略阳乌鸡体重和产蛋数性状遗传参数估计
党李苹,周雯馨,刘瑞芳,白云,王哲鹏
略阳乌鸡体重和产蛋数性状遗传参数估计
党李苹,周雯馨,刘瑞芳,白云,王哲鹏
(西北农林科技大学动物科技学院,陕西杨凌 712100)
【】略阳乌鸡是陕西省特有的家禽品种,该品种具有体型大、肉质好、氨基酸含量丰富、对林地散养适应性强的优点,但也存在生长缓慢,产蛋性能差的不足。为此,在系统测定略阳乌鸡生产性能的基础上,开展对体重和产蛋性状遗传参数的估计,阐明遗传效应对上述性状的调控作用及性状间的遗传关系,期望为这些性状的选育奠定理论基础。对略阳乌鸡蛋用系一世代71个半同胞家系799只公鸡和804只母鸡6、10、14、20周龄和开产日龄体重进行测定,以个体为单位记录从开产到31、35和40周龄产蛋数,以四分位数±1.5倍四分位距为界删除异常值,更正性别记录错误。以性别为固定效应,育种值为随机效应用单变量动物模型估计各性状遗传力,用双变量动物模型估计性状间遗传相关,用逆伽玛分布指定育种值和残差项方差先验分布,用贝叶斯算法执行130 000次迭代,弃去前30 000次迭代结果,以100为间隔抽取1 000个估计值获得方差和协方差后验分布,计算各性状遗传力和性状间遗传相关。用R语言PerformanceAnalytics软件包chart.Correlation命令计算性状间表型相关。略阳乌鸡6、10、14、20周龄公鸡体重为(0.56±0.07)、(1.07±0.13)、(1.56±0.19)、(1.97±0.21)kg,母鸡体重为(0.47±0.06)、(0.86±0.12)、(1.18±0.16)、(1.48±0.19)kg,开产体重为(1.68±0.23)kg;31、35和40周产蛋数为(31.2±11.5)、(42.5±16.7)和(54.7±20.4)枚。体重遗传力随年龄逐渐减小,分别为0.74、0.76、0.63、0.54和0.52,置信区间在0.25—0.33之间。产蛋数遗传力为0.27、0.25和0.26,置信区间在0.35—0.42之间。10、14、20周龄体重间维持中等偏上(0.52—0.68)的遗传相关性,但6周龄体重(0.21—0.52)和开产体重(0.21—0.46)与各时间点体重遗传相关性较弱。体重间遗传相关系数置信区间在0.13—0.34之间。在3个时间点产蛋数间遗传相关系数趋近于1,置信区间在0.03—0.06之间。体重和产蛋数间遗传相关系数均不显著。在时间维度上,各体重性状间表型相关系数在0.43—0.90之间(<0.001),产蛋数性状间表型相关系数在0.79—0.94之间(<0.001)。6—20周龄体重与产蛋数间维持了弱(0.023—0.15)正相关关系,但开产体重与产蛋数存在弱负相关关系(-0.17—-0.14)。首次估计了略阳乌鸡品种特异性的遗传参数,发现略阳乌鸡体重性状主要受遗传效应调控,而产蛋数性状受环境影响更大,阐明了早期与晚期性状间、体重与产蛋数间的遗传关系,为早期选种和多性状育种奠定了理论基础。这些结果建议在略阳乌鸡群体中对体重性状表型选择有望取得良好效果,但对产蛋数性状应建立纯系利用杂种优势;体重和产蛋数间不存在负遗传相关关系,可同时对两个性状选育提高。
略阳乌鸡; 体重; 产蛋数; 遗传力; 遗传相关
0 引言
【研究意义】略阳乌鸡是陕西省特有的家禽品种。该品种具有体型大、肉质好、氨基酸含量丰富、对林地散养适应性强等优点,但也存在生长速度缓慢、产蛋性能差的不足[1-3]。略阳乌鸡生产性能差增大了养殖成本,降低了乌鸡产品的市场竞争力,对乌鸡资源的开发利用产生了极为不利的影响。选育是提高略阳乌鸡生产性能的措施之一,而遗传参数估计是制定适宜选育方案的基础[4]。【前人研究进展】生长和产蛋作为现代家禽生产中两个重要的经济性状,已有大量研究对各时间点体重和产蛋数遗传力及彼此间遗传关系进行估计。但不同研究得出结果存在较大差异,以开产体重和开产后12周产蛋数为例,前者遗传力范围在0.32—0.57之间[4-7],而后者遗传力在0.099—0.47之间[4,8-9]。体重和产蛋数性状在时间维度上的遗传关系尽管在数值上仍有较大差异,但在方向上却保持了较为稳定的正相关关系[4-6,10-13]。但在体重和产蛋数性状间,不同研究得出的结论并不一致[6-12],主流观点认为二者维持了负相关的关系[4,6,11-12],但也有少数研究发现二者相关性的方向并不固定[7,9],甚至有相反的结论得出[10]。【本研究切入点】鸡的体重和产蛋数受多基因调控[14-15],而且环境因素还可以通过影响表观修饰水平影响基因的表型效应[16-17]。因此,不同品种的体重和产蛋性状遗传基础可能各不相同,即使相同,在不同环境下也可能有不同的表型[18]。这种遗传基础的复杂性就决定了不同研究很难取得相同的结果,也使得遗传参数跨品种应用受到很大限制。【拟解决的关键问题】本研究以略阳乌鸡蛋用系育种核心群1 603只一世代个体为对象,对6、10、14、20周龄和开产日龄5个时间点的体重及31、35和40周龄3个时间点的产蛋数进行测定,估计略阳乌鸡特异性遗传参数,分析这些性状的遗传基础和性状间的遗传关系,为略阳乌鸡体重和产蛋性状的选育奠定理论基础。
1 材料与方法
1.1 略阳乌鸡养殖方式和表型数据采集和筛选
略阳乌鸡遗传参数估计所用样本为略阳乌鸡蛋用系育种核心群一世代1 603只略阳乌鸡,公鸡799只,母鸡804只。这些个体来自71个半同胞家系。一世代在2017年3月25日入孵,4月15日出雏,系谱孵化,出雏时佩戴翅号,记录家系信息,在2018年2月14日淘汰。养殖地点为陕西略阳龙佳农业科技发展有限公司。1—3周饲养密度为60只/m2,4—6周密度为40只/m2。第一周育雏温度为33—35℃,之后每周降低2—3℃,四周脱温。第一周光照时间为18 h,之后每周减少1.5 h,7—14周维持9 h恒定光照,15周开始每周增加0.5—1 h,至25周光照时间达16 h,之后维持16 h恒定光照。笼养育雏,60日龄后转入产蛋鸡舍,单笼饲养。华秦全价料饲喂,自由采食、饮水。
在6、10、14、20周龄和开产测定乌鸡体重,在开产后记录每只鸡每天产蛋情况,直到40周龄。各时间点体重以下四分位数(first quantile)-1.5×四分位距(interquantile range)为下限,以上四分位数(third quantile)+1.5×四分位距(interquantile range)为上限,删除异常值。各时间点产蛋数除了按上述标准删除异常值外,还删除了各时间点产蛋数低于10枚的样本。这些样本产蛋数低可能因为疾病而非自身遗传因素所致。
1.2 各性状遗传力和性状间遗传相关估计的方法
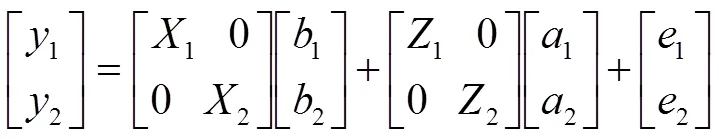
各性状间表型相关系数用R语言PerformanceAnalytics软件包chart.Correlation命令计算。
2 结果
2.1 各时间点体重与产蛋数的描述统计
本研究共对1 603只略阳乌鸡的体重和产蛋数进行测定,这些个体共来自71个半同胞家系、333个全同胞家系,各性状实际测定样本量及样本所来自家系数量见表1。性别对体重的影响随年龄而增大,在20周龄时略阳乌鸡公鸡体重比母鸡大33%(表1),表明公鸡有更强的生长势。公鸡屠体指标优于母鸡,且蒸煮损失、系水力、风味、多汁性等肉品质性别差异显著[22-23]。因此,这些结果建议公鸡的肉用潜力更为突出。略阳乌鸡属一种偏肉用型的地方鸡。当前我国多数黄羽肉鸡品种达2 kg以上上市体重在49—120 d左右。略阳乌鸡14周龄公鸡体重为(1.56±0.19)kg,母鸡体重(1.18±0.16)kg,明显低于当前多数黄羽肉鸡同期体重。略阳乌鸡达到2 kg上市体重,公鸡至少需要20周,母鸡至少需要25周以上。略阳乌鸡除了生长速度慢、产蛋性能差以外,也表现出较大的群体变异性,各时间点体重的变异系数在10.7%—14.0%之间,而产蛋数性状表现出更大的群体变异性,其变异系数在36.8%以上(表1)。
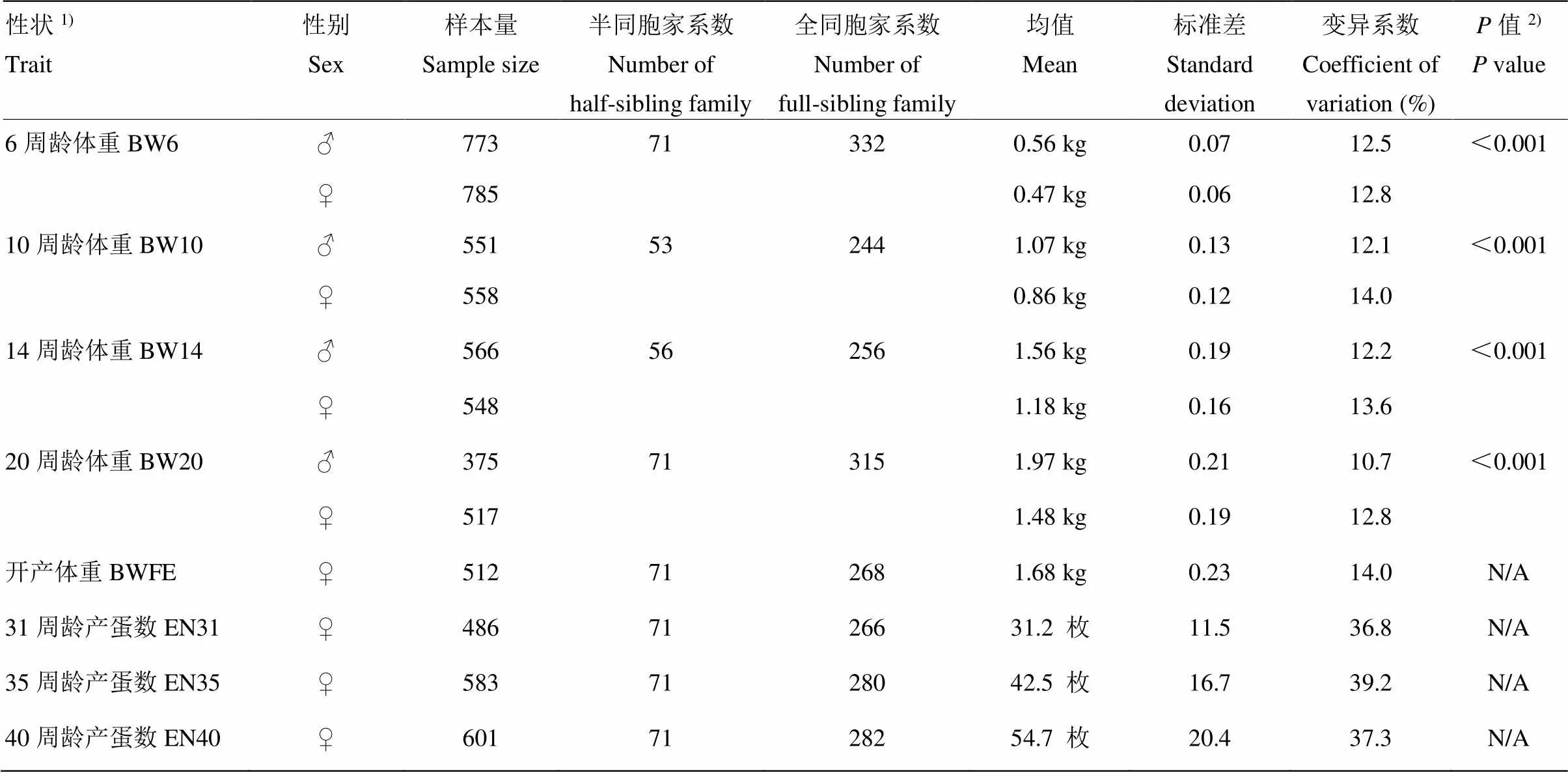
表1 5个时间点体重与3个时间点产蛋数描述统计
1)BW6-BWFE=body weight at 6, 10, 14, 20 weeks of age and first egg, EN31-EN40= number of eggs from age at first egg to 31, 35 and 40 weeks of age.2)公鸡和母鸡6-20周龄体重差异显著性检验值。N/A表示对本性状不适用Numbers in the column arevalues of significance test of body weight at 6, 10, 14 and 20 week between cocks and hens. N/A represents non applicable for the traits
2.2 5个时间点体重和3个时间点产蛋数遗传力估计结果
略阳乌鸡6、10、14、20周龄和开产体重遗传力在0.52—0.76之间,属中等偏上遗传力性状(图1)。31、35和40周龄产蛋数遗传力在0.25—0.27之间,属低遗传力性状(图1)。体重遗传力随周龄逐渐降低,但产蛋数遗传力随周龄变化不明显(图1)。在遗传力估计精度方面,6、10、14和20周龄体重遗传力估计置信区间(置信上限-置信下限)在0.25—0.33之间,要低于3个产蛋数遗传力估计区间(0.35—0.42),表明本研究对体重遗传力估计的精度要高于产蛋数性状(图1)。但是,开产体重遗传力估计精度(0.81-0.33=0.48)不及其余4个体重性状(图1)。在6、10、14和20周龄体重遗传力估计时所有的公鸡、母鸡样本均被用到,遗传力是以性别为固定效应被估计得来,而开产体重和产蛋数遗传力估计仅有母鸡样本可用。因此,样本量减少是遗传力估计精度下降的一个主要原因。
2.3 5个时间点体重与3个时间点产蛋数遗传与表型相关
各时间点体重均表现出正遗传相关性,遗传相关系数在0.21—0.68之间,置信区间在0.13—0.34之间,相关强度随体重测定间隔的延长而减弱(表2)。10、14和20周龄体重间维持了中等偏上的相关性,但与6周龄体重的相关性相对较弱(表2)。开产体重与各时间点体重均呈现出较弱的遗传相关性(表2)。31、35和40周龄产蛋数遗传相关系数趋近于1,且相关强度不受时间间隔的影响,表明产蛋性状调控基因的作用较为恒定(表2)。产蛋数和体重间遗传相关系数95%置信区间均覆盖0,表明二者遗传相关关系未得到统计学证据的支持(表2)。
在时间维度上,体重和产蛋数表型相关的方向、随测定间隔的变化趋势与遗传相关保持一致,但相关强度有所不同(表2)。体重表型相关强度均高于遗传相关(表2),但产蛋数表型相关要略低于遗传相关。体重与产蛋数间均显示出弱正相关关系,但开产体重与产蛋数显示出弱负相关关系(表2)。
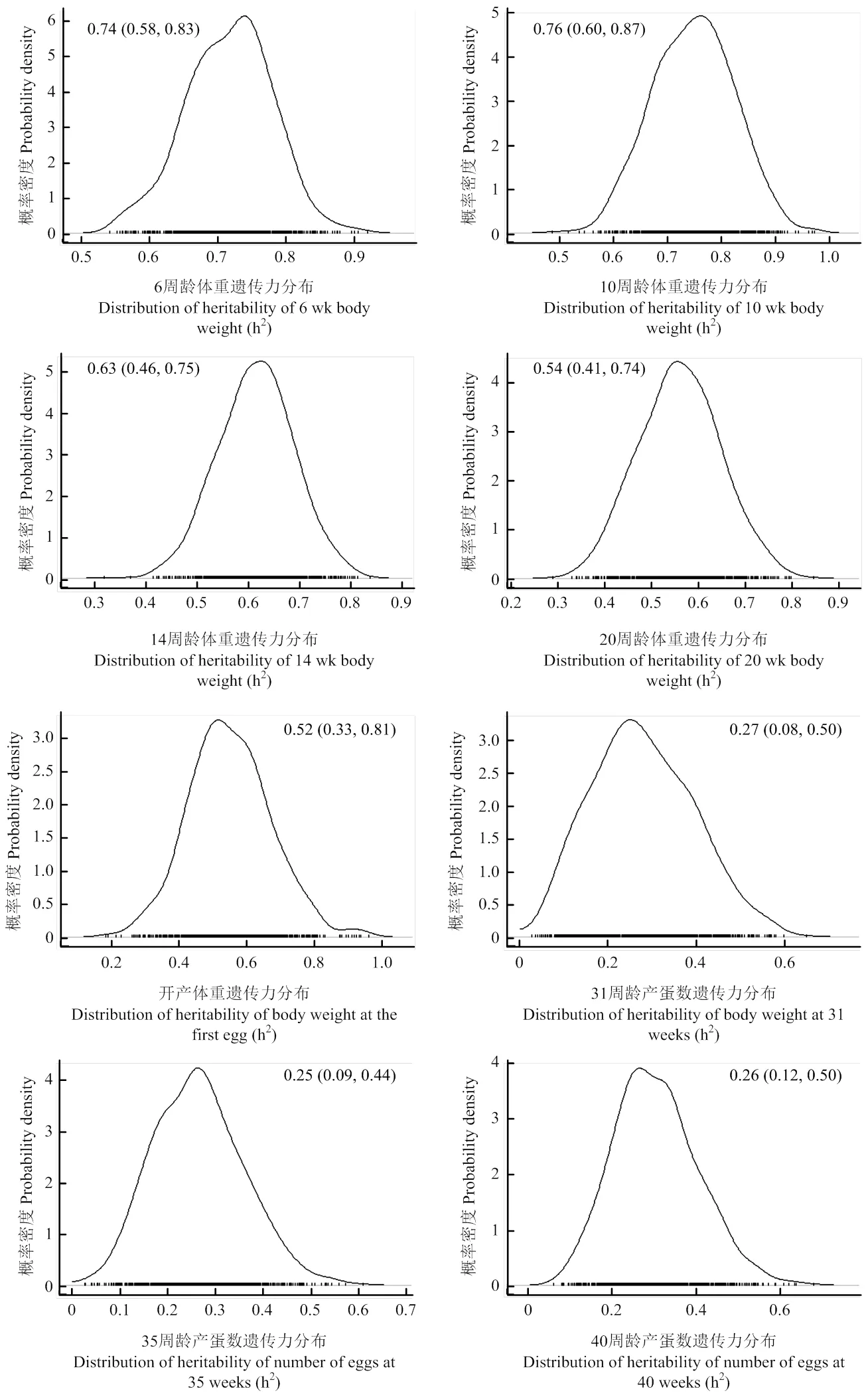
图中曲线描绘出1 000个后验遗传力概率分布,图上数字指各性状后验遗传力众数,括号中的数字给出各性状后验遗传力95%置信区间
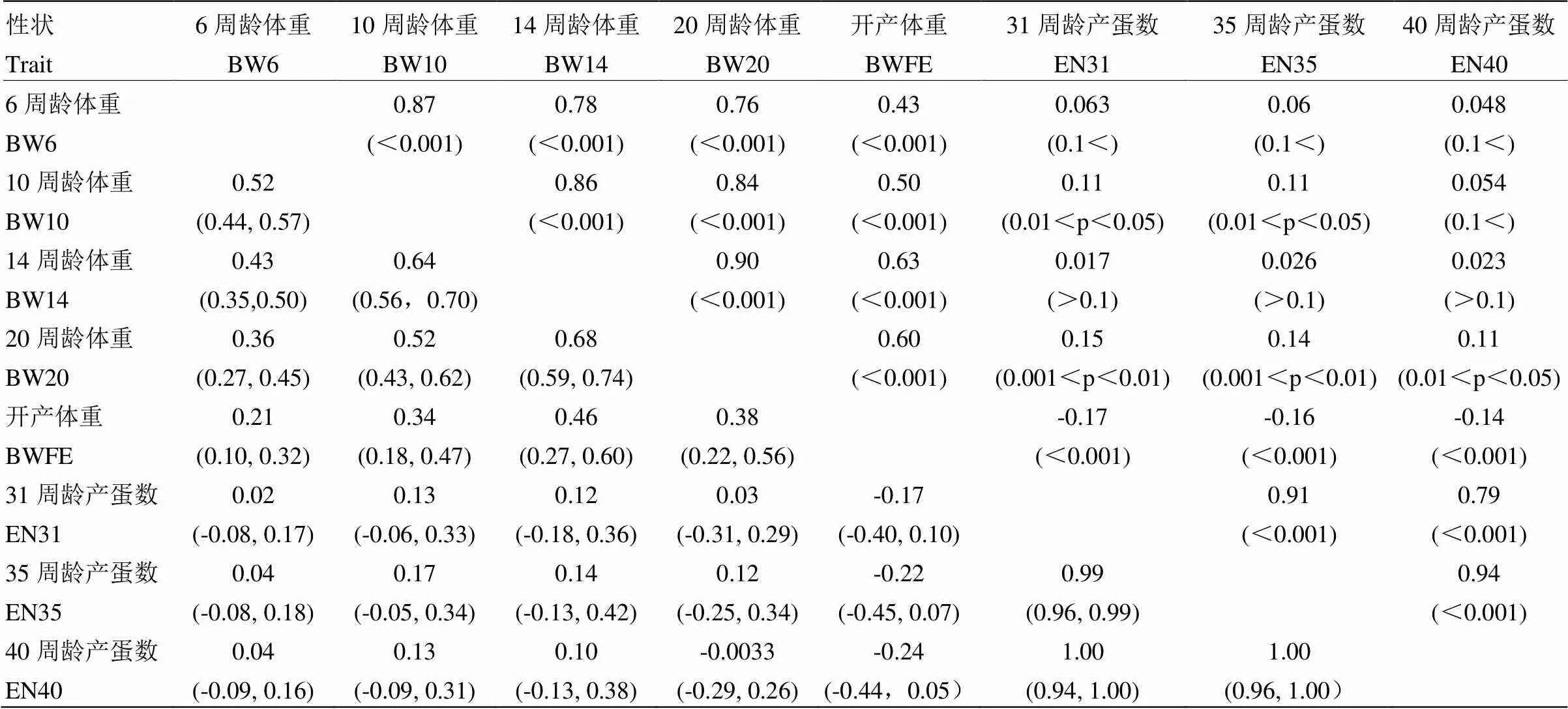
表2 5个时间点体重和3个时间点产蛋数间的遗传和表型相关
左下角数字为不同性状之间的遗传相关系数,括号中的数字为95%置信区间;右上角的数字为性状之间表型相关系数,括号中的数字为相关系数显著性检验值
Numbers at below diagonal represent genetic correlation, and numbers in parentheses show 95% confidence intervals of genetic correlation. The ones at above diagonal are phenotypic correlation coefficients between pair-wise traits, and numbers in the parentheses arevalues of the significance test to correlation coefficients. BW6-BWFE=body weight at 6, 10, 14, 20 weeks old and first egg, EN31-EN40= number of eggs from first to 31, 35 and 40 weeks old
3 讨论
3.1 略阳乌鸡体重遗传力高于前人估计结果
体重作为一个重要的经济性状,众多学者在不同品种中曾对各个时期的体重遗传力进行估计。其中,6周龄体重遗传力估计结果在0.15—0.24之间[10,24],8、12和16周龄体重遗传力在0.16—0.43之间[4,5,9-12],20周龄遗传力在0.3—0.68之间[11-12,24],开产体重遗传力在0.37—0.57之间[4,6,7,11]。本研究估计的6、10和14周龄体重遗传力在0.63—0.76之间,高于前人估计结果。20周龄遗传力和开产体重遗传力虽然在前人报道的范围内,但仍高于多数结果[4,6-7,11-12,24]。略阳乌鸡体重遗传力高表明体重QTL的效应较大且处于分离状态,这为体重QTL定位提供了一个天然的性状分离群体。
3.2 略阳乌鸡体重遗传力随年龄逐渐降低
家禽体重遗传力随年龄有一定变化趋势,但不同研究得出的结果并不相同。ADEYINKA等[5]在尼日利亚裸颈鸡、SAATEI等[25]在日本鹌鹑中均发现体重遗传力随年龄有逐渐降低的趋势,但SINGH等[24]在印度地方鸡和ASLAM等[26]在火鸡中却发现体重遗传力随年龄有升高的趋势。SANG等[6]发现不同的品种趋势不同,而LWELAMIRA等[12]观察到体重遗传力随年龄基本保持恒定。本研究发现略阳乌鸡体重遗传力随年龄逐渐降低,说明体重的QTL效应随年龄在逐渐减弱,而环境效应对体重的影响在逐渐增大。因此,对略阳乌鸡14周龄以前体重性状选育而言,个体表型值选择有望取得理想选育效果,但对开产体重和20周龄以上体重而言,要充分考虑对环境效应的控制,家系、育种值选择等有望取得更好的选育效果。
3.3 略阳乌鸡产蛋数性状遗传力较低
产蛋数是本研究关注的另一个经济性状。本研究发现略阳乌鸡31、35和40周龄产蛋数遗传力在0.25—0.27之间,与前人报道基本一致。研究者曾对来自世界各地18个品种不同时期产蛋数遗传力进行估计,所得遗传力的最大值为Kamali等[8]报道的伊朗地方鸡开产后12周产蛋数遗传力(0.4),最小值为郭军等[27]报道的如皋黄鸡开产后2个月产蛋数遗传力(0.05),平均值为0.24±0.10[6-9,11,27-29]。这些结果说明鸡产蛋数QTL效应较小,但在进化上较为保守,在时间上较为恒定。对略阳乌鸡产蛋性能选育而言,直接对性状选育难度较大,而通过配套系杂交利用杂种优势有望取得更好的选育效果。
3.4 早期体重和晚期体重存在正遗传相关关系
略阳乌鸡5个时间点体重遗传相关在0.21—0.68之间,低于埃塞俄比亚(0.67—0.99)、泰国(0.55—0.99)、伊朗(0.57—0.99)和坦桑尼亚(0.60—0.93)地方鸡估计结果,但相关强度随时间间隔延迟逐渐减弱的变化规律与前人报道一致[4,10-12]。本研究推测这种变化规律与略阳乌鸡体重QTL效应随年龄逐渐减弱有关,说明在略阳乌鸡群体中依据早期体重对后期体重进行间接选择具有可行性,但选择的准确性会随着时间跨度的延长而降低。与其他时间点体重间遗传相关相比,涉及开产体重的遗传相关往往更低[4,11]。本研究也观察到这种结果。开产时间是影响开产体重的一个重要因素。因此,在开产时间得不到有效校正的前提下对开产体重实施早期选种很难取得理想选育效果。
3.5 不同阶段产蛋数存在正遗传相关关系
本研究发现略阳乌鸡不同时间点产蛋数间维持较强正相关性。这一结果与埃塞俄比亚地方鸡研究结果(0.8—0.98)一致,但高于灵昆鸡(0.571)和洛克鸡(-0.21—0.7)[10,29-30]。这种结果说明调控略阳乌鸡产蛋性能的QTL尽管效应微弱,但调控作用较为恒定,不易受产蛋时间的影响。当前,在略阳乌鸡产蛋性能选育中存在的一个技术难题是确定适宜的留种时间。留种太早,产蛋性能测定时间短,选择可能不准确;留种时间太晚,略阳乌鸡产蛋性能下降严重,繁育难度加大。鉴于产蛋数性状间表现出的强正相关性,本研究建议依据31周龄产蛋数对整个产蛋期产蛋性能进行早期选择具有较高的准确性。
3.6 体重和产蛋数遗传关系不显著
由体重和产蛋数间负遗传相关产生的肉种鸡矛盾(broiler-breeder paradox, BBP)不仅是困扰白羽肉种鸡育种的一个技术难题[31],而且在众多地方鸡中也广泛被报道[4,6,11-12]。这种负相关关系的分子基础与快速生长对卵泡招募(follicle recruitment)产生的负效应有关,而限制饲喂能明显缓解这种负效应[32]。但是,本研究在略阳乌鸡群体中并未观察到这种遗传上的负相关性。DANA等[10]在埃塞俄比亚地方鸡、KAMALI等在伊朗地方鸡[8]、TONGSIRI等[7]在洛岛红和白洛克和GHAZIKHANI SHAD等[9]在伊朗地方鸡中均报道了类似的结果。这些研究说明鸡生长性状对繁殖性能产生的负遗传效应只有在生长强度超过一定阈值时才会出现;在低于这个阈值时,二者遗传关系并不明显,甚至呈现出正相关关系。这可理解为繁殖系统的正常发育是以体况的正常发育为基础。因此,在当前略阳乌鸡的生长强度下,对体重的选育不会对产蛋性能产生负效应。相反,生长速度加快可能为生殖系统发育乃至后期的产蛋创造更好的体况条件。这种观点得到体重与产蛋数间表型相关结果的支持。
3.7 表型相关与遗传相关保持一致
本研究发现各性状间表型相关和遗传相关在相关性的方向和随测定间隔的变化趋势上显示出高度的一致性。这说明环境效应和遗传效应在以同样的方向、以同样的调控通路影响略阳乌鸡在各个时期的生长和产蛋[33],也说明依据早期性状测定结果对后期生长和产蛋性能间接选择具有可行性。而表型相关和遗传相关在相关强度上的差异则很可能由遗传相关估计误差引起[34]。
4 结论
略阳乌鸡6—20周龄体重维持了较高的遗传力(0.52—0.76),表明遗传效应在略阳乌鸡生长调控方面起关键作用,但调控效应随年龄逐渐减弱。因此,对略阳乌鸡体重性状选育,在14周龄以前个体表型选择有望取得良好选育效果,但在14周龄以后应充分考虑对环境效应的控制。略阳乌鸡产蛋数遗传力较低(0.25—0.27),直接选育难度较大,而配套系杂交是更有效的育种手段。在时间维度上,体重和产蛋数均表现出较强的正相关性,说明早期选种具有可行性。体重和产蛋数间未出现负遗传关系,可同时对两种性状选育提高。
[1] 刘福柱, 刘景星, 魏忠义. 略阳鸡及其杂种的肉用性能和胴体品质研究. 西北农林科技大学学报(自然科学版), 1990(1): 66-70.
Liu F Z, Liu J X, Wei Z Y. Study on meat performance and carcass quality of Lueyang chicken and its hybrids., 1990(1): 66-70. (in Chinese)
[2] 刘福柱, 魏忠义, 刘景星. 略阳鸡与泰和鸡的肉质比较. 中国畜牧杂志, 1992, 28(3): 28-30.
Liu F Z, Wei Z Y, LIU J X. Comparison of meat quality between Lueyang chicken and Taihe chicken., 1992, 28(3): 28-30. (in Chinese)
[3] Wang Z P, Meng G H, Li N, Yu M F, LIANG X W, MIN Y N, LIU F Z, GAO Y P. The association of very low-density lipoprotein receptor (VLDLR) haplotypes with egg production indicates VLDLR is a candidate gene for modulating egg production., 2016, 39(3): 380-391. DOI: 10.1590/1678-4685- GMB-2015-0206.
[4] NIKNAFS S, NEJATI-JAVAREMI A, MEHRABANI-YEGANEH H, FATEMI S A. Estimation of genetic parameters for body weight and egg production traits in Mazandaran native chicken., 2012, 44(7): 1437-1443. DOI: 10.1007/ s11250-012-0084-6.
[5] ADEYINKA I A, ONI O O, NWAGU B I, ADEYINKA F D. Genetic parameter estimates of body weights of naked neck broiler chickens., 2006, 5(6):589-592. DOI: 10.3923/ijps.2006.589.592.
[6] SANG B D, KONG H S, KIM H K, CHOI C H, KIM S D, CHO Y M, SANG B C, LEE J H, JEON G J, LEE H K. Estimation of genetic parameters for economic traits in Korean native chickens., 2006, 19(3): 319-323. https://doi.org/10.5713/ajas.2006.319.
[7] TONGSIRI S, JEYARUBAN M G, VAN DER WERF J. Genetic parameters for egg production traits in purebred and hybrid chicken in a tropical environment., 2015, 56(6): 613-620. doi: 10.1080/00071668.2015.1099614.
[8] KAMALI M A, GHORBANI S H, MORADI SHARBABAK M, ZAMIRI M J. Heritabilities and genetic correlations of economic traits in Iranian native fowl and estimated genetic trend and inbreeding coefficients., 2007, 48(4): 443-448. DOI: 10.1080/00071660701505013.
[9] Ghazikhani Shad A, Nejati Javaremi A, Mehrabani Yeganeh H. Animal model estimation of genetic parameters for most important economic traits in Iranian native fowls., 2007, 10(16): 2787-2789. DOI: 10.3923/pjbs.2007.2787.2789.
[10] DANA N, VANDER WAAIJ E H, VAN ARENDONK J A. Genetic and phenotypic parameter estimates for body weights and egg production in Horro chicken of Ethiopia., 2011, 43(1): 21-28. DOI: 10.1007/s11250-010-9649-4.
[11] TONGSIRI S, JEYARUBAN G M, HERMESCH S, VAN DER WERF J H, LI L, CHORMAI T. Genetic parameters and inbreeding effects for production traits of Thai native chickens., 2019, 32(7): 930-938. DOI: 10.5713/ajas.18.0690.
[12] LWELAMIRA J, KIFARO G C, GWAKISA P S. Genetic parameters for body weights, egg traits and antibody response against Newcastle Disease Virus (NDV) vaccine among two Tanzania chicken ecotypes., 2009, 41(1): 51-59. DOI: 10.1007/s11250-008-9153-2.
[13] ZEREHDARAN S, VEREIJKEN A J, VAN ARENDONK J, VAN DER WAAIJT E H. Estimation of genetic parameters for fat deposition and carcass traits in broilers., 2004, 83(4): 521-525. DOI: 10.1093/ps/83.4.521.
[14] GOTO T, TSUDZUKI M. Genetic mapping of quantitative trait loci for egg production and egg quality traits in chickens: A review., 2017, 54:1-12. DOI:10.2141/ jpsa.0160121.
[15] ONO T, OHARA K, ISHIKAWA A, KOUGUCHI T, NAGANO A J, TAKENOUCHI A, IGAWA T, TSUDZUKI M. Mapping of quantitative trait loci for growth and carcass-related traits in chickens using a restriction-site associated DNA sequencing method., 2019, 56(3):166-176. DOI: 10.2141/jpsa.0180066.
[16] Furrow R E, Christiansen F B, Feldman M W. Environment-sensitive epigenetics and the heritability of complex diseases., 2011, 189(4):1377-87. DOI: 10.1534/genetics. 111.131912.
[17] ZHAO X L, REN W S, SIEGEL P B, LI J, WANG Y, YIN H D, ZHANG Y, LAI S, SHU G, ZHU Q. Meat quality characteristics of chickens as influenced by housing system, sex, and genetic line interactions., 2018, 17(2): 462-468. https://doi.org/10.1080/1828051X.2017.1363639.
[18] MACKAY T F. Q&A: Genetic analysis of quantitative traits., 2009, 8(3):23. DOI: 10.1186/jbiol133.
[19] Wilson A J, Réale D, Clements M N, Morrissey M M, Postma E, Walling C A, Kruuk L E, Nussey D H. An ecologist's guide to the animal model., 2010, 79(1): 13-26. DOI: 10.1111/j.1365-2656.2009.01639.x.
[20] HADFIELD J D. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package., 2010, 33(2): 1-22. DOI: 10.18637/jss.v033.i02.
[21] HYNDMAN R J. Computing and graphing highest density regions., 1996, 50(2): 120-126. DOI: 10.2307/ 2684423.
[22] ZHAO X L, REN W S, SIEGEL P B, LI J, WANG Y, YIN H D, ZHANG Y, LAI S, SHU G, ZHU Q. Meat quality characteristics of chickens as influenced by housing system, sex, and genetic line interactions., 2018, 17(2):462-468. DOI: 10.1080/1828051X.2017.1363639.
[23] HUSSEIN E O S, SULIMAN G M, AL-OWAIMER A N, AHMED S H, ABUDABOS A M, ABD EL-HACK M E, TAHA A E, SAADELDIN I M, SWELUM A A. Effects of stock, sex, and muscle type on carcass characteristics and meat quality attributes of parent broiler breeders and broiler chickens., 2019, 98(12):6586-6592. DOI: 10.3382/ps/pez464.
[24] SINGH M K, KUMAR S, SHARMA R K, SINGH S K, SINGH B, SINGH D V. Genetic parameter estimates for juvenile body weight in indigenous Uttara chickens., 2019, 53(4): 429-434. DOI: 10.18805/ijar.B-3560.
[25] SAATEI M, DEWI I A P, AKSOY R, KIRMIZIBAYRAK T, ULUTAS Z. Estimation of genetic parameter for weekly live weight in one to one sire and dam pedigree recorded Japanese quail. Montpellier: 7th World Congress on Genetic Applied to Livestock Production, 2002.
[26] ASLAM M L, BASTIAANSEN J W, CROOIJMANS R P, DUCRO B J, VEREIJKEN A, GROENEN M A. Genetic variances, heritabilities and maternal effects on body weight, breast meat yield, meat quality traits and the shape of the growth curve in turkey birds., 2011, 12(1): 14. DOI: 10.1186/1471-2156-12-14.
[27] 郭军, 王克华, 曲亮, 沈曼曼, 窦套存, 胡玉萍. 基于随机回归模型的如皋黄鸡产蛋数遗传参数分析. 西北农林科技大学学报(自然科学版), 2016, 44(5): 8-12. doi: 10.13207/j.cnki.jnwafu.2016.05.002.
GUO J, WANG K H, QU L, SHEN M M, DOU T C, HU Y P. Random-based genetic parameter analysis of number of egg of Rugao yellow., 2016, 44(5): 8-12. doi: 10.13207/j.cnki.jnwafu.2016.05.002. (in Chinese)
[28] SZYDŁOWSKI M, SZWACZKOWSKI T. Bayesian segregation analysis of production traits in two strains of laying chickens., 2001, 80(2): 125-131. DOI: 10.1093/ps/80.2.125.
[29] 熊化鑫, 张丽萍, 任晋东, 唐军旺, 周树和, 林兴钦, 卢立志. 灵昆鸡产蛋性状与遗传参数分析. 中国农学通报, 2016, 32(11): 6-10.
XIONG H X, ZHANG L P, REN J D, TANG J H, ZHOU S H, LIN X Q, LU L Z. Egg production trait of Lingkun chicken and genetic parameter analysis.2016, 32 (11): 6-10. (in Chinese)
[30] FERREIRA P B, RORATO P R N, BREDA F C, MICHELOTTI V T, ROSA A P, MACEDO A. Genotypic parameters for egg production in pure breed hens by using random regression model., 2017, 47(5): e20141631.http://dx.doi.org/10.1590/0103-8478cr20141631.
[31] DECUYPERE E, HOCKING P M, TONA K, ONAGBESAN O, BRUGGEMAN V, JONES E, CASSY S, RIDEAU N, MÉTAYER S, JEGO Y. Broiler breeder paradox: a project report., 2006, 62(3): 443-453. https://doi.org/10.1017/ S0043933906001073.
[32] JOHNSON P A, KENT T R, URICK M E, TREVINO L S, GILES J R. Expression of anti-Mullerian hormone in hens selected for different ovulation rates., 2009, 137(5): 857. DOI: 10.1530/REP- 08-0406.
[33] SODINI S M, KEMPER K E, WRAY N R, TRZASKOWSKI M. Comparison of genotypic and phenotypic correlations: Cheverud’s conjecture in humans., 2018, 209:941-948. DOI: 10.1534/ genetics.117.300630.
[34] CHEVERUD J M. A comparison of genetic and phenotypic correlations., 1988, 42(5): 958-968. doi: 10.1111/j.1558- 5646.1988.tb02514.x.
Estimation of Genetic Parameters of Body Weight and Egg Number Traits of Lueyang Black-Boned Chicken
DANG LiPing, ZHOU WenXin, LIU RuiFang, BAI Yun, WANG ZhePeng
(College of Animal Science and Technology, Northwest A&F University, Yangling 712100, Shaanxi)
【】Lueyang black-boned chicken (LBC) is an indigenous breed originating from Shaanxi province of China. The breed has large adult body weight, high meat quality, abundant contents of amino acids in meat and excellent adaptation to the free-range system in the forest. But the breed has poor performance of growth and egg production. We measured body weight (BW) and egg number (EN) of LBC at different ages. Based on these phenotypic data we evaluated genetic parameters of BW and EN traits. The aim of this study is to elucidate effect of genetic factors on these traits and genetic relationship among them, thus forming a theory foundation to breeding programs of LBC.【】BWs at 6, 10, 14, 20 weeks of age and first egg of 799 cocks and 804 hens from 71 half-sibling families of generation 1 of LBC breeding population and individual ENs from first egg to 31, 35 and 40 weeks of age were measured. Data lower than 1stquantile-1.5×interquantile range and higher than 3rdquantile + 1.5×interquantile range was deleted prior to statistical analysis. Errors in sex records were corrected. Heritabilities were estimated using a univariate animal model where sex was designed as the fix effect, and additive genetic effect was designed as random effect. Genetic correlations among traits were estimated using a bivariate animal model. Inverse gamma distributions were used as the prior distributions for variance components of random and residual effects.Posterior distributions of variance components were obtained based on 1 000 posterior estimates which were produced using Bayesian algorithm by running 130 000 iterations of which the first 30 000 were discarded in ‘burn-in’ period and the rest was sampled every 100 iterations. Heritabilities and genetic correlations were calculated according to posterior variance components. Phenotypic correlation among traits was calculated using chart.Correlation command in PerformanceAnalytics library of R.【】BWs of LBC cocks were (0.56±0.07), (1.07±0.13), (1.56±0.19), (1.97±0.21) kg and BWs of hens were (0.47±0.06), (0.86±0.12), (1.18±0.16), (1.48±0.19) and (1.68±0.23) kg at 6, 10, 14, 20 weeks and first egg. ENs were 31.2±11.5, 42.5±16.7 and 54.7±20.4 at 31, 35 and 40 weeks. Heritabilities of BWs at 6, 10, 14, 20 and first egg that were 0.74, 0.76, 0.63, 0.54 and 0.52 generally decreased with increasing age. Ninety-five percent confidence interval of BW heritabilities ranged from 0.25 to 0.33. Heritabilities of ENs at 31, 35 and 40 weeks were 0.27, 0.25 and 0.26 with 95% confidence interval varying between 0.35 and 0.42. Genetic correlation among 6, 10 and 14 week BWs were moderate to high (0.52-0.68). But BWs at 6 week (0.21-0.52) and first egg (0.21-0.46) showed relatively week genetic relationship with other BWs. Ninety-five percent confidence interval of genetic correlation coefficients among BW traits ranged from 0.13 to 0.34. Genetic correlation coefficients among EN traits approached to 1 with 95% confidence interval ranging from 0.03 to 0.06. BWs and ENs did not show any significant genetic relationship. On the temporal dimension phenotypic correlation coefficients varied between 0.43 and 0.90 (<0.001) for BW traits and between 0.79 and 0.94 (<0.001) for EN traits. There was weak (0.023-0.15) positive correlation between BWs at 6-20 weeks and ENs. But the BW at first egg showed weak negative correlation (-0.17-0.14) with ENs. 【】This represents the first study specifically estimating genetic parameters for LBC. The data shows that BW traits of LBC are predominately determined by genetic factors. In contrast, ENs are more influenced by environmental factors. Results from genetic correlation analysis elucidated the genetic relationship between early and late traits, and between growth and reproduction in LBC, which provide a solid foundation for early selection and multiple-trait breeding programs. High heritabilities showed by BWs suggest that the phenotypic selection of BWs could significantly increase the growth rate of LBC. However it might be more reasonable to breed pure lines and take advantage of heterosis to increase egg yield of LBS. Genetic independence between BWs and ENs suggests that it is feasible to simultaneously improve two traits in the LBC.
Lueyang black-boned chicken; body weight; egg number; heritability; genetic correlation

10.3864/j.issn.0578-1752.2020.17.018
2019-09-12;
2020-04-24
陕西省自然科学基础研究计划(2018JM3002)、2019年中央高校基本科研业务费(2452019202)、中国国家留学基金(201906305010)
党李苹,Tel:18829353060;E-mail:dangliping2017@163.com。通信作者王哲鹏,Tel:15619295726;E-mail:wangzhepeng-001@163.com
(责任编辑 林鉴非)

