1991. Fisheries Ecology of the Bohai Sea and the Yellow Sea. China Ocean Press, Beijing, 505p. (in Chinese)
Chen J J, Xu Z L. 2011. Spatial-temporal pattern to fi shing ground of white croaker in Bohai, Yellow and East China Seas. Journal of Natural Resources, 26(4): 666-673, https://doi.org/10.11849/zrzyxb.2011.04.012. (in Chinese with English abstract)
Chen J S. 2006. Theories of River Water Quality and Water Quality of Chinese Rivers. Science Press, Beijing, 292p. (in Chinese)
Crampton J S. 1995. Elliptic Fourier shape analysis of fossil bivalves: some practical considerations. Lethaia, 28(2): 179-186, https://doi.org/10.1111/j.1502-3931.1995.tb01611.x.
de Carvalho B M, Vaz-dos-Santos A M, Spach H L, Volpedo A V. 2015. Ontogenetic development of the sagittal otolith of the anchovy, Anchoa tricolor, in a subtropical estuary. Scientia Marina, 79(4): 409-418, https://doi.org/10.3989/scimar.04218.31A.
Ferguson G J, Ward T M, Gillanders B M. 2011. Otolith shape and elemental composition: complementary tools for stock discrimination of mulloway ( Argyrosomus japonicus) in southern Australia. Fisheries Research, 110(1): 75-83, https://doi.org/10.1016/j.fi shres.2011.03.014.
Gauldie R W. 1988. Function, form and time-keeping properties of fi sh otoliths. Comparative Biochemistry and Physiology Part A: Physiology, 91(2): 395-402, https://doi.org/10.1016/0300-9629(88)90436-7.
Han Z Q, Gao T X, Yanagimoto T, Sakurai Y. 2008. Deep phylogeographic break among white croaker Pennahia argentata (Sciaenidae, Perciformes) populations in Northwestern Pacifi c. Fisheries Science, 74(4): 770-780, https://doi.org/10.1111/j.1444-2906.2008.01588.x.
Jaramillo A M, Tombari A D, Durá V B, Rodrigo M E, Volpedo A V. 2014. Otolith eco-morphological patterns of benthic fi shes from the coast of Valencia (Spain). Thalassas, 30(1): 57-66.
Ju P L, Yang L, Lu Z B, Yang S Y, Du J G, Zhong H Q, Chen J, Xiao J M, Chen M R, Zhang C Y. 2016. Age, growth, mortality and population structure of silver croaker Pennahia argentata (Houttuyn, 1782) and red bigeye Priacanthus macracanthus Cuvier, 1829 in the northcentral Taiwan Strait. Journal of Applied Ichthyology, 32(4): 652-660, https://doi.org/10.1111/jai.13053.
Kuhl F P, Giardina C R. 1982. Elliptic Fourier features of a closed contour. Computer Graphics and Image Processing, 18(3): 236-258, https://doi.org/10.1016/0146-664X(82)90034-X.
Li X Z, Liu L S, Li B Q. 2010. Macrobenthos in China Sea: Research and Practice. China Ocean Press, Beijing, 378p. (in Chinese)
Libungan L A, Pálsson S. 2015. ShapeR: an R package to study otolith shape variation among fi sh populations. PLoS One, 10(3): e0121102, https://doi.org/10.1371/journal.pone.0121102.
Lin L S, Cheng J H, Ling J Z, Zhang H Y. 2006. First capture sizes of major commercial fi shes in the East China Sea Region. Journal of Fishery Sciences of China, 13(2): 250-256. https://doi.org/10.3321/j.issn:1005-8737.2006.02. 014. (in Chinese with English abstract)
Lohmann G P, Schweitzer P N. 1990. On eigenshape analysis. In: Rohlf F J, Bookstein F L eds. Proceedings of the Michigan Morphometrics Workshop. University of Michigan Museum of Zoology, Ann Arbor, Michigan. p.147-166.
Lombarte A, Lleonart J. 1993. Otolith size changes related with body growth, habitat depth and temperature. Environmental Biology of Fishes, 37(3): 297-306, https://doi.org/10.1007/BF00004637.
Lombarte A, Morales-Nin B. 1995. Morphology and ultrastructure of saccular otoliths from fi ve species of the genus Coelorinchus (Gadiformes: Macrouridae) from the Southeast Atlantic. Journal of Morphology, 225(2): 179-192, https://doi.org/10.1002/jmor.1052250204.
Lombarte A. 1992. Changes in otolith area: sensory area ratio with body size and depth. Environmental Biology of Fishes, 33(4): 405-410, https://doi.org/10.1007/BF00010955.
Longmore C, Fogarty K, Neat F, Brophy D, Trueman C, Milton A, Mariani S. 2010. A comparison of otolith microchemistry and otolith shape analysis for the study of spatial variation in a deep-sea teleost, Coryphaenoides rupestris. Environmental Biology of Fishes, 89(3-4): 591-605, https://doi.org/10.1007/s10641-010-9674-1.
Montanini S, Stagioni M, Valdrè G, Tommasini S, Vallisneri M. 2015. Intra-specifi c and inter-specifi c variability of the sulcus acusticus of sagittal otoliths in two gurnard species (Scorpaeniformes, Triglidae). Fisheries Research, 161: 93-101, https://doi.org/10.1016/j.fi shres.2014.07.003.
Naya M J G, Tombari A, Volpedo A, Gómez S E. 2012. Size related changes in sagitta otoliths of Australoheros facetus (Pisces; Cichlidae) from South America. Journal of Applied Ichthyology, 28(5): 752-755, https://doi.org/10. 1111/j.1439-0426.2012.02006.x.
Petursdottir G, Begg G A, Marteinsdottir G. 2006. Discrimination between Icelandic cod ( Gadus morhua L.) populations from adjacent spawning areas based on otolith growth and shape. Fisheries Research, 80(2-3): 182-189, https://doi.org/10.1016/j.fi shres.2006.05.002.
Popper A N, Ramcharitar J, Campana S E. 2005. Why otoliths? Insights from inner ear physiology and fi sheries biology. Marine and Freshwater Research, 56(5): 497-504, https://doi.org/10.1071/MF04267.
Stransky C, Murta A G, Schlickeisen J, Zimmermann C. 2008. Otolith shape analysis as a tool for stock separation of horse mackerel ( Trachurus trachurus) in the Northeast Atlantic and Mediterranean. Fisheries Research, 89(2): 159-166, https://doi.org/10.1016/j.fi shres.2007.09.017.
Stransky C. 2014. Morphometric outlines. In: Cadrin S X, Kerr L A, Mariani S eds. Stock Identifi cation Methods: Applications in Fishery Science. 2ndedn. Academic Press, New York. p.129-140.
Sun S, Sun X X. 2011. Atlas of Long-Term Changes in the Jiaozhou Bay Ecosystem. China Ocean Press, Beijing, 809p. (in Chinese)
Torres G J, Lombarte A, Morales-Nin B. 2000a. Sagittal otolith size and shape variability to identify geographical intraspecifi c diff erences in three species of the genus Merluccius. Journal of the Marine Biological Association of the United Kingdom, 80(2): 333-342, https://doi.org/10.1017/S0025315499001915.
Torres G J, Lombarte A, Morales-Nin B. 2000b. Variability of the sulcus acusticus in the sagittal otolith of the genus Merluccius (Merlucciidae). Fisheries Research, 46(1-3): 5-13, https://doi.org/10.1016/S0165-7836(00)00128-4.
Tsai K E. 2009. Study of the acoustic characters of eleven soniferous fi sh in the western coastal waters of Taiwan. National Sun Yat-sen University, Guangzhou. 68p. (in Chinese)
Tuset V M, Lombarte A, Assis C A. 2008. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Scientia Marina, 72(S1): 7-198, https://doi.org/10.3989/scimar.2008.72s17.
Tuset V M, Lombarte A, González J A, Pertusa J F, Lorente M J. 2003a. Comparative morphology of the sagittal otolith in Serranus spp. Journal of Fish Biology, 63(6): 1 491-1 504, https://doi.org/10.1111/j.1095-8649.2003.00262.x.
Tuset V M, Lozano I J, González J A, Pertusa J F, García-Díaz M M. 2003b. Shape indices to identify regional diff erences in otolith morphology of comber, Serranus cabrilla (L., 1758). Journal of Applied Ichthyology, 19(2): 88-93, https://doi.org/10.1046/j.1439-0426.2003.00344.x.
Volpedo A, Echeverría D D. 2003. Ecomorphological patterns of the sagitta in fi sh on the continental shelf off Argentine. Fisheries Research, 60(2-3): 551-560, https://doi.org/10. 1016/S0165-7836(02)00170-4.
Waessle J A, Lasta C A, Favero M. 2003. Otolith morphology and body size relationships for juvenile Sciaenidae in the Río de la Plata estuary (35-36°S). Scientia Marina, 67(2): 233-240, https://doi.org/10.3989/scimar.2003.67n2233.
Zar J H. 2010. Biostatistical Analysis. 5thedn. Prentice Hall, New Jersey, 944p.
 Journal of Oceanology and Limnology2020年5期
Journal of Oceanology and Limnology2020年5期
- Journal of Oceanology and Limnology的其它文章
- Distribution, sources and burial fl ux of sedimentary organic matter in the East China Sea*
- Photoelectrochemical cathodic protection of Cu 2 O/TiO 2 p-n heterojunction under visible light*
- Antioxidant bisabolane-type sesquiterpenoids from algalderived fungus Aspergillus sydowii EN-434*
- Calcium isotopic signatures of depleted mid-ocean ridge basalts from the northeastern Pacifi c*
- Application of confocal laser Raman spectroscopy on marine sediment microplastics*
- Corrosion behavior of Q235B carbon steel in simulated seawater pumped storage system under operational conditions*
