氧化石墨烯和五价砷在改性多孔介质中的共迁移特征
孟庆涛,管 多,姜延吉,孙慧敏,殷宪强,王 农
氧化石墨烯和五价砷在改性多孔介质中的共迁移特征
孟庆涛1,2,3,管 多3,姜延吉3,孙慧敏3,殷宪强3※,王 农4
(1.中国科学院西北生态环境资源研究院,兰州 730000;2. 中国科学院大学,北京 100049;3. 西北农林科技大学资源环境学院,杨凌 712100;4.农业农村部环境保护科研监测所,天津 300191)
砷是农田土壤重金属污染的主要元素之一,在砷污染农田土壤的修复过程中往往忽视纳米颗粒能够使结合态的砷重新释放,导致有效态砷浓度升高,探究土壤中黏土矿物对氧化石墨烯(Graphene Oxide,GO)和五价砷(As(V))在多孔介质中迁移行为的影响,对进一步完善农田土壤砷修复理论以及提高农作物产量、保护人体健康具有重要意义。该研究利用蒙脱石和高岭石改性石英砂,通过砂柱迁移试验系统地研究了GO、As(V)和GO-As(V)在填加0%、10%、30%和50%的蒙脱石和高岭石改性石英砂柱中的迁移行为。研究结果表明,随着高岭石和蒙脱石改性石英砂填加比例的增加,GO和As(V)的迁移能力均呈降低趋势,且GO和As(V)在不同条件下的迁移曲线均存在显著差异(0.05);GO在50%高岭石和蒙脱石改性石英砂柱中的回收率相对于石英砂柱分别下降了14%和17%,As(V) 分别下降了15%和12%;在共迁移试验中,GO和As(V)在石英砂柱中回收率分别上升至99%和100%。分析表明,As(V)在蒙脱石改性石英砂柱中的迁移能力大于高岭石改性石英砂,而GO与之相反;当GO和与As(V)共迁移时,二者在介质中的迁移能力均大于其单独迁移。本研究表明GO、As(V)释放到土壤后,能够加速As(V)的迁移,造成土壤砷污染的扩大化。
重金属;土壤;水;氧化石墨烯;高岭石;蒙脱石;五价砷;迁移
0 引 言
五价砷(As(V))是环境中常见的污染物之一[1]。在各种生产实践活动中As(V)会以不同的方式进入农田土壤中,农田土壤中As(V)浓度的升高引起了人们对农作物质量安全的担忧[2]。针对农田土壤中As(V)的污染现状及其可能的环境风险,众多学者开展了农田As(V)污染修复的研究[3-4],但是在农田土壤修复过程中,往往忽视了土壤中的纳米颗粒对As(V)的释放、迁移的影响。有相当多的证据表明,非水相流动胶体可以将As(V)输送相当长的距离[5-6]。Ishak等[5]的研究证明土壤浸出液中As(V)的浓度与浊度相关。当用去离子水冲洗土柱时,氧化铁胶体可以促进As(V)的迁移[6]。
氧化石墨烯(Graphene Oxide,GO)是一种具有特殊二维结构的碳基纳米材料。目前,GO广泛应用于电子、生物、储能和光学等众多领域[7]。众多含氧官能团和高比表面积性质使GO表现出良好的水溶性和对污染物产生极强的吸附能力[8-9],GO进入环境后可作为污染物的载体而携带污染物在地下水环境中迁移[10-11]。例如,一些砂柱迁移试验探究了GO和污染物的协同迁移行为,GO能够释放滞留在砂柱中二价铅(Pb(II))、二价镉(Cd(II))、二价铜(Cu(II))和六价铀(U(VI))等重金属污染物,并促进其迁移[12-14]。另外,Liu等[15]研究表明GO也可促进有机污染物的迁移。
综上,GO对As(V)在土壤介质中的迁移也具有重要影响,GO可以改变As(V)与土壤的结合形式,增加As(V)在土壤中的移动性与生物有效性。但As(V)与GO在介质中迁移行为的研究鲜见报道。黏土矿物是土壤中活性较强的固体成分,在影响污染物的迁移转化方面起着重要的作用[16-19]。Selim等[20]发现砷在土壤中的运移主要受固体基质表面吸附-解吸过程的控制。Constantinos等[21]和Indranil等[22]研究表明溶液中存在黏土矿物胶体时会抑制GO的迁移,其抑制能力最高是高岭石,其次是蒙脱石和伊利石。但这些研究是探究溶液中存在黏土矿物胶体时对GO和As(V)迁移的影响,而针对介质表面固定黏土矿物对GO和As(V)共迁移影响的研究还未开展,关于不同黏土矿物间对GO和As(V)运移的差异研究目前也处于空白。因此,本研究以GO、As(V)在蒙脱石、高岭石改性石英砂柱中的迁移为研究对象,深入分析GO、As(V)在黏土矿物改性介质中的共迁移行为,揭示GO、As(V)与土壤黏土矿物之间的相互作用,加深GO对As(V)在土壤介质中迁移影响的认知和理解,以期进一步完善纳米颗粒对土壤中As(V)迁移的理论,为土壤As(V)污染修复和毒性控制提供依据,并促进对农田作物和人体健康的有效保护。
1 材料与方法
1.1 材料制备
氧化石墨烯(GO)购自先丰纳米公司(中国南京)。将20 mg的 GO加入1 L蒸馏水中,使用超声波清洗器(KQ-500DE,昆山超声波仪器公司,中国)超声分散120 min,制得20 mg/L GO储备液,调节储备液pH值为6。将83.2 mg Na3AsO4加入1 L蒸馏水中配置20 mg/L As(V)储备液。将83.2 mg Na3AsO4加入1 L的GO储备液中,制得20 mg/LGO-As(V)储备液(迁移试验前超声分散均匀)。使用蒸馏水配制pH值为6、CNaCl浓度为1 mmol/L的背景溶液。使用Zetasizer nano ZS90(Malvern Instruments Inc.,UK)测定GO的Zeta电位和水合粒径。GO的水合粒径为(165.49±20.5) nm,Zeta电位为 (−21.3±0.6) mV;GO-As(V)的Zeta电位为(−26.7±0.4) mV。
1.2 柱迁移介质的制备和表征
将石英砂(周至县秦丰石英砂厂)筛分至0.4~0.8 mm,用蒸馏水搓洗、浸泡24 h,之后依次分别用酸碱搓洗、浸泡24 h,以除去石英砂表面的金属离子和胶体物质[23],再使用蒸馏水淘洗石英砂,105 ℃下烘干。
蒙脱石、高岭石改性石英砂的制备[24]:在1 L的蒸馏水中加入10 g的黏土矿物,搅拌,加入50 mL H2O2去除黏土矿物中的有机物,通过重力沉降获得直径小于2m的黏土矿物悬浮液。在悬浮液中加入500 mg/L聚乙烯醇溶液100 mL。将黏土-聚合物复合溶液与石英砂混合静置24 h,之后在80 ℃下烘干24 h,用去离子水洗涤以除去未附着在石英砂表面的黏土矿物和聚乙烯醇,之后100 ℃下烘干24 h备用。
运用扫描电子显微镜X射线能谱仪(Scanning Electron Microscope and Energy Dispersive Spectrometer,SEM-EDS,S-4800,日本)对改性石英砂的表面特征进行表征。表征结果如图1所示,石英砂表面涂覆的高岭石呈单体颗粒间分离,大小不同,多边形块状结构。石英砂表面涂覆的蒙脱石颗粒层层堆叠,呈无定形片状,具有孔状结构。材料中的铝元素(Al)和硅元素(Si)含量高表明高岭石和蒙脱石颗粒附着在了石英砂表面(图2)。
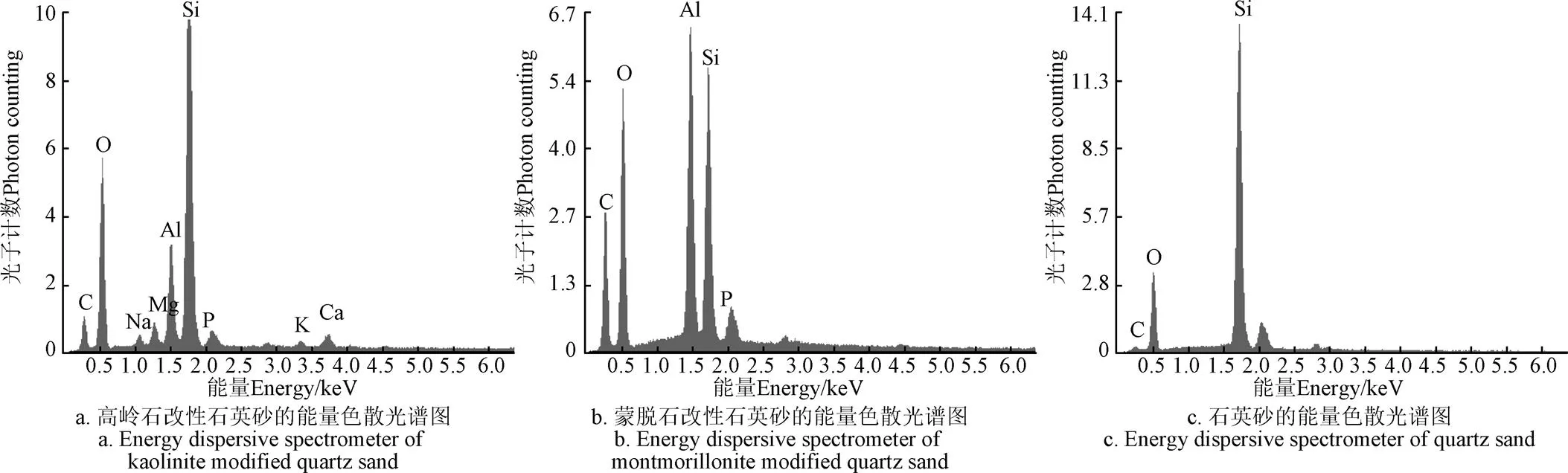
注:C为碳;O为氧;Na为钠;Mg为镁;Al为铝;Si为硅;P为磷;K为钾;Ca为钙。
1.3 砂柱迁移试验
在高15 cm、内径3 cm的有机玻璃柱中,填充石英砂、改性石英砂进行GO、As(V)的迁移试验。为最大限度减少分层,排空砂柱中的空气,采用湿法填装石英砂柱[23]。在湿法填装过程中,共加入48 mL蒸馏水,迁移柱的孔隙体积(Pore Volume,PV)设定为48 mL,孔隙率为0.45。试验前,使用蒸馏水持续冲洗砂柱,直至出流液中不含可见杂质。
将填装比例0%、10%、30%和50%的改性石英砂(蒙脱石、高岭石)与未改性石英砂混匀,采用湿法装填迁移砂柱。迁移试验分为2个部分,1)用NaCl背景溶液(浓度为1 mmol/L,pH值为6)冲洗砂柱以平衡迁移条件,之后分别输入60 mL的20 mg/LGO、20 mg/L As(V),最后将冲洗液切换为背景溶液继续冲洗,直至出流液中无GO、As(V);2)用NaCl背景溶液冲洗以平衡迁移条件,将60 mL的GO-As(V)(20 mg/LGO、20 mg/L As(V))混合溶液输入砂柱,之后再将冲洗液切换为背景溶液继续冲洗,直至出流液中无GO、As(V)。砂柱迁移试验重复2次。
试验流速通过蠕动泵(HL-2B,上海沪西分析仪器有限公司)控制为1 mL/min。使用自动收集器(EBS-20,上海沪西分析仪器有限公司)收集出流液。通过紫外-可见分光光度计(UV-2800,上海尤尼柯仪器有限公司)在波长229 nm下测定GO的浓度[25]。As(V)的浓度使用火焰石墨炉原子吸收光谱仪(PinAAciie 900F,美国珀金埃尔默有限公司)进行测定。使用Zetasizer nano ZS90测定0%、10%、30%和50%改性石英砂表面的Zeta电位[26]。测定方如下:迁移试验前,从砂柱中取出完整的石英砂置于背景溶液中,超声10 min后浸泡6 h,测定溶液的Zeta电位。纯石英砂的Zeta电位为(−19.4±0.8) mV;10%、30%和50%高岭石改性石英砂的Zeta电位分别为(−21.4±1.6)、(−22.45±0.4)和(−26.4±0.7) mV;10%、30%和50%蒙脱石改性石英砂的Zeta电位分别为(−23.2±1.3)、(−29.6±2.4)和(−37.5±0.9) mV。
1.4 数据分析
Darjaguin-Landau-Verwey-Overbeek(DLVO)理论用于计算GO和石英砂表面的相互作用力[12],通常用来解释胶体在多孔介质中的迁移行为。 DLVO能(ΔDLVO,kT)是范德华作用能(ΔEL,kT)和静电作用能(ΔLW,kT)两相互作用能的总和,计算如式(1)所示。
ΔDLVO=ΔLW+ΔEL(1)
Microsoft Excel软件用于处理标准偏差。通过质量守恒计算As(V)和GO的回收率(%)。不同因素对As(V)和GO迁移影响的差异使用t检验的配对样本检验进行分析,显著水平设置为0.05。
2 结果与分析
2.1 五价砷、氧化石墨烯在改性石英砂中的单独迁移
As(V)在高岭石、蒙脱石改性石英砂柱中的迁移曲线如图3所示。
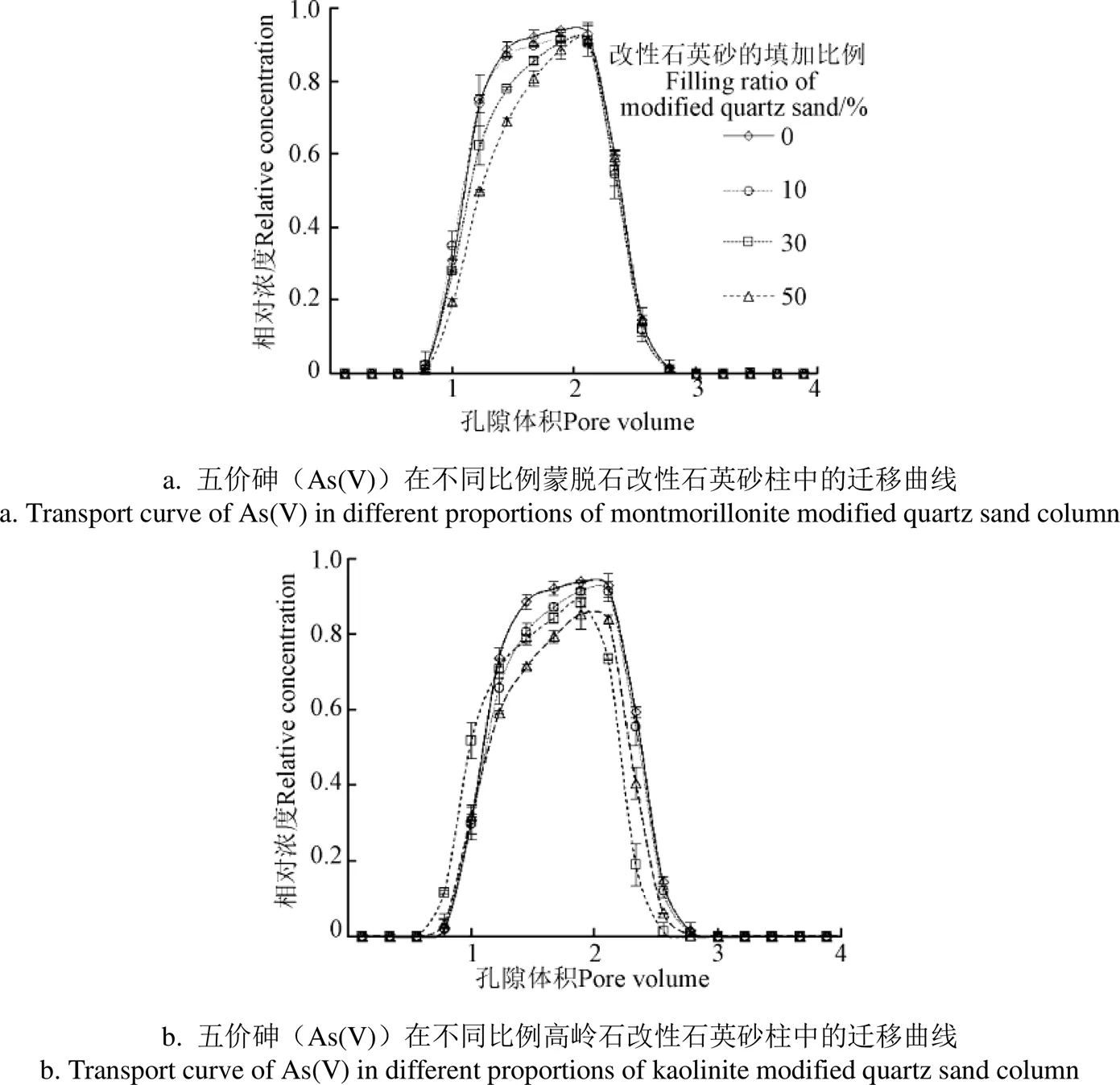
图3 不同改性石英砂填加量下五价砷(As(V))的迁移曲线
As(V)在蒙脱石比例为0%、10%、30%和50%的改性石英砂柱中呈现出不同的迁移曲线(图3a)。纯石英砂柱中,在PV为0.8处As(V)被检出,之后As(V)浓度迅速升高,在PV为2处达到峰值,相对浓度达0.95,表明As(V)在纯石英砂柱中的迁移性极强。对比图3a和图3b 中的迁移曲线发现,随着砂柱中蒙脱石和高岭石改性石英砂填加比例的增大,As(V)在砂柱中的沉积量逐渐增加。As(V)在比例为0%、10%、30%和50%蒙脱石改性石英砂柱中的回收率分别为94%、92%、88%和82%,在相应比例高岭石改性石英砂柱中的回收率分别为94%、89%、83%和79%(表1)。As(V)在50%蒙脱石改性石英砂柱中的回收率相对于纯石英砂柱下降了12%,在高岭石改性石英砂柱的回收率下降了15%,通过t检验的配对样本检验进行差异分析发现在不同比例间As(V)的迁移均呈显著性差异(0.05)。
GO在不同质量比例的蒙脱石、高岭石改性石英砂柱中的穿透曲如图4所示。在纯石英砂柱中,GO几乎全部流出砂柱,表明GO在纯石英砂中不受阻滞作用。GO在10%蒙脱石改性石英砂柱中的迁移曲线与纯石英砂柱中的迁移曲线差异不显著(0.05),表明较低含量的蒙脱石改性石英砂对GO的迁移影响较小。GO在50%蒙脱石改性石英砂柱中的回收率相对于纯石英砂柱下降了17%,经过t检验-配对样本检验进行差异分析发现,GO在纯石英砂和10%蒙脱石改性石英砂中的迁移曲线与30%和50%蒙脱石改性石英砂柱中的迁移曲线呈显著性差异(0.05)。结果表明随着蒙脱石改性石英砂填加量的进一步增加,GO受到的阻滞作用愈加明显。
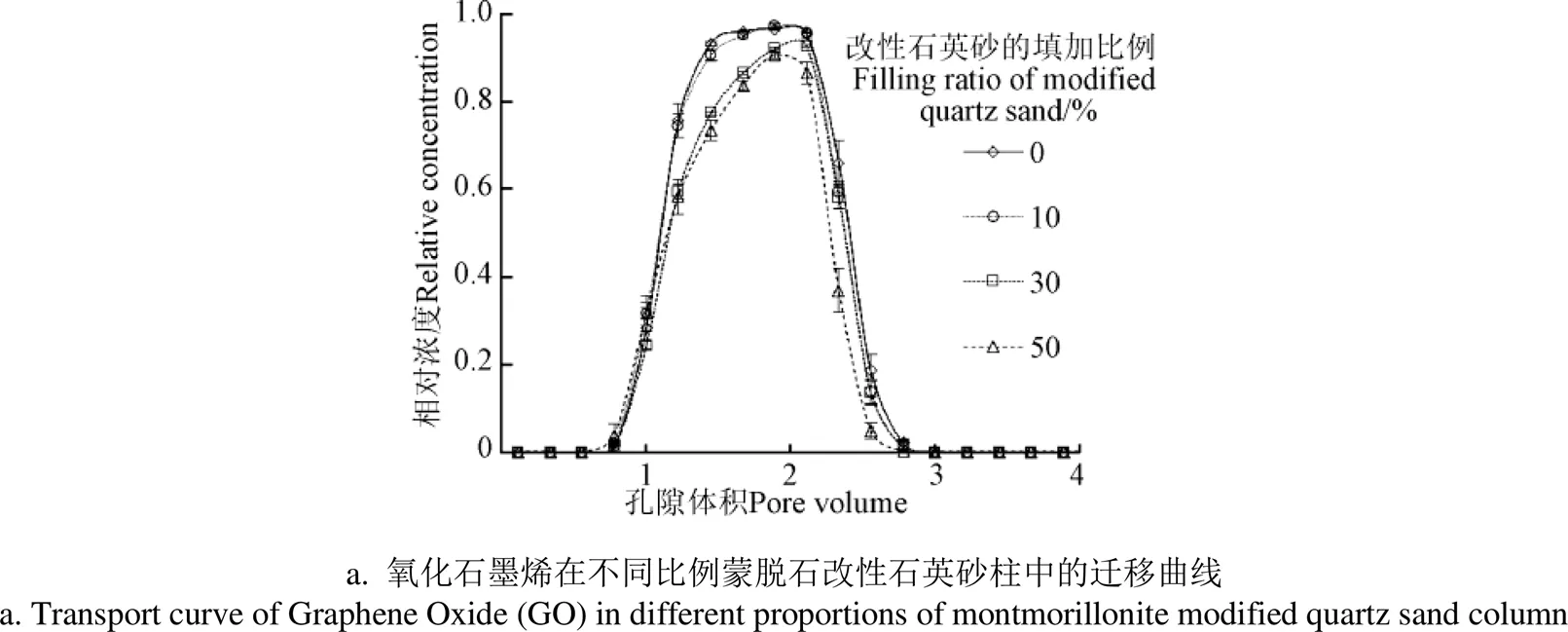
与填加不同比例蒙脱石改性石英砂的结果类似,随着高岭石改性石英砂所占比例的增加,GO的迁移能力也随之下降(图4b),根据质量平衡计算,GO在高岭石改性石英砂填加比例为0%、10%、30%和50% 的砂柱中的回收率分别为96%、91%、87%和82%(表1)。GO在不同比例高岭石改性石英砂柱中的迁移曲线均存在显著差异(0.05)。
2.2 氧化石墨烯与五价砷在改性石英砂中的共迁移
GO与As(V)在蒙脱石和高岭石改性石英砂柱中的共迁移曲线(图5),其中图5a和图5b分别为GO-As在高岭石和蒙脱石改性石英砂柱中GO的迁移曲线。在纯石英砂柱中,GO的回收率为99%(表1),相比于单独迁移的96%,回收率有所上升,但两者的穿透曲线无显著差异(0.05)。10%、30%和50%填加比例的高岭石改性石英砂中GO的回收率分别为96%、95%和93%;蒙脱石改性石英砂中GO的回收率分别为97%、90%和84%(表1)。可知GO-As(V)共迁移中GO在砂柱中具有很强的迁移性,且迁移能力高于单独迁移的GO。
GO-As(V)在高岭石和蒙脱石改性石英砂柱中As(V)的迁移曲线见图5c和图5d。As(V)的迁移曲线与GO保持一致,在PV为0.8处被检测出来,之后浓度迅速升高,在PV为2处出流液浓度达到峰值。根据质量守恒计算,As(V)在填加0%、10%、30%和50%高岭石改性石英砂柱中的回收率依次为100%、97%、91%和85%,蒙脱石改性石英砂柱中的回收率依次为100%、98%、94%和92%(表1),表明高岭石改性石英砂对As(V)的滞留能力高于蒙脱石改性石英砂。
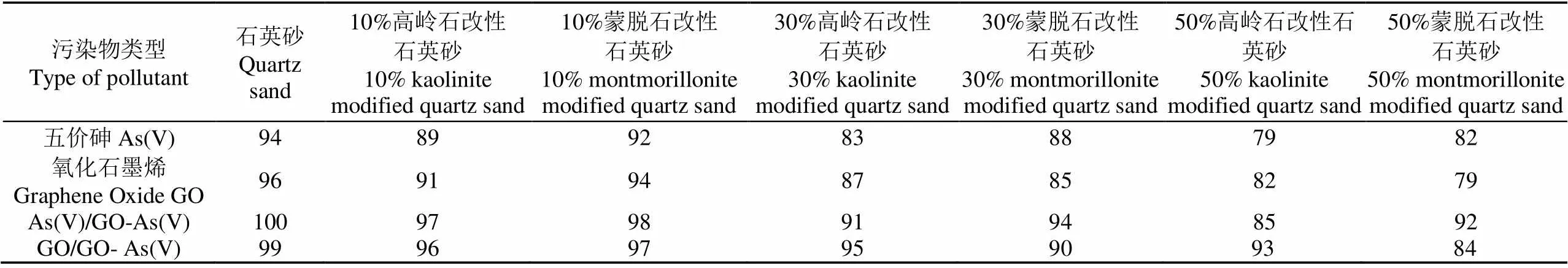
表1 五价砷(As(V))和氧化石墨烯在填加不同比例改性石英砂柱中的回收率
注:As(V)/GO-As(V)为氧化石墨烯与五价砷共迁移中的五价砷,GO/GO-As(V) 为氧化石墨烯与五价砷共迁移中的氧化石墨烯。
Note: As(V)/GO-As(V) is the As(V) in the co-transport of Graphene Oxide (GO) and As(V), GO/GO-As(V) is the Graphene Oxide (GO) in the co-transport of Graphene Oxide (GO) and As(V).

图5 氧化石墨烯与五价砷(As(V))在改性石英砂柱中的共迁移曲线
2.3 DLVO理论分析
GO与改性石英砂之间的DLVO相互作用能谱如图6所示。
随着改性石英砂质量比例的增加,GO和高岭石及蒙脱石改性石英砂之间的能垒逐渐增大,表明随着改性石英砂比例的增加,GO与介质表面的排斥力增大,不易沉积于介质表面。图6a中展示出GO和介质之间的能垒均大于20 kT,表明GO颗粒难以通过布朗运动而沉积于介质的表面。同时,GO与蒙脱石改性石英砂之间的能垒大于GO与高岭石改性石英砂之间的能垒,表明GO颗粒在蒙脱石改性石英砂柱中更不容易通过布朗运动克服能垒而永久性地沉积在石英砂表面[21]。GO与纯石英砂、10%蒙脱石改性石英砂、30%蒙脱石改性石英砂、50%蒙脱石改性石英砂、10%高岭石改性石英砂、30%高岭石改性石英砂以及50%高岭石改性石英砂的二次能量最小值分别为−0.112、−0.103、−0.094、−0.091、−0.102、−0.093和−0.089 kT。表明随着蒙脱石改性石英砂、高岭石改性石英砂比例增加,GO和高岭石及蒙脱石改性石英砂之间的二次能量最小值逐渐增大。
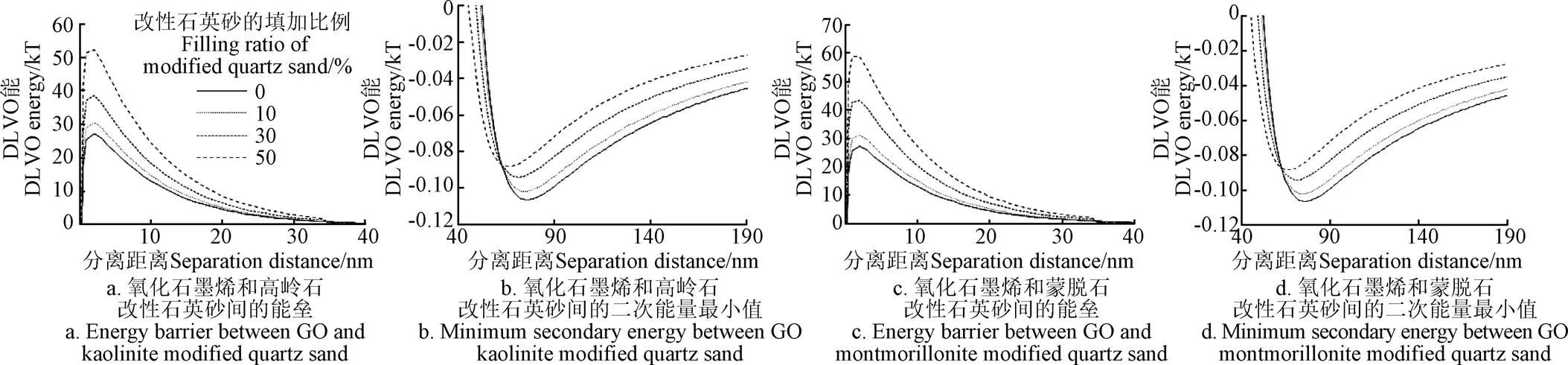
图6 氧化石墨烯与不同比例改性石英砂的DLVO能谱图
2.4 黏土矿物对氧化石墨烯和五价砷迁移的影响
土壤中污染物的移动性主要是由土壤的吸附特性控制,黏土矿物是土壤中活性较强的固体成分,在影响污染物的迁移转化方面起着重要的作用[16-19]。As(V)在黏土矿物改性石英砂柱中的迁移结果显示,As(V)的迁移能力受到了抑制。Lin等[27]指出蒙脱石和高岭石外表面的边缘断键,像Si-OH和Al-OH,让其外部边缘拥有类似氧化物的特性,黏土矿物表面可以吸附As(V)。Eleonora等[28]和Ladeira等[29]等研究发现,As(V)与蒙脱石周围的≡Al-OH官能团结合形成单核或双核双配位络合基团,从而吸附在蒙脱石的表面。As(V)在0%、10%、30%和50%比例蒙脱石和高岭石改性石英砂柱中的迁移能力逐渐降低,是因为随着蒙脱石和高岭石改性石英砂填加量的增加,砂柱中供As(V)沉积的位点也随之增加。尽管As(V)在不同比例高岭石改性石英砂柱中的迁移规律与在蒙脱石改性石英砂柱中的基本一致,但经过t检验分析发现,As(V)在2种改性石英砂柱中的迁移行为存在着差异性(0.05),As(V)在高岭石改性石英砂柱中的整体回收率均低于在蒙脱石改性石英砂柱中的回收率。这是因为高岭石晶体破裂所形成的正电荷多于蒙脱石,整体呈现出的Zeta电位大于蒙脱石,因而As(V)在高岭石改性石英砂柱的滞留量更多[30]。由测定的Zeta电位可以得知蒙脱石表面的负电性更大,对As(V)的排斥作用更强,因而As(V)的出流更多。这与Yin等[31]对蒙脱石改性石英砂柱中Pb(II)和Cd(II)在迁移的研究现象一致:蒙脱石改性石英砂柱中Pb(II)和Cd(II)的沉积量大于高岭石改性石英砂柱中的沉积量。此外,与单独迁移的As(V)相比,GO-As(V)混合液As(V)的迁移能力明显提升(0.05)。主要是因为GO能够作为As(V)的载体携带As(V)迁移。这与目前的一些研究结果类似,溶液中存在GO颗粒时,Pb(II)、Cd(II)、Cu(II)和U(VI)在介质中的迁移能力均有提升[12-14]。
GO和介质之间的能垒大于15 kT,且与不同比例蒙脱石改性石英砂间的二次能量最小值均小于高岭石改性石英砂。根据DLVO理论,当能垒大于15 kT时,GO在介质表面上的沉积行为通常会发生在二次能量最小值处,二次能量最小值越小,越容易发生沉积[12]。可以推测出相对于高岭石改性石英砂,GO在蒙脱石改性石英砂中更容易沉积。GO在黏土矿物改性石英砂柱中的迁移结果显示,高岭石改性石英砂中对GO的阻滞能力小于蒙脱石改性石英砂,表明试验结果符合DLVO理论的预测。在Yin等[31]研究中同样发现蒙脱石改性石英砂对GO的迁移能力的抑制作用大于高岭石改性石英砂,主要是因为涂覆在石英砂表面的蒙脱石相比于高岭石褶皱更多,比表面积更大,砂柱中的吸附位点更多,使得GO滞留在砂柱中。随着蒙脱石和高岭石改性石英砂填加量的进一步增加,GO受到的阻滞作用愈加明显,是因为黏土矿物进一步增大了石英砂的比表面积,提供了更多的沉积位点[31]。
GO-As(V)共迁移结果显示出GO在砂柱中具有很强的迁移性,且迁移能力高于单独迁移的GO。主要是因为GO负载As(V)后,其Zeta电位比GO更低,因而与介质表面间的静电排斥力更大,导致GO的迁移能力增强。与GO单独迁移结果类似的是随着高岭石和蒙脱石改性石英砂填加量的增大,GO受到的阻滞作用均逐渐增大,且GO在蒙脱石改性石英砂柱中受到的阻滞作用更为明显,表明在GO-As(V)迁移过程中,GO与黏土矿物间的相互作用起着主导作用[31]。通过分析讨论As(V)与GO的共迁移结果可知,当溶液中同时存在GO和As(V)时,二者在介质中的迁移能力均大于其单独迁移,因此当GO、As(V)共同释放到土壤中后,会加速As(V)的迁移,造成地下水As(V)污染。
3 结 论
1)五价砷(As(V))在填加0%、10%、30%和50%蒙脱石改性石英砂柱中的回收率依次为94%、92%、88%和82%,在相同比例高岭石改性石英砂柱中的回收率依次为94%、89%、83%和79%,显示出高岭石和蒙脱石改性石英砂的含量越高,As(V)在砂柱中的沉积量愈多,As(V)随着蒙脱石和高岭石改性石英砂填加比例的增加,迁移能力逐渐降低,氧化石墨烯(Graphene Oxide,GO)亦是同样的结果;
2)As(V)在不同性质介质中的迁移能力从大到小为纯石英砂、蒙脱石改性石英砂、高岭石改性石英砂,而GO的迁移能力从大到小则为纯石英砂、高岭石改性石英砂、蒙脱石改性石英砂;
3)当溶液中同时存在GO和As(V)时,GO的Zeta电位由−21.3下降至−26.7 mV,主要原因是As(V)的存在增加了GO表面携带的负电荷,增大了与介质表面间的排斥力,另一方面说明GO可以作为As(V)的载体携带As(V)进行迁移,因此GO和As(V)在高岭石和蒙脱石改性石英砂中的迁移能力均大于其单独迁移。
综上所述,GO、As(V)共同释放到农田土壤中后,会加速As(V)的迁移,造成农田土壤砷污染的扩大化,不利于土壤砷污染的修复。
[1]董双快,徐万里,吴福飞,等. 铁改性生物炭促进土壤砷形态转化抑制植物砷吸收[J]. 农业工程学报,2016,32(15):204-212.
Dong Shuangkuai, Xu Wanli, Wu Fufei, et al. Fe-modified biochar improving transformation of arsenic form in soil and inhibiting its absorption of plant[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(15): 204- 212. (in Chinese with English abstract)
[2]姜晓璐,邹滨,汤景文,等. 广东省东南部菜地水田砷含量空间分布[J]. 农业工程学报,2016,32(23):263-268.
Jiang Xiaolu, Zou Bin, Tang Jingwen, et al. Spatial distribution of As in vegetable field and paddy in southeast of Guangdong province[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(23): 263-268. (in Chinese with English abstract)
[3]焦常锋,常会庆,王启震,等. 碳酸钙和壳聚糖联用对高pH值石灰性土壤砷污染的钝化[J]. 农业工程学报,2020,36(11):234-240.
Jiao Changfeng, Chang Huiqing, Wang Qizhen, et al. Passivation effects of calcium carbonate and chitosan on arsenic pollution in high pH calcareous soil[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(11): 234-240. (in Chinese with English abstract)
[4]吴福飞,贾宏涛,董双快,等. 铁改性生物炭抑制土壤中As的迁移[J]. 农业工程学报,2020,36(6):215-222.
Wu Fufei, Jia Hongtao, Dong Shuangkuai, et al. Inhibition effect of iron modified biochar on migration of As in soil[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(6): 215-222. (in Chinese with English abstract)
[5]Ishak C F, Seaman J C, Miller W P, et al. Contaminant mobility in soils amended with fly ash and flue-gas gypsum: Intact soil cores and repacked columns[J]. Water Air & Soil Pollution, 2002, 134(1): 285-303.
[6]DouOvá B, Martaus A, Filippi M, et al. Stability of arsenic species in soils contaminated naturally and in an anthropogenic manner[J]. Water Air & Soil Pollution, 2008, 187(1): 233-241.
[7]Farid A, Debora F R. Investigation of acute effects of graphene oxide on wastewater microbial community: A case study[J]. Journal of Hazardous Materials, 2013, 256-257: 33-39.
[8]Bai Jing, Sun Huimin, Yin Xiaojie, et al. Oxygen- content-controllable graphene oxide from electron-beam- irradiated graphite: Synthesis, characterization, and removal of aqueous lead [Pb(II)][J]. ACS Applied Materials & Interfaces, 2016, 8(38): 25289-25296.
[9]Yang Kaijie, Chen Baoliang, Zhu Xiaoying, et al. Aggregation, adsorption, and morphological transformation of graphene oxide in aqueous solutions containing different metal cations[J]. Environmental Science & Technology, 2016, 50(20): 11066-11075.
[10]Aniruddha M, Li Yuanyuan, Bikash M, et al. Selective adsorption of organic dyes on graphene oxide: Theoretical and experimental analysis[J]. Applied Surface Science, 2019, 464: 170-177.
[11]Franois A P, Faria F, Menachem E. Environmental applications of graphene-based nanomaterials[J]. Chemical Society Reviews, 2015, 44(16): 5861-5896.
[12]Jiang Yanji, Zhang Xiongxiong, Yin Xianqiang, et al. Graphene oxide-facilitated transport of Pb2+and Cd2+in saturated porous media[J]. Science of the Total Environment, 2018(631/632): 369-376.
[13]Zhou D D, Jiang X H, Lu Y, et al. Cotransport of graphene oxide and Cu(II) through saturated porous media[J]. Science of the Total Environment, 2016, 550: 717-726.
[14]Zhao Kang, Chen Chong, Cheng Tao, et al. Graphene oxide-facilitated uranium transport and release in saturated medium: Effect of ionic strength and medium structure[J]. Environment Pollution, 2019, 247: 668-677.
[15]Liu Xianwei, Li Meng, Liu Fuyang, et al. Cotransport of graphene oxides/reduced graphene oxides with BPA in both bare and iron oxides coated quartz sand[J]. Science China Technological Sciences, 2019, 62(11): 1-11.
[16]Li Yanrong. Effects of particle shape and size distribution on the shear strength behavior of composite soils[J]. Bulletin of Engineering Geology & the Environment, 2013, 72(3/4): 371-381.
[17]Roberto R F, Lidia L F, Cecilia I C, et al. Particle-size distribution in soils: A critical study of the fractal model validation[J]. Geoderma, 2006, 134(3): 320-334.
[18]Zhao Lijuan, Jose R P, Hernandez J A, et al. Transport and retention behavior of ZnO nanoparticles in two natural soils: Effect of surface coating and soil composition[J]. Journal of Nano Research, 2012, 17: 229-242.
[19]Morillo E, Undabeytia T, Maqueda C, et al. The effect of dissolved glyphosate upon the sorption of copper by three selected soils[J]. Chemosphere, 2002, 47(7): 740-752.
[20]Selim H M. Modeling the transport and retention of arsenic (V) in soils[J]. Soil Science Society of America Journal. 1992, 70(5): 1677-1687.
[21]Constantinos V C, Nikolaos P S, Nikolaos G K. Cotransport of graphene oxide nanoparticles and kaolinite colloids in porous media[J]. Transport in Porous Media, 2017, 119(1): 181-204.
[22]Indranil C, Matthew C D, Nikhita D M, et al. Response to comment on “colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment”[J]. Environmental Science & Technology, 2013, 47(12): 6288-6296.
[23]孙慧敏,殷宪强,曹秀蓉. 离子强度对蒙脱石胶体在饱和多孔介质中运移与释放的影响[J]. 环境科学学报,2012,32(5):1120-1125.
Sun Huimin, Yin Xianqiang, Cao Xiurong. The effect of solution ionic strength on the transport and release of montmorillonite colloid in saturated porous media[J]. Acta Scientiae Circumstantiae, 2012, 32(5): 1120-1125. (in Chinese with English abstract)
[24]Philip R J, Sun Ning, Menachem E. Colloid transport in geochemically heterogeneous porous media: Modeling and measurements[J]. Environmental Science & Technology, 1996, 30(11): 3284-3293.
[25]Xia Tianjiao, Lin Yixuan, Guo Xuetao, et al. Co-transport of graphene oxide and titanium dioxide nanoparticles in saturated quartz sand: Influences of solution pH and metal ions[J]. Environment Pollution, 2019, 251(8): 723-730.
[26]Jorge J, Flury M. Humic acid-, ferrihydrite-, and aluminosilicate-coated sands for column transport experiments[J]. Colloids & Surfaces A Physicochemical & Engineering Aspects, 2006, 273(1/3): 90-96.
[27]Lin Z, Puls R W. Adsorption, desorption and oxidation of arsenic affected by clay minerals and aging process[J]. Environmental Geology, 2000, 39(7): 753-759.
[28]Eleonora D, Ciminelli V S T, Weidler P G, et al. Arsenic sorption onto soils enriched in Mn and Fe minerals[J]. Clays & Clay Minerals, 2003, 51(2): 197-204.
[29]Ladeira A C Q, Ciminelli V S T, Duarte H A, et al. Mechanism of anion retention from EXAFS and density functional calculations: Arsenic (V) adsorbed on gibbsite[J]. Geochimica Et Cosmochimica Acta, 2001, 65(8): 1211-1217.
[30]韩永华. 高岭石、蒙脱石表面性质及其分散机理的量子化学研究[D]. 北京:中国矿业大学,2017.
Han Yonghua. Quantum Chemistry Study on Surface Properties and Dispersion of Kaolinite and Montmorillonite[D]. Beijing: China University of Mining and Technology, 2017. (in Chinese with English abstract)
[31]Yin Xianqiang, Jiang Yanji, Tan Yuihui, et al. Co-transport of graphene oxide and heavy metal ions in surface-modified porous media[J]. Chemosphere, 2019,218:1-13.
Co-transport characteristics of graphene oxide and pentavalent arsenic in modified porous media
Meng Qingtao1,2,3, Guan Duo3,Jiang Yanji3, Sun Huimin3, Yin Xianqiang3※, Wang Nong4
(1,730000,; 2100049;3.712100,;4300191,)
Arsenic is one of the main elements of heavy metal pollution in farmland soil. In the process of remediation of arsenic-contaminated farmland soil, it is often overlooked that nanoparticles can re-release the bound arsenic, leading to an increase in the effective arsenic concentration. Due to its high specific surface area and strong adsorption capacity, Graphene Oxide (GO) can be used as a carrier of pollutants to carry pollutants and migrate in groundwater environments. However, researches on the transport behavior of anions Arsenic (As(V)) and GO have not been reported. Clay minerals are more active solid components in the soil and play an important role in affecting the migration and transformation of pollutants. Exploring the influence of clay minerals in the soil on the transport behavior of GO and As(V) in porous media is of great significance for improving the theory and model of the fate and transport of nanoparticles and As(V) in the soil, and protecting the soil-groundwater environment. In this study, the influence of clay minerals on the transport behavior of GO and pentavalent As(V) in porous media was investigated. The montmorillonite and kaolinite were used to modify the quartz sand, and the surface characteristics of the modified quartz sand were characterized by a scanning electron microscope and energy dispersive spectrometer. The migration behavior of GO, As(V) and GO-As(V) in 0%, 10%, 30%, and 50% montmorillonite and kaolinite modified quartz sand column was systematically studied by sand column transport experiment. The difference of the effect of different addition ratios on the transport of As(V) and GO was analyzed with the t-test (paired sample test), and the transport behavior of GO colloids in porous media was explained with Darjaguin-Landau-Verwey-Overbeek (DLVO) theory. The research results showed that the kaolinite particles coated on the surface of quartz sand were separated between particles, and the size was different. The montmorillonite particles coated on the surface of the quartz sand were stacked layer by layer in the shape of an amorphous sheet with a pore-shaped structure. GO and As(V) alone had high mobility in porous media. GO and As(V) both had high mobility in pure quartz sand column, and the recovery rates were 96% and 94%, respectively. The proportion of kaolinite and montmorillonite modified quartz sand added was increased to 10%, 30%, and 50%. The migration ability of GO and As(V) all showed a decreasing trend, there were significant differences in the migration curves of GO and As(V) under different conditions (<0.05). The recovery rate of GO in the 50% kaolinite modified quartz sand column was 14% lower than that of the quartz sand column, and the recovery rate in the montmorillonite modified quartz sand column was reduced by 17%, while the As(V) decreased by 15% and 12% respectively. When both GO and As(V) existed in the solution, the Zeta potential of GO decreased from -21.3 to -26.7 mV. The presence of As(V) increased the negative charge carried on the GO surface and increased the repulsive force with the surface of the medium. On the other hand, it showed that GO could be used as a carrier of As(V) to carry As(V) for migration. Therefore, the migration ability of GO and As(V) in kaolinite and montmorillonite modified quartz sand was greater than their transport alone. The analysis showed that the mobility of As(V) in the montmorillonite modified quartz sand column was greater than that of kaolinite modified quartz sand, while the mobility of GO was opposite. When both GO and As(V) existed in the solution, the mobility of both in the medium was greater than their transport alone. The transport behavior of GO in packing modified quartz sand with different proportions was consistent with the DLVO theory. This study showed that they could accelerate the transport of As(V) and caused the expansion of soil arsenic pollution after GO and As(V) being released into the porous media.
heavy metals; soils; water; graphene oxide; kaolinite; montmorillonite; As(V); transport
孟庆涛,管多,姜延吉,等. 氧化石墨烯和五价砷在改性多孔介质中的共迁移特性[J]. 农业工程学报,2020,36(17):142-148.doi:10.11975/j.issn.1002-6819.2020.17.017 http://www.tcsae.org
Meng Qingtao, Guan Duo, Jiang Yanji, et al. Co-transport characteristics of graphene oxide and pentavalent arsenic in modified porous media[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(17): 142-148. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2020.17.017 http://www.tcsae.org
2020-04-25
2020-07-22
国家重点研发计划项目(2018YFC1800600);国家自然科学基金面上项目(41877018,41771260);陕西省重点研发项目(2018ZDXM-SF-030,2017SF-377)
孟庆涛,博士生,主要从事水土环境污染控制与生态修复研究。Email:mqt@nwafu.edu.cn
殷宪强,博士,教授,主要从事环境污染治理领域的纳米材料的环境效应、微量元素的迁移转化、土壤重金属污染修复、有机污染物的降解等研究。Email:xqyin@nwsuaf.edu.cn
10.11975/j.issn.1002-6819.2020.17.017
X8
A
1002-6819(2020)-17-0142-07

