Syntheses,Crystal Structures and Photoluminescence Properties of Two Cadmium(II) Coordination Polymers
LIN Xiaoying (林小英), ZHONG Qinhua(钟琴华), SU Ting(苏 婷), SHI Ronghui(史荣会), CHEN Jun(陈 君), LIU Zhipeng(刘志鹏), GONG Lingzhu(龚凌诸)
School of Ecological Environment and Urban Construction,Fujian University of Technology,Fuzhou 350118,China
Abstract: The solvothermal reaction of CdCl2·2.5H2O with terephthalic acid yields two new three-dimensional (3D) cadmium (II) coordination polymers(CPs),namely {[Cd(C8H4O4)Cl]·C2H8N}n and [Cd(C8H4O4)]n,we use the abbreviations CP1 and CP2 to refer to them,respectively. The compounds are characterized in detail by single-crystal X-ray diffraction,powder X-ray diffraction,IR spectroscopy,elemental analysis and thermogravimetric analysis(TGA). The results indicate that compounds CP1 and CP2 are 3D frameworks,and both the compounds feature a uninodal 4-connected framework with a dia net. The empty spaces of such 3D framework are filed by the other two identical frameworks,leading to a 3-fold interpenetrated network. In addition,the photochemical properties of the frameworks are also investigated. The two frameworks show strong solid-state fluorescence emission at 463 and 503 nm,respectively,which are obvious red-shift compared to the emission of ligand.
Key words: solvothermal synthesis; terephthalic acid; photoluminescence property; crystal structure
Introduction
Three-dimensional(3D) coordination polymers (CPs) constructed from metal atoms and ligands are of great interest for their intriguing architectures and potential applications in electrochemistry,photophysics,catalysis,adsorption and separation[1-4]. However,it is still a challenge to obtain CPs with desired structures and properties. An effective and facile approach for the synthesis of such complexes is the self-assembly of well-designed multidentate organic ligands and d-block metal ions or metal clusters under solvothermal conditions[5-8]. Thus,much attention has been paid to the design and synthesis of various multifunctional bridging ligands. Due to their wide range of coordination modes and versatile conformations when binding to metals,aromatic polycarboxylates ligands have attracted increasing interest,because they have rigid organic skeletons,excellent coordination capability andexible coordination patterns[9-12]. It is well known that the rigid terephthalic acid ligands (H2PTA) molecule can act as a versatile bridging ligand to ligate various d10metal ions[13-14]. cadmium(II),as a ds-block metal ion,owns large radius and tends to display flexible coordination behaviors,which provides unique opportunities for the formation of novel network structure[15]. Moreover,the unique 4d10electron configuration often gives rise to substantial photoluminescent processes in the cadmium(II)-luminescent CPs involving ligand-centered transitions (LLCT) and ligand-to-metal charge transfer (LMCT)[16]. In this spirit,our current synthetic strategy is to construct new CPs by combining H2PTA ligands with cadmium(II) metal ions.
Herein,we report the synthesis of two novel 3D CPs,namely {[Cd(C8H4O4)Cl]·C2H8N}nand [Cd(C8H4O4)]n,use the abbreviations CP1 and CP2 to refer to them respectively,which are self-assembly by CdCl2·2.5H2O and H2PTA under solvothermal condition. The compounds are characterized in detail by single-crystal X-ray diffraction,powder X-ray diffraction,IR spectroscopy,elemental analysis and thermogravimetric analysis (TGA). Impressively,both compounds feature 3-fold interpenetrated dia net. In addition,the luminescent properties of compounds CP1 and CP2 were investigated in the solid state at room temperature.
1 Experiments
1.1 Materials and instruments
All chemicals were commercially available sources of analytical grade and used without further purification. The elemental analyses of C,H and N were determined with a Vario EL III elemental analyzer (Elementar Analysensysteme GmbH,Germany). The Fourier transform infrared (FT-IR) spectra were recorded in the range of 4 000-400 cm-1with a Perkin-Elmer Spectrum One Spectrometer using KBr pellets (Thermo Nicolet Corporation,USA). Luminescent properties were tested with an FLS980 luminescence spectrometer (Edinburgh Instruments,England). TGA experiments were done on a STA 409PG Jupiter thermogravimetric analyzer (Netzsch,Germany) in flowing nitrogen with the sample heated in an Al2O3crucible at a heating rate of 10 K·min-1. All powdered X-ray diffraction data were collected on a D8 Advance-Bruker diffractometer (Bruker,Germany) using Cu-Kαradiation (λ= 0.154 059 8 nm) at 40 kV and 40 mA in the range 5.00°≤2θ≤50.00°.
1.2 Synthesis of {[Cd(C8H4O4)Cl]·C2H8N}n and [Cd(C8H4O4)]n
1.2.1Synthesisof{[Cd(C8H4O4)Cl]·C2H8N}n
A mixture of H2PTA (0.1 mmol,0.017 g) and CdCl2·2.5 H2O (0.1 mmol,0.023 g) was solved inN,N-Dimethylformamide(DMF,10 mL),and 0.2 mL HCl (36%) was added. The mixture was stirred for half an hour at room temperature and transferred to a 25 mL Teflon-lined stainless-steel autoclave heated at 100 ℃ for 72 h,and then naturally cooled to room temperature. Colourless block crystals of CP1 suitable for X-ray analyses were obtained,washed with DMF and dried in air. Yield: 43% (based on H2PTA),elemental analysis for CP1: C 33.51%,H 3.35%,N 3.91%; Found: C 33.85%,H 3.58%,N 4.14%. FT-IR (KBr,cm-1): 3 432 (m),3 124 (m),2 973 (w),2 923 (w),1 682 (s),1 556 (s),1 501 (m),1 390 (s),1 294 (m),1 097 (m),1 011 (s),840 (s),749 (s),527 (s).
1.2.2Synthesisof[Cd(C8H4O4)]n
A mixture of H2PTA (0.13 mmol,0.022 g) and CdCl2·2.5H2O (0.39 mmol,0.090 g) was solved in DMF (8 mL),trimesitinic acid (0.13 mmol,0.028 g) and 0.2 mL HNO3(2 mol·L-1) was added. The mixture was stirred for half an hour at room temperature and transferred to a 25 mL Teflon-lined stainless-steel autoclave heated at 150 ℃ for 72 h,and then naturally cooled to room temperature. Colourless block crystals of CP2 suitable for X-ray analyses were obtained,washed with DMF and dried in air. Yield: 56% (based on H2PTA),elemental analysis for CP2: C 43.57%,H 1.81%; Found: C 45.49%,H 1.96%,N 1.58%. FT-IR (KBr,cm-1): 3 305 (m),3 089 (w),2 978 (w),1 688 (s),1 608 (s),1 395 (s),1 287 (m),1 085 (w),823 (m),769 (s).
1.3 Single crystal structure determination
Single crystals of CP1 and CP2 suitable for X-ray analyses were stacked to a fiberglass. Data collections were performed on a Rigaku CCD Saturn 724 (Rigaku Corporation,Japan) at 293 K,which were equipped with graphite monochromatic Mo-Kαradiation (λ= 0.071 073 nm). The intensity data sets were collected with theωscan technique and reduced by CrystalClear software[17]. The structure was solved by direct methods with the SHELXTL crystallographic software package[18]and refined by full-matrix least-squares refinement onF2. Non-hydrogen atoms were located by difference Fourier maps and subjected to anisotropic refinement. The hydrogen atoms were added according to theoretical models. The crystallographic data and structural refinements are summarized in Table 1. The selected bond lengths and bond angles are listed in Tables 2-5.
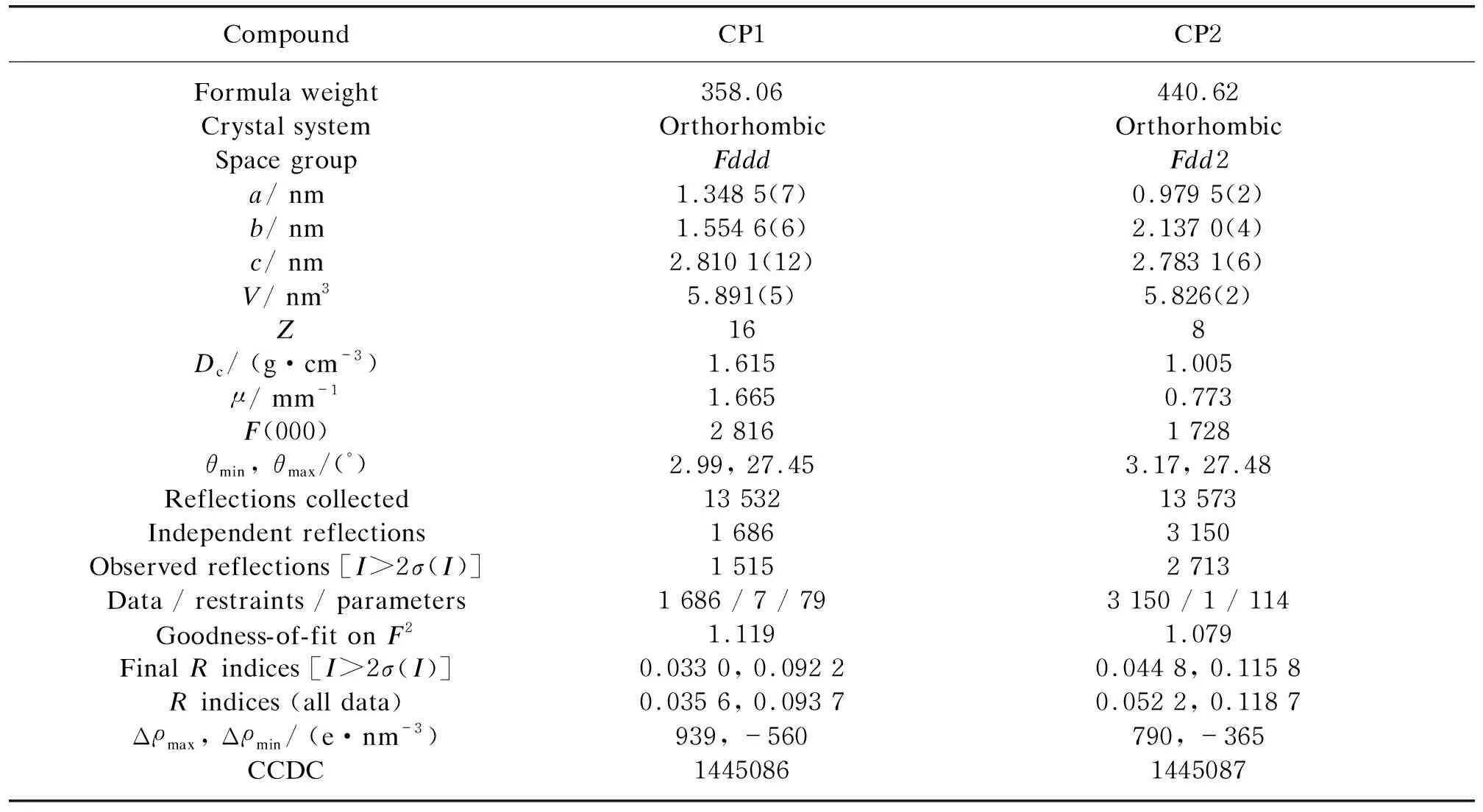
Table 1 Crystallographic data and structural refinements for CP1 and CP2
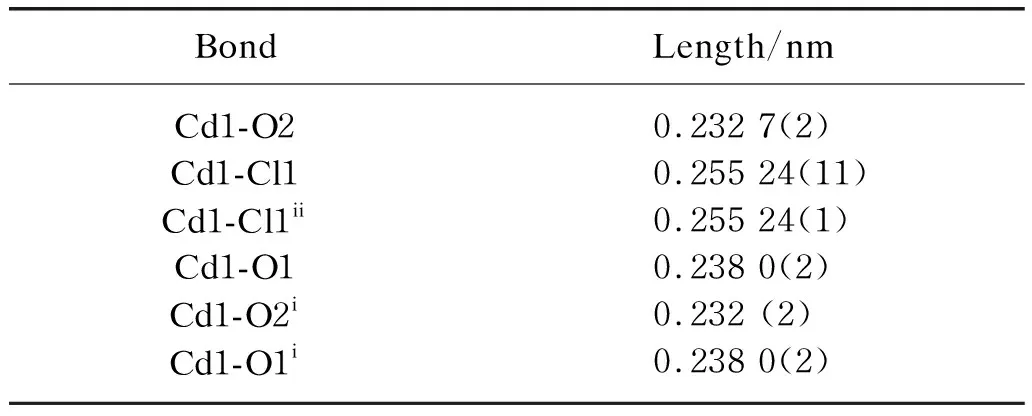
Table 2 Selected bond lengths for CP1
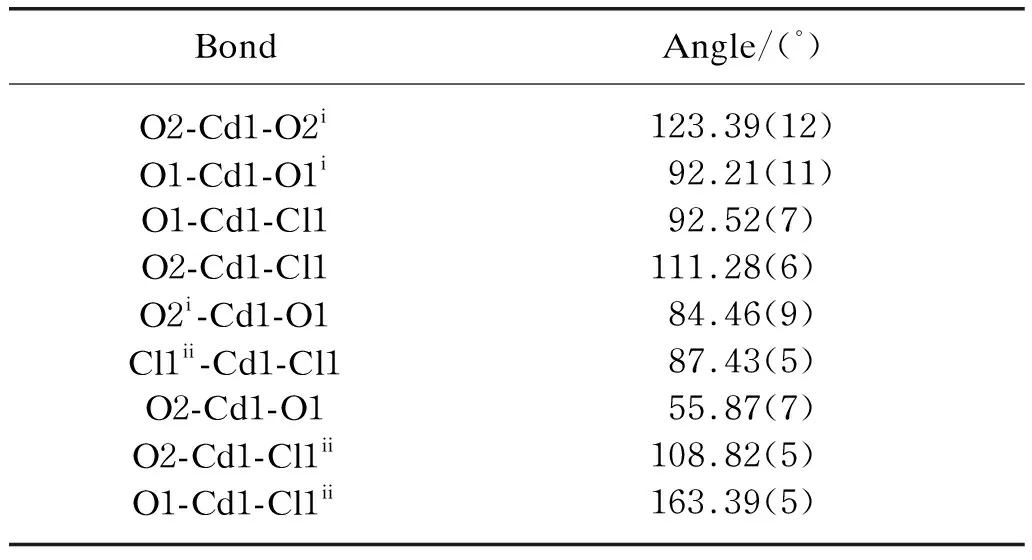
Table 3 Selected bond angles for CP1

Table 4 Selected bond lengths for CP2
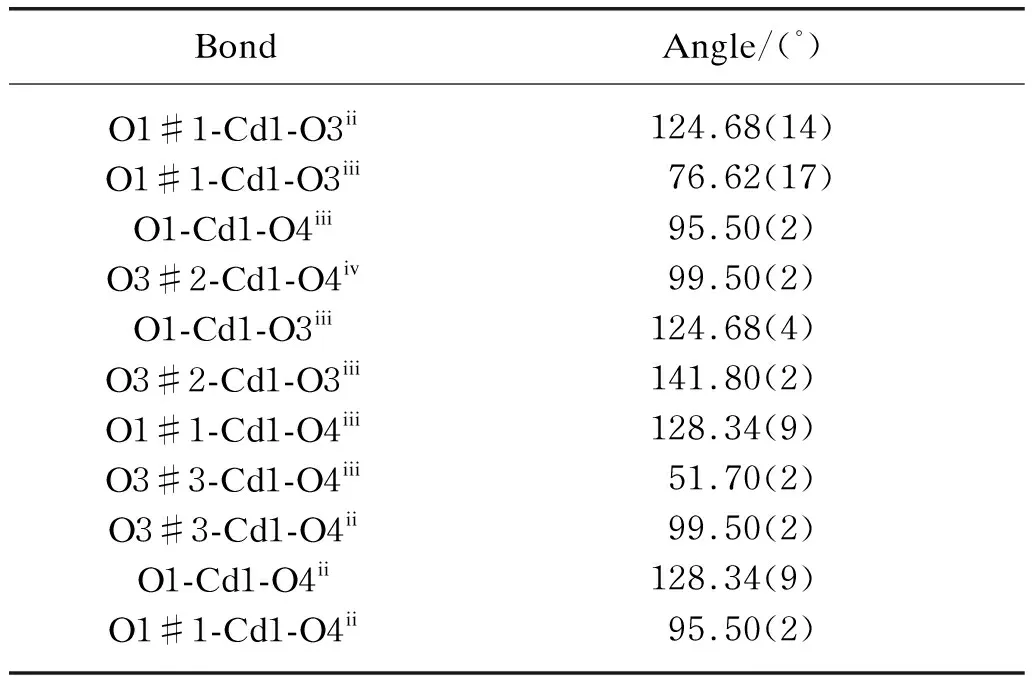
Table 5 Selected bond angles for CP2
2 Results and Discussion
2.1 Structure description
X-ray crystallographic analysis shows that CP1 crystallizes in the orthorhombic space groupFdddand shows a 3D framework structure. As plotted in Fig. 1(a),the asymmetric unit contains a half crystallographically independent cadmium(II) cation (Cd1),half deprotonated terephthalic acid ligands (denoted PTA2-),one chloride anion,and half of protonated dimethylamine. The protonated dimethylamine may derive from the decomposition of DMF solvent.
Atom Cd1 is 6-coordinated by two chloride anion (Cl1 and Cl1ii) and four carboxylate O atoms (O1,O2,O1iand O2i) from two PTA2-ligands,forming a distorted octahedron. The Cd1-O1/O2 bond lengths are in the range of 0.232 7(3)-0.238 6(3) nm,and fall within the typical range of Cd-O bond distances according to the reported literature[12,19]. The Cd1-Cl1 bond length is 0.255 44(12) nm,which is similar to the values reported for Cd complex. The PTA2-ligand is fully (i.e. doubly) deprotonated and adoptedμ2-O,O′,O″,O‴ coordination mode,bridging two cadmium(II) cations from two Cd2(μ2-Cl)2secondary building units (SBUs). The dihedral angles between the planes of the carboxylate groups at PTA2-and their adjacent benzene ring is 4.5(2)°. The chloride anion ligand in CP1 bridges two cadmium(II) cations,i.e. the inversion-related Cd1 and Cd1iicentres are joined into a dinuclear complex Cd2(μ2-Cl)2SBUs with Cd…Cd distance is 0.369 0(3) nm.
In CP1,each PTA2-ligands is connected to two Cd2(μ2-Cl)2SBUs through the same coordination modes. The dinuclear Cd2(μ2-Cl)2SBU connects to four neighbouring congeners through four PTA2-ligands,forming a 3D framework [Fig. 1(b)]. To simplify this structure,each Cd2(μ2-Cl)2SBU was treated as a 4-connected node and PTA2-ligands were treated as linkers. The whole framework of CP1 can then be topologically represented as a uninodal 4-connected diamond network with a Schläfli symbol of 66(Fig. 2) according to the Reticular Chemistry Structure Resource(RCSR) symbol[20]. The empty spaces of the 3D framework are interlaced with two equivalent frameworks to form a 3-fold interpenetrated network.

Fig.1 Crystallographic structure of CP1: (a) asymmetric unit with 50% thermal ellipsoids; (b) 3D framework (the protonated dimethylamine and all H atoms are omitted for clarity)
X-ray crystallographic analysis shows that CP2 crystallizes in the orthorhombic space groupFdd2 and also features a 3D framework structure. There are one independent cadmium(II) ions and one PTA2-ligand in the asymmetric unit [ Fig. 3(a)]. Each cadmium(II) center is bound to six car boxylate oxygen atoms (O1,O1ii,O3i,O4i,O3iiiand O4iii) from four individual PTA2-ligands in a distorted octahedral geometry,the Cd1-O bond lengths are in the range of 0.237 5(3)-0.243 3(5) nm,and fall within the normal range of Cd-O bond distances according to the literatures[12,19]. The cadmium(II) atom can be regarded as a 4-connected tetrahedral node. The PTA2-ligand functions as an extended linker,bridging two cadmium(II) centers. As shown in Fig. 3(b),the presence of a huge adamantane cage is apparent. All of the Cd…Cd edge separations equal 1.144 2(2) nm and the Cd…Cd…Cd angles range from 100.10° to 124.133°,in which an imaginary sphere with a diameter of up to 1.1 nm could be accommodated.

Fig. 3 Crystallographic structure of CP2: (a) asymmetric unit with 50% thermal ellipsoids; (b) a huge adamantane cage; (c) 3D framework (all H atoms are omitted for clarity )
A topology analysis using the TOPOS software package[21]reveals that compound CP2 consists of a 66dia 3-fold interpenetrated framework which exclusively involves translationally equivalent dia nets (Fig. 4). Interpenetration is an important and essentially ubiquitous phenomenon in the crystal engineering of huge frameworks. To the best of our knowledge,the report on terephthalic acid entangled in CPs with a 3-fold interpenetration mode is rather limited.
2.2 Powder X-ray diffraction and TGA
The experimental powdered X-ray diffraction patterns of CP1 and CP2 agree well with the simulated ones based on the single-crystal X-ray data (Fig. 5),indicating that both compounds are in a pure phase. As TGA curves shown in Fig. 6,less than 2% weight loss is

Fig. 4 Topology diagrams of CP2: (a) 3-fold interpenetration; (b) topologic dia net
observed before 200 ℃,indicating that CP1 almost does not contains low-boiling-point solvent molecules. The weight loss from 200 ℃ to 260 ℃ may be attributed to the decomposition of protonated dimethylamine (calculated 12.8%). Then the following weight loss from 280 ℃ corresponds to the collapse of CPs. Compound CP2 shows a weight loss from room temperature to 180 ℃,which is corresponding to the release of free solvent molecule,and the resulting dried framework is stable up to 250 ℃. With continuous heating,compound CP2 begins to decompose.
2.3 Luminescent property
The solid-state luminescence of compound CP1 and CP2 are investigated at room temperature (Fig. 7). When excited by 320 and 330 nm light,compounds CP1 and CP2 display dominant emission bands at 463 and 503 nm,respectively,which are significantly red-shift compared with the emission band at 380 nm of free ligand H2PTA[22]. This phenomenon can be attributed to ligand-to-metal charge transfer between the π systems and the d orbitals of the cadmium(II) centres[23]. It needs to note that the CP emission intensities are much higher than the free ligand ones. This phenomenon was also observed in other reported d10metal carboxyl complexes. Reasons for this might be ascribed to that the strong interactions of cadmium(II) and carboxyl lead to effective charge transfer between the aromatic π systems and the metal center.

Fig. 7 Solid state emission spectra
3 Conclusions
In summary,we have successfully prepared two novel 3D PTA2--based cadmium(II) CPs using the solvothermal method. Interestingly,both compounds possess a 3-fold interpenetrated 3D framework. Moreover,the two frameworks show strong solid-state fluorescence emission characteristics which may make them good candidates for luminescent materials.
 Journal of Donghua University(English Edition)2020年3期
Journal of Donghua University(English Edition)2020年3期
- Journal of Donghua University(English Edition)的其它文章
- Analysis of Outlets Retail Market Based on Chinese Tourists
- Fraud Identification of Chinese Listed Companies—an Improvement Based on M-Score
- Structure and Properties of Degradable Polybutylene Succinate Fibers
- Hierarchical Visualized Multi-level Information Fusion for Big Data of Digital Image
- Encryption and Decryption of Color Images through Random Disruption of Rows and Columns
- Clothes Keypoints Detection with Cascaded Pyramid Network
