miRNA与2型糖尿病关系的研究进展
张方华 高珊 王秀秀 杨颖 王晔 姚民秀

[摘要] 2型糖尿病的主要病因是由多种因素引起的胰岛素缺乏和胰岛素抵抗。microRNA(miRNA)是很多生理过程及病理生理过程的重要调节因子,在多个生物学过程中发挥关键作用,如细胞增殖、分化、凋亡和癌变等。有研究表明,miRNA通过作用于多个通路促进胰岛素分泌或调节胰岛素抵抗,异常miRNA表达可能是2型糖尿病的潜在发病机制。本文就目前所知与2型糖尿病发病机制有关的miRNA进行综述。
[关键词] 微RNAs;糖尿病,2型;胰岛素抗药性;综述
[中图分类号] R587.1 [文献标志码] A [文章编号] 2096-5532(2020)05-0619-05
doi:10.11712/jms.2096-5532.2020.56.138 [开放科学(资源服务)标识码(OSID)]
[ABSTRACT] Type 2 diabetes is primarily caused by insulin deficiency and insulin resistance resulting from composite factors such as gene, environment, and diet. MicroRNAs (miRNAs) are important regulators of numerous physiological and pathophysiological processes. They play critical roles in various biological processes, including cell proliferation, differentiation, apoptosis, and carcinogenesis. It has been recently revealed that miRNAs promote insulin secretion or regulate insulin resistance by acting on multiple pathways, and abnormal miRNAs expression plays a potentially pathological role in type 2 diabetes. This article is a review of the currently known miRNAs related to the pathogenesis of type 2 diabetes.
[KEY WORDS] microRNAs; diabetes mellitus, type 2; insulin resistance; review
糖尿病在世界范圍内呈爆发性增长,国家糖尿病联盟(IDF)2015年的数据表明,全球成人糖尿病的患病率已达到8.8%,20~79岁人群中有500万人死于糖尿病及其并发症。中国全国慢性非传染性疾病预防控制中心对170 287人的调查结果显示,糖尿病患病率高达10.9%,糖尿病前期患病率为35.7%[1]。糖尿病人群中90%为2型糖尿病,2型糖尿病的主要病因是由基因、环境及饮食等综合因素引起的胰岛素缺乏和胰岛素抵抗。糖尿病及其并发症是致残、降低病人生活质量及导致病人早亡的主要原因,对糖尿病的预防及控制迫在眉睫。
大约90%哺乳动物基因组包含非编码序列,microRNA(miRNA)是一种长为19~23个核苷酸的非编码小RNA。miRNA结合到mRNA的3′端非翻译区,对mRNA进行剪切、降解或去腺苷酸化调控,影响mRNA 的完整性,或者通过干扰其翻译进行转录后调控。miRNA在转录后水平调控成千上万个基因,在多个生物学过程中发挥关键作用,如细胞增殖、分化、凋亡和癌变等。有关研究结果表明,miRNA在糖尿病、胃癌及慢性心力衰竭病人中均呈差异性表达[2-4]。miRNA通过作用于多个通路促进胰岛素分泌或调节胰岛素抵抗,可能成为治疗糖尿病的新靶点。因此,明确miRNA在糖尿病发病中的分子作用机制,可能为糖尿病的进一步治疗提供新的靶点。本文主要就目前所知与2型糖尿病发病机制有关的miRNA进行综述。
1 miRNA在β细胞中的作用
2004年,POY等[5]首次报道了miR-375可直接调节胰岛分泌。miR-375直接靶向作用于3′-磷酸肌醇依赖性激酶1 mRNA,从而降低胰岛素分泌,3′-磷酸肌醇依赖性激酶1 mRNA是胞内磷脂酰肌醇激酶(PI3K)途径中的关键分子;降低miR-375水平可促进胰岛素分泌。miR-375敲除小鼠表现出高糖血症伴β细胞数量的减少。在糖尿病ob/ob小鼠中可观察到miR-375表达升高。在2型糖尿病病人的胰岛细胞中也发现miR-375的表达升高[6]。这些研究结果表明,miR-375不仅能调节葡萄糖稳态(例如胰岛素基因表达)和胰岛素分泌(通过对胞吐作用的影响),而且在胰腺β细胞的发育、维持和存活中也起重要作用。
miR-9可以负向调节胰岛素的分泌,其作用机制是以靶向作用homeobox2的切口,增加Rab3/Rab27 GTPase效应物granuphilin水平,阻止质膜上β细胞分泌颗粒物,从而抑制胰岛素分泌。miR-124a可负向调节葡萄糖诱导的胰岛素分泌,在2型糖尿病病人的胰腺中其表达是上调的[7]。miR-124a2靶向作用于Mtpn和Foxa2 mRNA[7],而Foxa2是胰腺发育的主要调控因子,它通过作用于下游靶点胰腺和十二指肠同源盒1及与胰岛素分泌和葡萄糖代谢相关的基因发挥生物学作用。miR-29可以靶向作用于单羧酸盐转运蛋白1,从而影响胰岛素的释放。miR-29亚种型通过减少Onecut2的表达、增强granuphilin的表达,达到抑制MIN6细胞系和胰岛细胞胰岛素释放。因此,miR-29负向调控葡萄糖刺激的胰岛素分泌。miR-29作为胰岛素刺激的葡萄糖代谢和脂质过氧化的重要调节因子,参与2型糖尿病的发生[8]。miR-182是β细胞特异的miRNA。胰岛β细胞中miR-182的表达水平显著高于胰腺α细胞。通过靶向下调β细胞内α细胞特异性基因和调节胰高血糖素合成的重要转录因子cMaf的表达抑制α细胞表型的形成,可维持β细胞表型的稳定性[9]。miR-182可能对胰岛β细胞表型的维持及功能的调控有重要作用,其表达异常可能参与了糖尿病的发病过程。目前所知与β细胞功能相关的miRNA见表1。
2 miRNA和胰岛素抵抗
研究证实,miRNA参与肥胖病人脂肪细胞的基因表达。Goto-Kakizaki大鼠是一种目前已成熟的2型糖尿病大鼠模型[29],其骨骼肌、肝脏和脂肪组织中miR-29表达增加。在脂肪细胞衍生的3T3-L1细胞系中也发现,高糖血症或者高胰岛素血症均可诱导miR-29的表达[30]。miR-29可调控由FOXA2介导的脂质代谢基因表达,如PPARGC1A、HMGCS2和ABHD5[31]。3T3-L1细胞中miR-29过表达可使胰岛素诱导的葡萄糖摄取减少,导致胰岛素抵抗。胰岛素抵抗的3T3-L1脂肪细胞中miR-320的表达显著上调,miR-320表达增强有助于改善胰岛素的敏感性。应用miR-320拮抗剂干预后,胰岛素抵抗明显增强[32]。miR-320与糖尿病小鼠的胰腺组织学改变密切相关,其相关信号通路为PI3K/Akt信号通路[33]。
miR-103和miR-107在肥胖小鼠中呈高表达状态。沉默miR-103和miR-107可改善脂肪组织和肝脏的胰岛素抵抗[34]。miR-103/107存在于脂肪或肝脏组织中,其靶基因小窝蛋白-1是一种胰岛素受体(Insr)必不可少的调控因子。miR-103/107通过靶向作用于小窝蛋白-1,导致胰岛素抵抗[35]。miR-143在高脂饮食诱导的肥胖小鼠肠系膜脂肪组织及db/db小鼠的肝脏中表达上调[22,36]。在糖尿病前期,miR-103的表达已明显升高。糖尿病小鼠miR-103和miR-143的表达较非糖尿病小鼠明显升高[35]。降低miR-103和miR-143的表达,血糖水平随之降低[37]。
miRNA let-7可下调骨骼肌中胰岛素-PI3K-mTOR信号通路的蛋白,如Insr、胰岛素样生长因子1受体(Igf1r)、Irs2、Pik3ip1、Akt2、Tsc1和Rictor。缺乏肌肉特异性RNA结合蛋白Lin28a的小鼠和诱导let-7转基因小鼠表现出葡萄糖耐量异常[38]。糖尿病合并动脉硬化的病人let-7表达下降,增强let-7的表达有助于改善血管内皮炎性反应[39]。广泛敲除let-7家族,可改善肝脏和肌肉的胰岛素敏感性,其部分原因是通过Insr和Irs2表达水平的恢复[40]。
miR-122是肝脏特异性miRNA,与胰岛素抵抗、代谢综合征、糖尿病等密切相关[41]。血液中miR-122表达增强的糖耐量异常病人不会进展为糖尿病[42]。ob/ob小鼠和链脲佐菌素诱导的糖尿病小鼠肝脏中的miR-122表达水平均降低[43]。蛋白酪氨酸磷酸酶1b作为miR-122的另一个直接靶点,通过对Insr和Insr底物的酪氨酸残基去磷酸化抑制肝脏胰岛素信号通路。
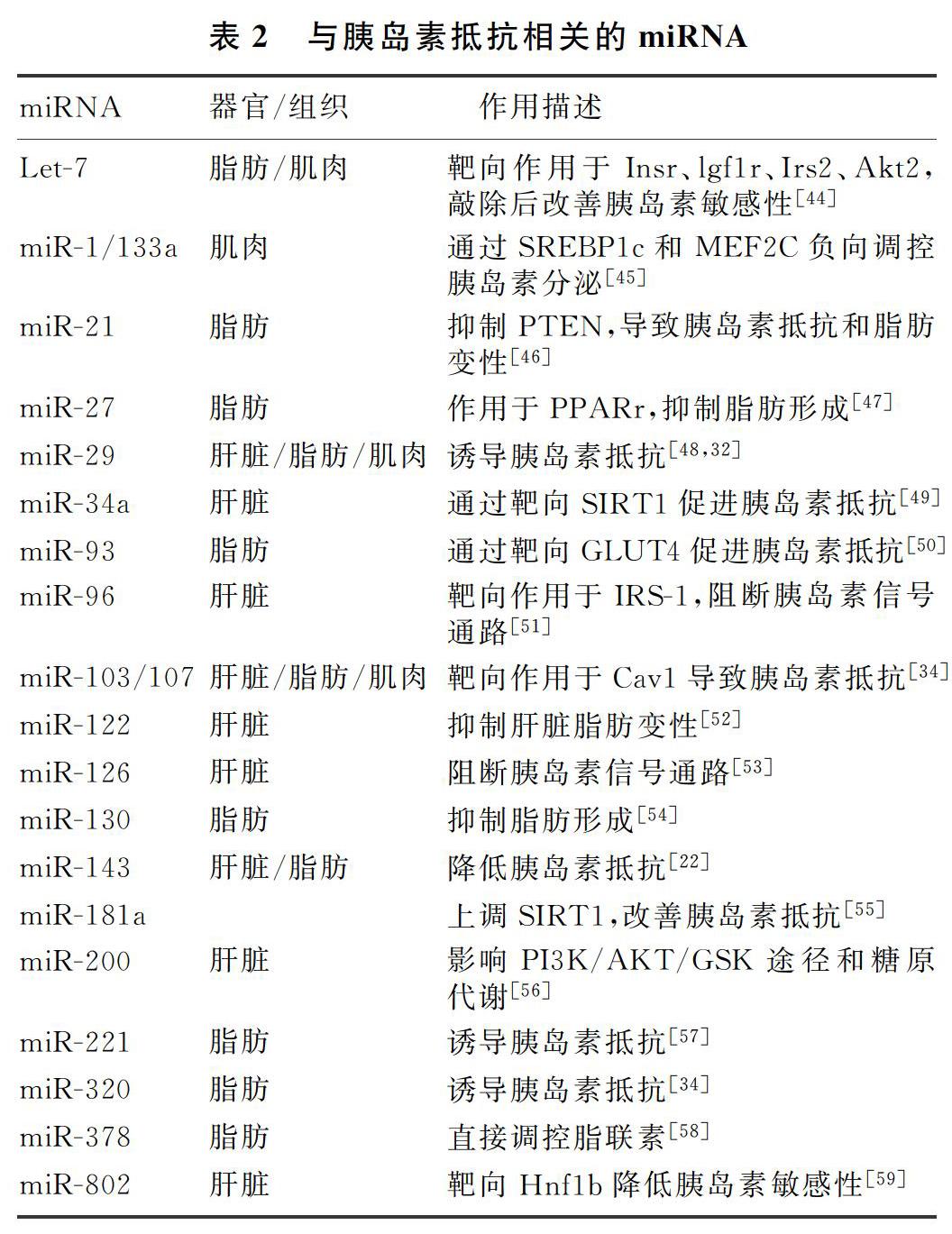
许多miRNA与胰岛素抵抗紧密相关,受营养、代谢和炎症状态等影响。随着临床技术的发展,miRNA靶向方法如小分子操作以及特异性抑制miRNA的表达和功能等为疾病治疗提供了新的方向,在体内试验确认其治疗特异性、有效性、安全性后,有望用于治疗多种临床疾病,尤其是治疗2型糖尿病和肥胖。目前所知与胰岛素抵抗相关的miRNA见表2。
3 与2型糖尿病相关的miRNA
来自意大利的一项关于动脉粥样硬化的前瞻性研究表明,在病人发生2型糖尿病前的数年,其血浆miR-15a、miR-28-3p、miR-29b、miR-126和miR-223等的表达水平已发生改变[60]。NIELSEN等[61]第一次报道了非1型糖尿病儿童与1型糖尿病儿童血清中的12种miRNA(miR-152、miR-30a-5p、miR-181a、miR-24、miR-148a、miR-210、miR-27a、miR-29a、miR-26a、miR-27b、miR-25和miR-200a)的表达水平不同。
近年来,2型糖尿病和其他疾病的基因学检测技术发生了巨大变化。现在高速发展的高通量基因组测序技术可检测单核苷酸多态性(SNPs)的精确信息,目前国际化的HapMap技术也可达到同样目的。近年来,全基因组关联研究(GWAS)通过测定基因组中的SNP位点,已经鉴定出了与2型糖尿病易感性相关的80多个基因[62]。
在应用GWAS的基础上,VAN DE BUNT等[63]鉴定出了调控2型糖尿病易感基因的miRNA,其表达差异可能构成2型糖尿病遗传基础的一部分。糖尿病的主要病理生理学特征是胰岛素分泌不足,许多已筛选出的易感基因与胰岛素分泌不足相关。VAN DE BUNT等[63]将在胰岛细胞中获得的384个miRNA与人类其他组织相对比发现,有40个miRNA在β细胞中特异性高表達。这40个特异性高表达的miRNA包括直接调控胰岛素分泌的miR-375及以往未曾报道的与糖尿病有关的miR-27b-3p和miR-192-5p。实际上,GWAS数据已经使用糖尿病遗传复制和荟萃分析的数据组合(DIAGRAM)进行了深入的分析[64]。2型糖尿病的几个易感位点就是通过DIAGRAM筛选基因组编码区胰岛表达的miRNA前体重叠区域发现的。最值得注意的是,胰腺β细胞特异性miRNA靶基因也是2型糖尿病的易感基因,如AP3S2、CNK16、NOTCH2、SCL30A8、VPS26A和WFS1等,这些基因的多态性均与胰岛素分泌减少有关[63]。
4 结语
许多研究结果都阐述了miRNA在代谢性疾病——糖尿病的病理生理学中发挥的功能作用,以及在建立和(或)维持β细胞特性及功能过程中发挥的作用。虽然已经在小鼠和人类中鉴定了数千种miRNA,并且证实几十个miRNA与糖尿病有关,但它们的确切作用机制在很大程度上仍然未完全明了,这有待于进一步研究。此外,近年来,随着对2型糖尿病病理生理机制的进一步研究,筛选出2型糖尿病的异常表达miRNA作为其潜在生物标志物成为研究热点。不断深入认识miRNA,进一步了解其功能特点,可为糖尿病潜在的发病机制和遗传易感性研究提供新的视点,尤其是在调控β细胞功能和胰岛素抵抗方面。
[參考文献]
[1] WANG L M, GAO P, ZHANG M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013[J]. JAMA, 2017,317(24):2515-2523.
[2] MELNIK B C. Milk exosomal miRNAs: potential drivers of AMPK-to-mTORC1 switching in β-cell de-differentiation of type 2 diabetes mellitus[J]. Nutr Metab, 2019,16:85.
[3] OHZAWA H, KUMAGAI Y, YAMAGUCHI H, et al. Exosomal microRNA in peritoneal fluid as a biomarker of peritoneal metastases from gastric cancer[J]. Ann Gastroenterol Surg, 2020,4(1):84-93.
[4] FOINQUINOS A, BATKAI S, GENSCHEL C, et al. Precli-nical development of a miR-132 inhibitor for heart failure treatment[J]. Nat Commun, 2020,11(1):633.
[5] POY M N, ELIASSON L, KRUTZFELDT J, et al. A panc-reatic islet-specific microRNA regulates insulin secretion[J]. Nature, 2004,432(7014):226-230.
[6] ZHAO H L, GUAN J, LEE H M, et al. Up-regulated panc-reatic tissue microRNA-375 associates with human type 2 diabetes through beta-cell deficit and islet amyloid deposition[J]. Pancreas, 2010,39(6):843-846.
[7] SEBASTIANI G, PO A, MIELE E, et al. MicroRNA-124a is hyperexpressed in type 2 diabetic human pancreatic islets and negatively regulates insulin secretion[J]. Acta Diabetol, 2015,52(3):523-530.
[8] MASSART J, SJGREN R J O, LUNDELL L S, et al. Altered miR-29 expression in type 2 diabetes influences glucose and lipid metabolism in skeletal muscle[J]. Diabetes, 2017,66(7):1807-1818.
[9] KLEIN D, MISAWA R, BRAVO-EGANA V, et al. Micro-RNA expression in alpha and beta cells of human pancreatic islets[J]. PLoS One, 2013,8(1):e55064.
[10] GURUNG B, MUHAMMAD A B, HUA X X. Menin is required for optimal processing of the microRNA let-7a[J]. J Biol Chem, 2014,289(14):9902-9908.
[11] WANG Y, LIU J, LIU C, et al. MicroRNA-7 regulates the mTOR pathway and proliferation in adult pancreatic β-cells[J]. Diabetes, 2013,62(3):887-895.
[12] LATREILLE M, HAUSSER J, STTZER I, et al. Micro-RNA-7a regulates pancreatic β cell function[J]. J Clin Investig, 2014,124(6):2722-2735.
[13] HU D Z, WANG Y, ZHANG H Y, et al. Identification of miR-9 as a negative factor of insulin secretion from beta cells[J]. Journal of Physiology and Biochemistry, 2018,74(2):291-299.
[14] SUN L L, JIANG B G, LI W T, et al. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression[J]. Diabetes Res Clin Pract, 2011,91(1):94-100.
[15] ZHANG Z W, ZHANG L Q, DING L, et al. MicroRNA-19b downregulates insulin 1 through targeting transcription factor NeuroD1[J]. FEBS Letters, 2011,585(16):2592-2598.
[16] BAGGE A, CLAUSEN T R, LARSEN S, et al. MicroRNA-29a is up-regulated in beta-cells by glucose and decreases glucose-stimulated insulin secretion[J]. Biochem Biophys Res Commun, 2012,426(2):266-272.
[17] LU H M, HAO L Y, LI S T, et al. Elevated circulating stea-ric acid leads to a major lipotoxic effect on mouse pancreatic beta cells in hyperlipidaemia via a miR-34a-5p-mediated PERK/p53-dependent pathway[J]. Diabetologia, 2016,59(6):1247-1257.
[18] BAROUKH N N, VAN OBBERGHEN E. Function of microRNA-375 and microRNA-124a in pancreas and brain[J]. FEBS J, 2009,276(22):6509-6521.
[19] HENNESSY E, CLYNES M, JEPPESEN P B, et al. Identifification of microRNAs with a role in glucose stimulated insulin secretion by expression profifiling of MIN6 cells[J]. Biochem Biophys Res Commun, 2010,396(2):457-462.
[20] NESCA V, GUAY C, JACOVETTI C, et al. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes[J]. Diabetologia, 2013,56(10):2203-2212.
[21] JORDAN S D, KRGER M, WILLMES D M, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism[J]. Nat Cell Biol, 2011,13(4):434-446.
[22] KANG M H, ZHANG L H, WIJESEKARA N, et al. Regulation of ABCA1 protein expression and function in hepatic and pancreatic islet cells by miR-145[J]. Arterioscler Thromb Vasc Biol, 2013,33(12):2724-2732.
[23] MELKMAN-ZEHAVI T, OREN R, KREDO-RUSSO S, et al. miRNAs control insulin content in pancreatic β-cells via downregulation of transcriptional repressors[J]. The EMBO Journal, 2011,30(5):835-845.
[24] TATTIKOTA S G, RATHJEN T, MCANULTY S J, et al. Argonaute2 mediates compensatory expansion of the pancrea-tic β cell[J]. Cell Metab, 2014,19(1):122-134.
[25] BAO L D, FU X D, SI M W, et al. MicroRNA-185 targets SOCS3 to inhibit beta-cell dysfunction in diabetes[J]. PLoS One, 2015,10(2):e0116067.
[26] LOCKE J M, DA SILVA XAVIER G, DAWE H R, et al. Increased expression of miR-187 in human islets from individuals with type 2 diabetes is associated with reduced glucose-stimulated insulin secretion[J]. Diabetologia, 2014,57(1):122-128.
[27] XU G L, CHEN J Q, JING G, et al. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204[J]. Nat Med, 2013,19(9):1141-1146.
[28] EL OUAAMARI A, BAROUKH N, MARTENS G A, et al. miR-375 targets 3′-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in panc-reatic beta-cells[J]. Diabetes, 2008,57(10):2708-2717.
[29] HE A, ZHU L, GUPTA N, et al. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes[J]. Mol Endocrinol, 2007,21(11):2785-2794.
[30] HERRERA B M, LOCKSTONE H E, TAYLOR J, et al. Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes[J]. Diabetologia, 2010,53:1099-1109.
[31] KURTZ C L, PECK B C E, FANNIN E E, et al. MicroRNA-29 fine-tunes the expression of key FOXA2-activated lipid metabolism genes and is dysregulated in animal models of insulin resistance and diabetes[J]. Diabetes, 2014,63(9):3141-3148.
[32] LING H Y, OU H S, FENG S D, et al. Changes in micro-RNA (miR) profile and effects of miR-320 in insulin-resistant 3T3-L1 adipocytes[J]. Clin Exp Pharmacol Physiol, 2009,36(9):e32-e39.
[33] MO F F, AN T, ZHANG Z J, et al. Jiang Tang Xiao ke gra-nule play an anti-diabetic role in diabetic mice pancreatic tissue by regulating the mRNAs and microRNAs associated with PI3K-Akt signaling pathway[J]. Frontiers in Pharmacology, 2017,8:795.
[34] TRAJKOVSKI M, HAUSSER J, SOUTSCHEK J, et al. MicroRNAs 103 and 107 regulate insulin sensitivity[J]. Nature, 2011,474(7353):649-653.
[35] VATANDOOST N, AMINI M, IRAJ B, et al. Dysregulated miR-103 and miR-143 expression in peripheral blood mononuclear cells from induced prediabetes and type 2 diabetes rats[J]. Gene, 2015,572(1):95-100.
[36] TAKANABE R, ONO K, ABE Y, et al. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet[J]. Biochemical and Biophysical Research Communications, 2008,376(4):728-732.
[37] EL-GHARBAWY R M, EMARA A M, ABU-RISHA S E S. Zinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in type-2 diabetes[J]. Biomedicine & Pharmacotherapy, 2016,84:810-820.
[38] ZHU H, SHYH-CHANG N, SEGR A V, et al. The Lin28/let-7 axis regulates glucose metabolism[J]. Cell, 2011,147(1):81-94.
[39] BRENNAN E P, WANG B, MCCLELLAND A, et al. Protective effect of let-7 miRNA family in regulating inflammation in diabetes-associated atherosclerosis[J]. Diabetes, 2017,66(8):2266-2277.
[40] DEIULIIS J A. MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics[J]. Int J Obes, 2005,2016,40(1):88-101.
[41] WILLEIT P, SKROBLIN P, MOSCHEN A R, et al. Circulating MicroRNA-122 is associated with the risk of new-onset metabolic syndrome and type-2-diabetes[J]. Diabetes, 2017,66(2):347-357.
[42] BRANDT S, ROOS J, INZAGHI E, et al. Circulating levels of miR-122 and nonalcoholic fatty liver disease in pre-pubertal obese children[J]. Pediatr Obes, 2018,13(3):175-182.
[43] LI S J, CHEN X, ZHANG H J, et al. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status[J]. J Lipid Res, 2009,50(9):1756-1765.
[44] FROST R J, OLSON E N. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs[J]. Proc Natl Acad Sci USA, 2011,108(52):21075-21080.
[45] GRANJON A, GUSTIN M P, RIEUSSET J, et al. The microRNA signature in response to insulin reveals its implication in the transcriptional action of insulin in human skeletal muscle and the role of a sterol regulatory element-binding protein-1c/myocyte enhancer factor 2C pathway[J]. Diabetes, 2009,58(11):2555-2564.
[46] LAWAN A, MIN K, ZHANG L, et al. Skeletal muscle-specific deletion of MKP-1 reveals a p38 MAPK/JNK/Akt signaling node that regulates obesity-induced insulin resistance[J]. Diabetes, 2018,67(4):624-635.
[47] KIM S Y, KIM A Y, LEE H W, et al. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression[J]. Biochem Biophys Res Commun, 2010,392(3):323-328.
[48] HUNG Y H, KANKE M, KURTZ C L, et al. Acute suppression of insulin resistance-associated hepatic miR-29 in vivo improves glycemic control in adult mice[J]. Physiol Genom, 2019,51(8):379-389.
[49] LEE J, PADHYE A, SHARMA A Y, et al. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition[J]. J Biol Chem, 2010,285(17):12604-12611.
[50] CHEN Y H, HENEIDI S, LEE J M, et al. miRNA-93 inhi-bits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance[J]. Diabetes, 2013,62(7):2278-2286.
[51] JEONG H J, PARK S Y, YANG W M, et al. The induction of miR-96 by mitochondrial dysfunction causes impaired glycogen synthesis through translational repression of IRS-1 in SK-Hep1 cells[J]. Biochem Biophys Res Commun, 2013,434(3):503-508.
[52] YANG Y M, SEO S Y, KIM T H, et al. Decrease of micro-RNA-122 causes hepatic insulin resistance by inducing protein tyrosine phosphatase 1B, which is reversed by licorice flavonoid[J]. Hepatology, 2012,56(6):2209-2220.
[53] CHENG Z H, LUO C, GUO Z L. LncRNA-XIST/micro-RNA-126 sponge mediates cell proliferation and glucose metabolism through the IRS1/PI3K/Akt pathway in glioma[J]. J Cell Biochem, 2020,121(3):2170-2183.
[54] LEE E K, LEE M J, ABDELMOHSEN K S, et al. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferato-ractivated receptor gamma expression[J]. Mol Cell Biol, 2011,31(4):626-638.
[55] GOK O, KARAALI Z, ERGEN A, et al. Serum sirtuin 1 protein as a potential biomarker for type 2 diabetes: increased expression of sirtuin 1 and the correlation with microRNAs[J]. J Res Med Sci: Off J Isfahan Univ Med Sci, 2019,24:56.
[56] DOU L, ZHAO T, WANG L L, et al. miR-200s contribute to interleukin-6 (IL-6)-induced insulin resistance in hepatocytes[J]. Journal of Biological Chemistry, 2013,288(31):22596-22606.
[57] PENG J, ZHOU Y, DENG Z, et al. miR-221 negatively regulates inflammation and insulin sensitivity in white adipose tissue by repression of sirtuin-1 (SIRT1) [J]. J Cell Biochem, 2018,119(8):6418-6428.
[58] ISHIDA M, SHIMABUKURO M, YAGI S, et al. Micro-RNA-378 regulates adiponectin expression in adipose tissue:a new plausible mechanism[J]. PLoS One, 2014,9(11):e111537.
[59] KORNFELD J W, BAITZEL C, KNNER A C, et al. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b[J]. Nature, 2013,494(7435):111-115.
[60] ZAMPETAKI A, KIECHL S, DROZDOV I, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes[J]. Circ Res, 2010,107(6):810-817.
[61] NIELSEN LB, WANG C, SRENSEN K, et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during di-sease progression[J]. Exp Diabetes Res, 2012,2012:896362.
[62] IMAMURA M, TAKAHASHI A, YAMAUCHI T N, et al. Genome-wide association studies in the Japanese population identify seven novel loci for type 2 diabetes[J]. Nat Commun, 2016,28(7):10531.
[63] VAN DE BUNT M, GAULTON K J, PARTS L, et al. The miRNA profile of human pancreatic islets and beta-cells and relationship to type 2 diabetes pathogenesis[J]. PLoS One, 2013,8(1):e55272.
[64] MORRIS A P, VOIGHT B F, TESLOVICH T M, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes[J]. Nat Genet, 2012,44(9):981-990.
(本文編辑 马伟平)

