Structure and Properties of Degradable Polybutylene Succinate Fibers
HU Jiewen(胡杰文), WANG Qianqian(王倩倩), ZHU Ping(朱 平)*
1 College of Textile & Clothing, Qingdao University, Qingdao 266071, China2 Institute of Functional Textiles and Advanced Materials, Qingdao University, Qingdao 266071, China3 State Key Laboratory of Bio-Fibers and Eco-Textiles, Qingdao University, Qingdao 266071, China
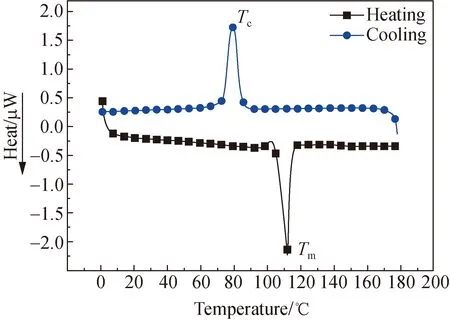
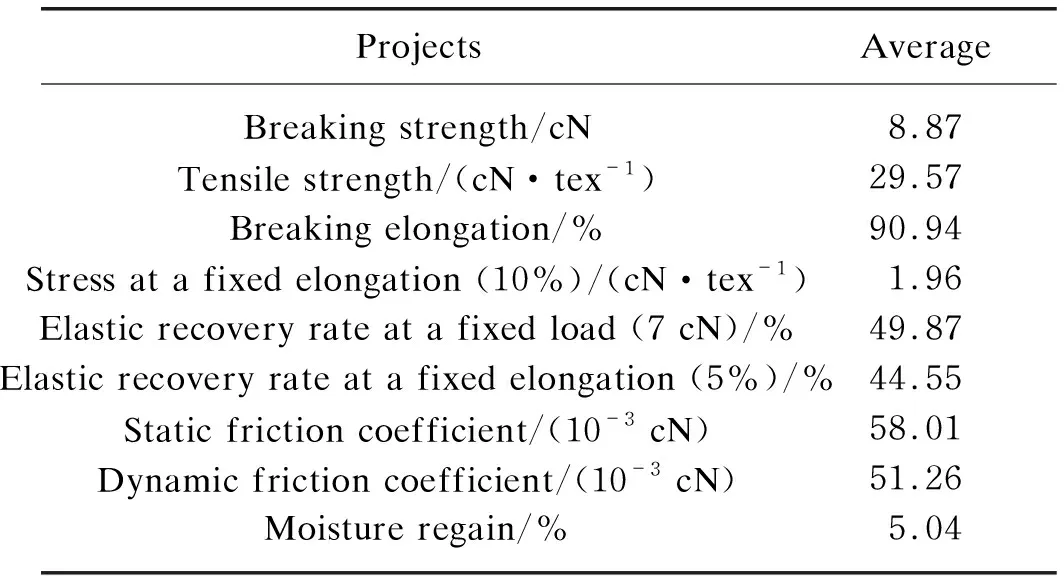
Abstract: Polybutylene succinate (PBS) fiber is a kind of synthetic fibers with excellent properties and biodegradability,which has been produced on a large scale in China. To investigate the application properties of PBS fibers,the structure and properties were systematically studied in this paper. The microstructures and thermal properties of PBS fibers were analyzed by Fourier transform infrared (FTIR) spectroscopy,X-ray diffraction (XRD),scanning electron microscopy (SEM),thermo gravimetric (TG) and differential scanning calorimetry (DSC). The mechanical properties,chemical stability and dyeing properties of PBS fibers were also studied. The results show that PBS fibers are α-crystalline with a crystallinity of 58.56%. PBS fibers have an excellent thermal stability and the initial temperature of thermal degradation is 370 ℃. The tensile strength,the elongation at break,the elastic recovery rate at a fixed elongation (5%) and the moisture regain rate of PBS fibers are 29.57 cN/tex,90.94%,44.55% and 5.04%,respectively. The chemical stability is as follows: alkali resistance Key words: polybutylene succinate (PBS) fiber; structure property; mechanical property; chemical stability; dyeing property Nowadays,plastics have not only been widely used in industrial and agricultural production and people’s daily life,but also caused a large number of wastes that are difficult to degrade[1-2]. Because the cost of recycling and reproduction of plastics is much higher than that of origin production,it is difficult to recycle,which will form a large number of permanent garbage[3-6]. The development of eco-friendly biodegradable plastics has become one of the strategies to solve the current “white pollution” problem[7-8]. Currently,the research of biodegradable plastics mainly includes polylactic acid (PLA)[9],polybutylene succinate (PBS)[10],polycaprolactone (PLC)[11],polyhydroxyalkanoate (PHA)[12],etc. It is well known that in addition to petroleum resources,PBS can also be obtained by biological resources[2,13],for example,by fermentation of sugar cane and grain,and then by polycondensation of the fermented material[14]. The thermal stability,biodegradability and processability of PBS are excellent[15-16],especially the excellent mechanical property comparable with isotactic polypropylene (PP) which indicate that PBS has broad prospects in the field of bioplastics[13]. The relatively simple production process of PBS can be produced by slightly modifying existing polyester equipment and saving the cost of equipment modification and upgradation[17]. The development of PBS has profound implications in the context of the depletion of non-renewable resources[18]. The weak intermolecular force and low melting point aliphatic polyester properties of PBS[19]limit its further application in film blowing,foaming and spinning[20-21]. The modification utilizing copolymerization and crosslinking reaction to tune its performance and lower its cost has always been a viable method[13]. Lietal.[20]obtained a PBS/solid epoxy (SE)/carboxyl-ended polyester (CP) blend by compression molding,which improved the weak intermolecular force and improved melt strength and melts viscosity. Lietal.[21]successfully foamed the PBS compound through chemical compression molding. Fanetal.[22]prepared a blend of PBS/poly(ethyleneglycol-co-cyclohexane-1,4-dimethanolterephthalate) by melt spinning to make the yield strength of PBS increase from 25.6 MPa to 39.8 MPa. Malinowskietal.[23]greatly improved PBS thermal stability and reduced PBS biodegradability by radiation-crosslinking trimethylallyl isocyanurate (TMAIC) concerning the low melting point of PBS. However,copolymerization and crosslinking of PBS fibers would cause the problem of incomplete degradaton,thus limiting the future application of PBS fibers[24]. Although PBS fibers have been industrially produced in China,the above problems still exist,which greatly limits its application in the fiber field. Pure PBS fibers have not been systematically studied. In this paper,the structure,thermal properties,physical properties,dyeing properties and chemical resistance of industrial PBS fibers were studied. The PBS fibers were characterized by Fourier transform infrared (FTIR) spectroscopy,X-ray diffraction (XRD),thermo gravimetric (TG),scanning electron microscopy (SEM) and differential scanning calorimetry (DSC). A single fiber strength tester was utilized to study the mechanical properties and chemical stability of PBS fibers. And the carrier dyeing of PBS fibers was carried out by disperse dyes. The research on the structure and performance of PBS fibers is expected to provide a theoretical basis for optimizing the industrial production of PBS fibers. PBS staple fibers with a linear density of 0.3 tex were obtained from Shaoxing Jiuzhou Chemical Fiber Co.,Ltd.,Shaoxing,China. Both hydrogen peroxide with a concentration of 30% and sulfuric acid with a concentration of 98% were purchased from Tianjin Hengxing Chemical Reagent Co.,Ltd.,Tianjin,China. Sodium silicate was purchased from Tianjin Dingshengxin Chemical Co.,Ltd.,Tianjin,China. Sodium hydroxide was purchased from Tianjin Jinte Chemical Co.,Ltd.,Tianjin,China. All the reagents mentioned above are of analytical purity. Holly oil was purchased from Hairui Natural Plant Co.,Ltd.,Ji’an,China. JYK-POW (commodity name) was obtained from Shanghai Jieyikang Chemical Technology Co.,Ltd.,Shanghai,China. Benzyl benzoate was obtained from West Asia Company,Linyi,China. The infrared absorption spectrum of the PBS fibers was measured with the Nicolet iS50 FTIR spectrometer in the ATR mode. The scanning range was 4 000-400 cm-1. The number of scans was 32,and the data point interval was 1.285 8 cm-1. The crystal structure of the PBS fibers was measured by the D8 Advance X-ray diffractometer of Brooke Company. The scanning range was 5°-50°,and the scanning speed was 2 (°)/min. The thermal propeties of the PBS fibers was tested by DSC 250 of TA Company. The PBS fiber sample was placed in a crucible,and the heat history was removed by heating from 0 ℃ to 180 ℃ for 3 min. Then the temperature was lowered to 0 ℃ and increased to 180 ℃ again. The heating rate was 10 ℃/min in the whole process. The thermal degradation process of PBS fibers was tested by using the synchronous thermal analyzer TG209F3 of the NETZSCH Company. The PBS fibers were put into a crucible and heated from room temperature to 600 ℃ at a rate of 10 ℃/min in nitrogen. Referring to the standards GB/T14337—2008 Testing method for tensile properties of man-made staple fibers,FZ/T 50007—2012 Test method for elasticity of polyurethane filament,and GB/T6503—2017 Man-made fiber-test method of moisture regain,the breaking strength and elongation,10% elongation at break,the load elasticity at 7 cN and the elastic recovery rate at 5% elongation of PBS fibers,as well as dynamic and static friction coefficients and the moisture regain rate were tested. PBS fibers were immersed in the different concentrations of sulfuric acid,sodium hydroxide and hydrogen peroxide solution. The solutions were then treated at 25 ℃,60 ℃ and 90 ℃ for a period of time. The breaking strength of PBS fiber after 30 min and 60 min treatment was tested. The morphological structure of PBS fibers before and after treatment was observed by the VEGS-3SBH SEM. The carriers of holly oil,benzyl benzoate and JYK-POW were selected to dye PBS fibers with disperse dyes. A series of orthogonal experiments with six factors and three levels were designed according to the influencing factors and levels of dyeing experiments in Table 1. The dyes,1 g/L diffuser NNO,carriers and 2 g/L hydrogen phosphate diamine were added into the dyeing bath with a bath ratio of 1∶10. The pH value was adjusted to 4-5 with acetic acid. Then PBS fibers were immersed in a dye bath. The dye bath was heated to 80-100 ℃ at a rate of 2 ℃/min. The dyeing temperature was kept for 40-60 min. The dyeing residue was taken to determine the dye uptake. Table 1 Influencing factors and levels of dyeing experiments The FTIR spectra of PBS fibers are shown in Fig. 1 (a). PBS fibers have absorption peaks at 2 945 cm-1,which is the absorption peak of —CH2— stretching vibration. The absorption peak at 1 308 cm-1is —CH2— bending vibration. The stretching vibration absorption peak at 1 718 cm-1is the carbonyl group in the ester group. And 1 151 cm-1is the strong absorption peak of C—O single group in the ester group[25]. These absorption peaks indicated the existence of a large number of esters. The XRD spectra of PBS fibers are shown in Fig.1(b). The PBS fibers have obvious diffraction peaks near the 2θdiffraction angles of 19.8°,22.6° and 29.1°. It is presumed that they are (020),(110) and (111) planes of monoclinic α-crystals[26-27]. As can be seen from the DSC curves of Fig. 1(c),the glass transition temperatureTgof PBS fibers is not obvious. During the heating process,the melting peakTmof PBS fibers appears near 111.2 ℃,and the melting enthalpy △Hmof PBS fibers is 64.59 J/g. In the cooling process,the fibers crystallized at about 89 ℃ and reached the maximum Tcnear 74.9 ℃. The theoretical melting enthalpyHm0of PBS is 102 J/g[28]. Thus,the crystallinityαiof PBS fibers can be expressed by the ratio of the melting heat of this material to the theoretical melting heat of the complete (100%) polymer. The formula is (1) From the TG and derivative thermo gravimetric (DTG) curves of PBS fibers shown in Fig. 1(d),it can be seen that the initial temperature of thermal degradation of PBS polyester is 370 ℃ and the termination temperature is 428 ℃. Among them,the maximum thermal degradation rate is reached at 395 ℃. This is mainly due to the cracking of ester bonds in PBS fibers[29]. (a) (c) According to the physical and mechanical properties of PBS fibers in Table 2,the average breaking strength of PBS fibers was about 8.87 cN. And the linear density is 0.3 tex. Compared with the other four fibers in Table 3[30],the breaking strength of PBS fibers was higher than that of cotton fibers,viscose fibers and wool,but far lower than that of nylon 6. The breaking elongation of PBS fibers was 90.94%,which was higher than that of four kinds of fibers. The initial modulus of PBS fibers was close to that of wool and nylon 6,which was smaller than that of cotton and viscose fibers. We can see that PBS fibers have strong toughness which are not easy to crack. Table 2 Physical and mechanical properties of PBS fibers Chemical fibers are usually blended with cotton fibers. If the stress at a fixed elongation (10%) of chemical fibers is lower than that of cotton,the tensile strength of the yarn is mainly contributed by cotton fibers with external forces. The stress at a fixed elongation (10%) of PBS fibers was 1.96 cN. When blending with cotton fibers,increasing the content of PBS fibers will increase the strength of the blended yarn[30]. Thus,PBS fibers are suitable for blending with cotton fibers. According to Table 2,the elastic recovery rate at a fixed load (7 cN) of PBS fibers and the elastic recovery rate at a fixed elongation (5%) are 49.87% and 44.55%,respectively. The values were similar to those of cotton fibers but higher than those of viscose fibers. Therefore,the PBS fiber was more flexible and durable than viscose fibers. The difference between the static friction coefficient and the dynamic friction coefficient of PBS fibers was larger than that of viscose fibers,so the hand feel of PBS fibers is slightly harder than viscose fibers. At room temperature,the moisture absorption properties of PBS fibers were weak,and the moisture regain rate of PBS fibers was only 5.04%. Table 3 Physical and mechanical properties of four kinds of fibers 2.3.1AcidresistanceofPBSfibers Figure 2 shows the strength change curve of PBS fibers treated with sulfuric acid for 30 min and 60 min at a temperature of 25 ℃ (noted as N),60 ℃ (noted as M) and 90 ℃ (noted as H). After 30 min treatment with 10%,20% and 30% sulfuric acid solution at 90℃,the breaking strength of the PBS fibers decreased by 29.43%,56.21% and 75.86% respectively,while the breaking strength decreased by 56.92%,75.89% and 100% after 60 min. This showed that the concentration of sulfuric acid solution and the treatment time had a greater impact on the breaking strength of PBS fibers. These results were mainly due to the acidic hydrolysis reaction of esters. The addition-elimination reaction of acyl-oxygen bonds in polyesters resulted in the formation of carboxylic acids and alcohols. However,carboxylic acids and esters can recombine to form esters under acidic conditions. Therefore,PBS fibers have certain acid resistance. Fig. 2 Breaking strength of PBS fibers treated with sulfuric acid 2.3.2AlkaliresistanceofPBSfibers Fig. 3 Breaking strength of PBS fibers treated with sodium hydroxide solution Figure 3 shows the breaking strength of PBS fibers treated with sodium hydroxide solution. It can be seen that the breaking strength of PBS fibers treated with 5 g/L sodium hydroxide decreased by 58% in 60 min at 90 ℃. However,when the concentration increased to 20 g/L,the PBS fibers were hydrolyzed into short flocs and the breaking strength could not be measured. Therefore,the concentration of sodium hydroxide had a significant effect on PBS fibers. Besides,the effect of temperature on the strength of fibers was also significant. At the same concentration and temperature,the difference in damage degree of fibers was between 10% and 20%,which indicated that the treatment time had little effect on the strength of fibers. In alkali catalysis,the nucleophilic reagent OH-was added with carbonyl carbon to form tetrahedral intermediates firstly,and then R2O-was eliminated to form a carboxylic acid[31]. The alkaline hydrolysis of esters could be carried out to the end because alkali neutralized the acid produced by the reaction and promoted the reaction to decompose. 2.3.3OxidationresistanceofPBSfibers Fig. 4 Breaking strength of PBS fibers treated withhydrogen peroxide solution 2.3.4SEMimagesofPBSfibersbeforeandafterchemicaltreatment Figures 5(a) and (b) show the images of PBS fibers magnified 1 000 times by SEM. The PBS fibers were cylindrical with a smooth surface and no skin-core structure. The radius of the PBS fibers was about 8.73 μm. From the SEM images of the PBS fibers treated in Figs. 5(c)-(d),it could be seen that the surface of the PBS fibers was severely damaged and a large number of cracks appeared after treatment with sulfuric acid and sodium hydroxide solution. However,after hydrogen peroxide treatment shown in Fig. 5 (e),there was no obvious damage,cracks and fracture on the surface of PBS fibers. Table 4 shows the dye uptake rate of 27 groups of disperse dyes in orthogonal experiments. Experiments proved that PBS fibers could be dyed with disperse dyes and the dyeing rates obtained by different carriers were quite different. Among the three carriers,the effect of holly oil was the most remarkable. Using holly oil as the carrier can make PBS obtain an excellent dyeing rate,up to 92%. The effect of JYK-POW was the second,and the dyeing rate can reach 60%-80%. However,the effect of benzyl benzoate as a carrier was not ideal due to the influence of temperature and the amount of carrier added. In addition,the dyeing rate of the three carriers increased with the increase of the temperature, time and amount[33]. Therefore,PBS fibers could be dyed well by disperse dyes and carriers,and the effect of carrier holly oil was the best. However,holly oil has stimulating odor,which has a negative impact on the environment. Although JYK-POW commercial carrier can not achieve the same dyeing rates as holly oil,it has environmental protection advantages. Therefore,JYK-POW is more likely to be used as a carrier to dye PBS fibers in the actual production process. (a) (d) Table 4 Dye uptake rate of disperse dyes in orthogonal experiments Through the study of the structure and performance of PBS fibers,the following conclusions are obtained. (1) It can be seen from the thermal analysis of PBS fibers that the glass transition temperature of PBS fibers is not obvious. The crystallization temperatureTc= 74.9 ℃,and its crystalline melting enthalpyΔHmis 64.59 J/g; the melting temperatureTm=111.2 ℃,and the crystallinity is 58.56%. PBS fibers have excellent thermal stability,the initial temperature of thermal degradation starts at 370 ℃,and the maximum thermal degration rate is reached at 395 ℃. (2) The breaking strength of PBS fibers is 8.87 cN,the breaking elongation is 90.94%,and the strength is 1.96 cN at 10% elongation,which is suitable for blending with cotton fibers. The physical properties of PBS fibers are better than that of cotton fibers,viscose fibers and wool. (3) The ester bonds in PBS fibers are hydrolyzed in acidic and alkaline solutions,and PBS fibers have certain acid resistance. The breaking strength of PBS fibers decreases with the increase of concentration,temperature and treatment time,but the oxidation resistance of PBS fibers is excellent. (4) PBS fibers could be dyed with disperse dyes carrier,and the carrier holly oil can achieve a dyeing rate of 92% with the best effect. However,considering environmental protection,JYK-POW commercial carrier is more suitable for the carrier dyeing of PBS fibers.Introduction
1 Experiments
1.1 Materials
1.2 Structural characterization of PBS fibers
1.3 Physical property test of PBS fibers
1.4 Chemical stability test of PBS fibers
1.5 Dyeing property test of PBS fibers

2 Results and Discussion
2.1 Structure and thermal properties of PBS fibers

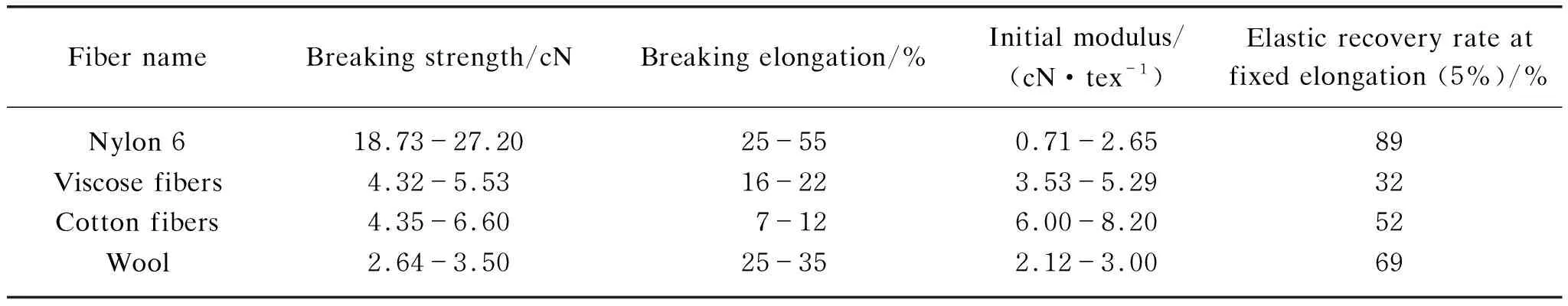
2.2 Physical properties of PBS fibers
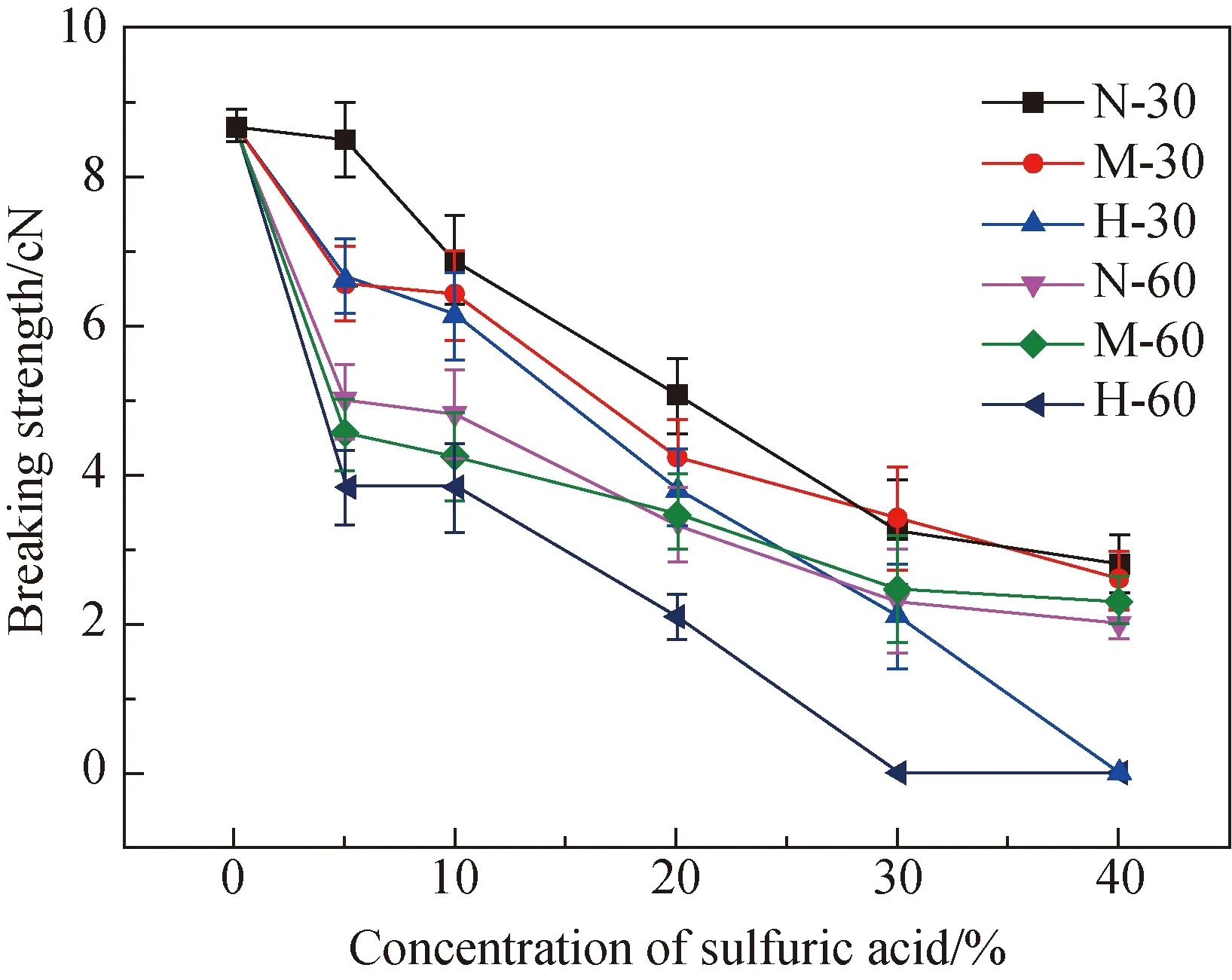
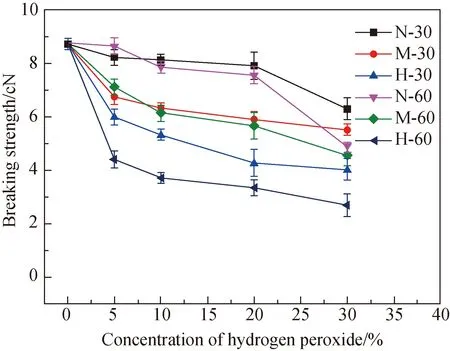
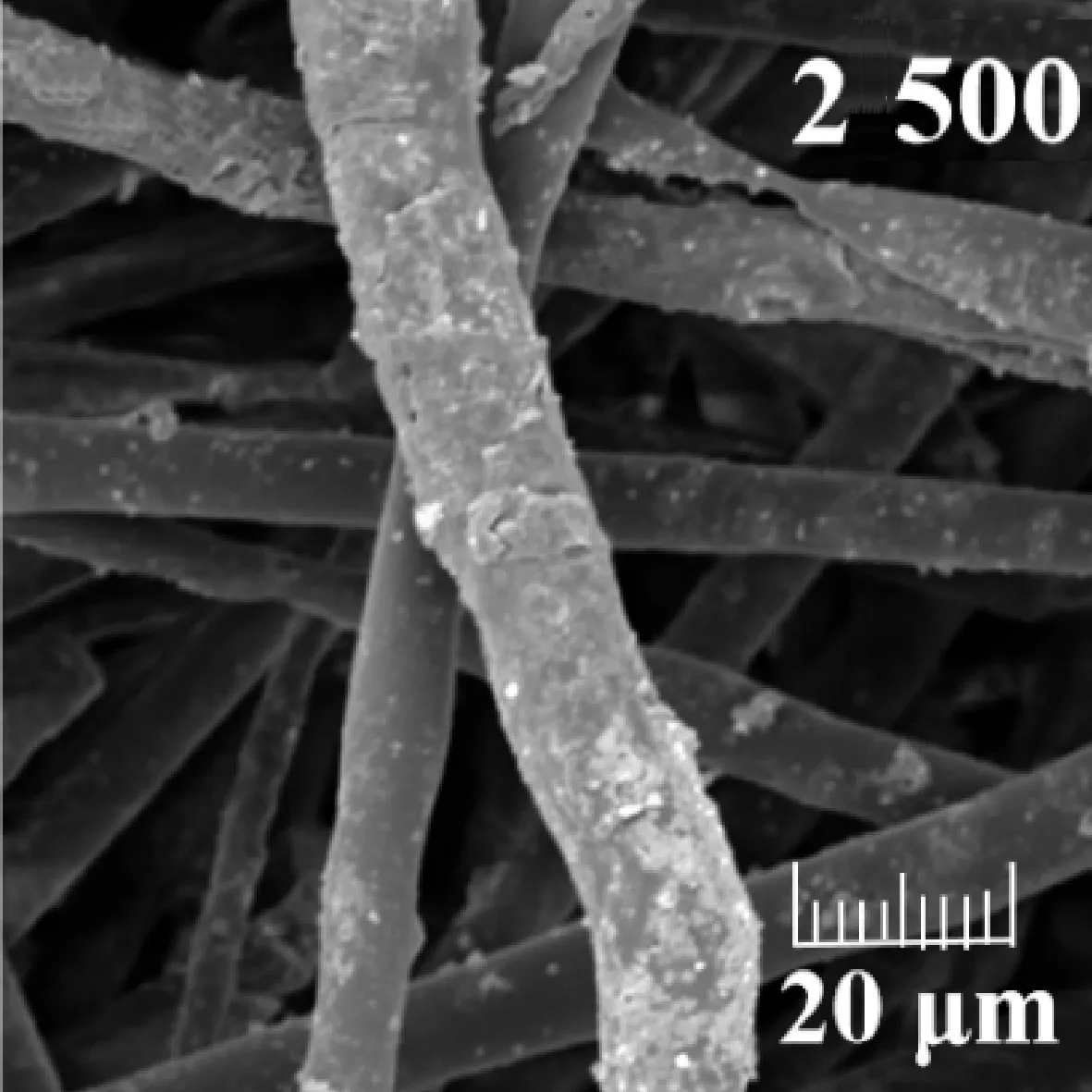
2.3 Chemical stability of PBS fibers

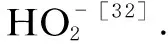
2.4 Dyeing properties of PBS fibers

3 Conclusions
 Journal of Donghua University(English Edition)2020年3期
Journal of Donghua University(English Edition)2020年3期
- Journal of Donghua University(English Edition)的其它文章
- Recent Progress for Gallium-Based Liquid Metal in Smart Wearable Textiles
- Accident Analysis and Emergency Response Effect Research of the Deep Foundation Pit in Taiyuan Metro
- Effect of Elastane on Physical Properties of 1×1 Knit Rib Fabrics
- Knowledge Graph Extension Based on Crowdsourcing in Textile and Clothing Field
- Analysis on the Application of Image Processing Technology in Clothing Pattern Recognition
- Clothes Keypoints Detection with Cascaded Pyramid Network
