山羊卵泡发育相关基因的筛选及分析
赵园园,李鹏飞,许勤智,安清明,孟金柱
山羊卵泡发育相关基因的筛选及分析
赵园园1,李鹏飞2,许勤智1,安清明1,孟金柱1
(1铜仁学院,贵州铜仁 554300;2山西农业大学生命科学学院,山西太谷 030801)
山羊第一卵泡波中的优势卵泡(dominant follicles, DF)和从属卵泡(subordinate follicles, SF)是整个卵泡发育过程中最为关键的两个阶段。随着卵泡的进一步发育,最终DF可能发育成为成熟卵泡,直到排卵;SF将走向闭锁,其中颗粒细胞的凋亡是导致卵泡发生闭锁的关键因素。然而目前对促进卵泡的优势化或导致其闭锁的分子机理尚不清楚。【】通过对山羊第一卵泡波中DF和SF颗粒细胞进行高通量测序,旨在筛选影响卵泡发育的关键基因,为深入探究卵泡发育的调控机制提供理论依据。选取10只1岁龄健康的贵州白山羊分别注射前列腺素F2α,使其同期发情,此后每天用B超检测并记录卵泡的生长情况,发情3 d后,统一屠宰并采集第一卵泡波中DF (直径4.5—6 mm)与SF (直径3 —4.5 mm),分别分离其中的颗粒细胞,提取总RNA、构建文库后通过Illumina Hiseq 2500平台进行测序。利用FastQC对测序产出raw reads进行质量评估并经过过滤后,获得品质较高的clean reads;使用Trinity对得到的clean reads进行重新组装,从而获得unigenes;使用CLC Genomics Workbench将unigenes与山羊RefSeq数据库进行比对获得mRNA;使用DESeq2 软件对获得的mRNA进行差异表达分析;分别采用goseq和kobas软件对得到的差异表达基因进行GO分析及KEGG信号通路分析;最终通过qRT-PCR对筛选出的可能影响卵泡发育的关键基因进行验证。分别对测序得到的raw reads进行过滤后,在DF颗粒细胞中获得43 217 934条clean reads,占raw reads的比例为95.19%;SF颗粒细胞中获得40 766 348条clean reads,占raw reads的比例为95.35%。将得到的unigenes与山羊的RefSeq 数据库进行比对后,共得到33 896条带有注释的转录本,再通过设定FPKM>1, q value<0.05,共在两种卵泡颗粒细胞中获得13 644个基因。设定参数:FPKM≥1,SF-FPKM/DF-FPKM>1,<0.05,获得695个差异表达mRNA,其中233个在SF颗粒细胞中表达显著上调,462个表达显著下调;对所获得695个差异表达mRNA进行GO功能富集分析,共分为三大类42组:其中生物学过程占47.6%,细胞组分占47.6%,分子功能占4.8%;KEGG信号通路分析,发现20条通路,其中与核糖体通路相关的基因富集最为显著。通过在Genecard中进行功能分析后,筛选6个可能与山羊卵泡发育密切相关的基因,其中、、在SF颗粒细胞中表现为上调;、、则表现为下调。qRT-PCR显示、、、、的表达趋势与高通量测序结果一致,且在从属卵泡颗粒细胞中的表达量极显著地高于优势卵泡(<0.01);、、在优势卵泡颗粒细胞中的表达量极显著地高于从属卵泡(<0.01)。、、和在优势卵泡和从属卵泡中表达量存在极显著差异,推测在山羊卵泡发育过程中可能促进卵泡的优势化或导致闭锁,对深入探究卵泡发育的调控机制具有重要意义。
山羊;高通量测序;卵泡发育;颗粒细胞
0 引言
【研究意义】哺乳动物卵泡发育是一个受多种因素调控的复杂生物学过程,在这个过程中有众多激素或者调控因子直接或间接参与[1]。通过基因敲除,在小鼠的卵巢中已经发现有 1 000 多个基因共同调控着卵泡的发育[2]。然而目前对促进山羊卵泡的优势化或导致其闭锁的分子机理尚不清楚。通过对山羊第一卵泡波中DF和SF颗粒细胞进行高通量测序,筛选出影响卵泡发育的关键基因,为深入探究卵泡发育的调控机制具有重要意义。【前人研究进展】在哺乳动物的一个发情周期中,一簇卵泡经过募集、选择及优势化过程发育成为排卵卵泡[3]。在卵泡的优势化过程中,细胞色素P450侧链裂解酶(Pytochrome P450 side chain lyase, P450scc)和细胞色素P450芳构化酶(cytochrome P450 aromatase, P450arom)mRNA能够促进卵泡的发育,推测P450arom和P450scc可能促进卵泡颗粒细胞分泌雌二醇(E2),从而参与负反馈调节作用并促进卵泡的优势化[4]。胰岛素样生长因子(insulin-like growth factor,IGF)会显著促进颗粒细胞分泌E2,并加快卵泡选择与优势化过程[5],然而成纤维细胞生长因子(fibroblast growth factor, FGF)与IGF的作用却相反,它通过抑制卵泡颗粒细胞分泌E2和抑制卵泡LH受体的表达从而抑制卵泡的选择与优势化过程[6]。卵泡一旦确定了优势化地位后,优势化卵泡会通过分泌E2和INH等调控因子维持自己的优势化地位[7]。LI等[8]通过对水牛不同大小卵泡的颗粒细胞进行高通量测序,发现免疫系统可能在卵泡的成熟和排卵过程中起了重要作用。TERENINA等[9]通过对猪的正常卵泡与闭锁卵泡颗粒细胞进行转录组测序,筛选出了等11个基因可能在卵泡颗粒细胞的增殖过程中起了抑制作用,从而引起卵泡闭锁。【本研究切入点】山羊第一卵泡波中的DF最终可能发育成为成熟卵泡,直到排卵;而SF则会受到各种调控因子而作用而走向闭锁,其中颗粒细胞的凋亡是导致卵泡发生闭锁的关键因素[10]。然而目前对促进卵泡的优势化或导致其闭锁的分子机理尚不清楚。【拟解决的关键问题】通过对山羊第一卵泡波中DF和SF颗粒细胞进行高通量测序,并通过qRT-PCR进行验证分析,筛选出影响卵泡发育的关键基因,为深入探究其调控卵泡发育机制提供理论依据。
1 材料与方法
本试验于2018年12月至2019年4月在铜仁学院完成。
1.1 试验动物及样品采集
在贵州省铜仁市沿河土家族自治县华珍牧业有限公司,选取10只1岁龄健康的贵州白山羊分别注射前列腺素F2α,使其同期发情,此后每天用B超检测并记录卵泡的生长情况,发情3 d后,统一屠宰并采集第一卵泡波中DF(直径4.5—6 mm)与SF(直径3—4.5 mm),迅速置于4℃灭菌杜氏磷酸缓冲液(DPBS)中,迅速运输到铜仁学院动物学实验室。将处于DPBS中的DF和SF分别放到盛有0.9%的生理盐水的培养皿上,使用眼科剪刀剪开并用细胞刮刀刮取位于卵泡内膜上的颗粒细胞(GCs)后置于-80℃冰箱中保存。
1.2 试验方法
1.2.1 总RNA提取、文库构建及测序 将保存在-80℃冰箱中的GCs取出后置于冰盒中解冻,加入1 mL Trizol(购自Invitrogen公司,美国)分别提取两种卵泡中GCs的总RNA,经RNeasy mini kit(购自QIAGEN公司,德国)纯化,Agilent Bioanalyzer 2100完整性检测,Qubit 2.0 Flurometer测量浓度后,交由北京诺和致源生物信息科技有限公司进行文库构建并通过Illumina Hiseq 2500平台测序。
1.2.2 数据处理及分析 FastQC(http://www. bioinformatics.babraha m.ac.uk/projects/fastqc/)用于对测序产出原始数据进行质量评估,然后清除原始数据(raw reads)中带接头的、低质量的reads,从而获得品质较高的有效读段(clean reads)。使用Trinity(http://trinityrnaseq.sourceforge.net/)对得到的clean reads进行重新组装,以便得到单一序列(singleton)及重叠群(contigs)此时得到的序列称为unigenes。使用CLC Genomics Workbench将unigenes与山羊RefSeq数据库进行比对。基于负二项分布的DESeq2软件用于差异表达mRNA的分析,之后使用goseq软件对得到的差异表达基因进行GO分析,使用kobas软件进行KEGG信号通路分析。
1.2.3 反转录及引物合成 通过EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix(购自北京全式金生物技术有限公司)将提取到的总RNA反转录成cDNA,条件为:42℃孵育15 min,85℃加热5 s失活TransScript RT与gDNA Remover。Primer 5.0设计引物(表1),使用作为内参基因,引物合成委托生工生物工程(上海)股份有限公司完成。
1.2.4 qRT-PCR分析 qRT-PCR用于验证贵州白山羊DF与SF GCs中差异表达mRNA的相对表达水平。采用3个样本重复,3个技术重复,通过TransStart® Tip Green qPCR SuperMix(购自北京全式金生物技术有限公司)对各基因进行相对定量分析,根据产品使用说明书构建20 μL PCR反应体系:2×Transstart®Tip Green qPCR super mix 10 μL,上下游引物各0.4 μL,cDNA 4 μL(100 ng),RNA-free H2O 5.2 μL。反应程序为:94℃预变性1 min;94℃ 10 s,60℃ 30 s,72℃ 10 s,45个循环。结果使用2△△CT法来计算各基因的相对表达情况。

表1 荧光定量引物基因列表
2 结果
2.1 RNA-seq数据分析
高通量测序得到的raw reads,经过滤带接头的、含N的及低质量的reads,最终在DF中获得43 217 934条clean reads,占95.19%;在SF中获得40 766 348条clean reads,占95.35%(图1)。将得到的clean reads比对到山羊RefSeq数据库中,共得到33 896条带有注释的转录本。设定FPKM >1, q value<0.05,在两种卵泡颗粒细胞中共获得13 644个基因,其中438个基因高表达(FPKM≥1),表2中列出了表达量排名前20的基因。

图1 DF和SF颗粒细胞中原始数据的分类

表2 SF和DF颗粒细胞中表达量最高的20个基因
2.2 差异表达基因筛选
在差异转录本分析过程中,将DF和SF的FPKM (Fragmentsperkilobaseoftranscriptpermillionfragmentsmapped)进行标准化,使用DESeq2软件对获得13 644个mRNA进行差异表达分析,设定参数:FPKM≥1,SF-FPKM/DF-FPKM>1,<0.05,共获得695个差异表达mRNA,其中233个在从属卵泡颗粒细胞中表达显著上调,462个则表达下调(图2)。
2.3 GO功能富集
通过goseq软件对得到的695个差异表达基因进行GO功能富集分析, 共分为三大类42组:其中生物学过程占47.6%,细胞组分占47.6%,分子功能占4.8% (图3)。
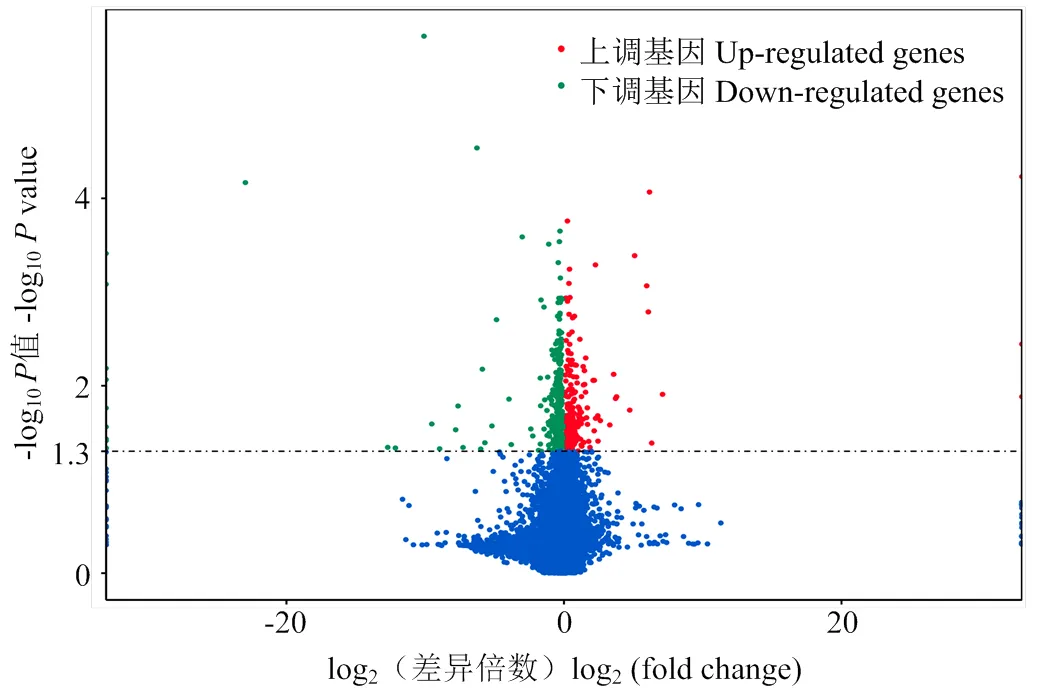
图2 DF和SF颗粒细胞中差异表达基因火山图

1:RNA加工;2:RNA拼接;3:核酸代谢过程;4:含碱基化合物的代谢过程;5:核糖核蛋白复杂生物合成;6:细胞大分子代谢过程;7:核糖体生物合成;8:杂环代谢过程;9:细胞芳香族化合物的代谢过程;10:细胞氮化合物代谢过程;11:基因表达;12:RNA代谢过程;13:核糖体小亚基生物合成;14:核糖体RNA加工;15:大分子代谢过程;16:氮化物代谢过程;17:ncRNA加工;18:ncRNA代谢过程;19:有机循环化合物代谢过程;20:核糖体RNA代谢过程;21:下拨核内腔;22:细胞核部分;23:细胞核;24:细胞内的细胞器;25:细胞器;26:细胞膜内腔;27:细胞内细胞器内腔;28:细胞器内腔;29:细胞内细胞器的部分;30:细胞内的;31:细胞内的部分;32:细胞器的部分;33:细胞内膜上细胞器;34:膜上细胞器;35:核质;36:核糖核蛋白复合体;37:胞质核糖体小亚基;38:胞质核糖体;39:核质部分;40:大分子复合体;41:RNA结合;42:mRNA结合
2.4 KEGG信号通路分析
通过kobas软件对差异表达基因进行KEGG信号通路分析,共发现20条通路(图4),其中与核糖体通路相关的基因最为显著富集,达到50个;另外,还发现5个基因参与了卵母细胞的减数分裂。
2.5 qRT-PCR验证分析
通过功能分析,我们筛选出6个可能与山羊卵泡发育密切相关的基因(表3)。其中、、在SF颗粒细胞中表现为上调;、、则表现为下调。QRT-PCR结果显示、、、、的表达趋势与高通量测序结果一致,且在SF颗粒细胞中的表达量极显著地高于DF(<0.01);、、在DF颗粒细胞中的表达量极显著地高于SF(<0.01),的荧光定量结果虽然与高通量测序结果的表达趋势相反,但不存在显著差异(>0.05)(图5)。

图4 DF和SF颗粒细胞中差异表达基因KEGG通路富集散点图

图5 候选基因在山羊DF和SF颗粒细胞中的相对表达量(**表示P<0.01)
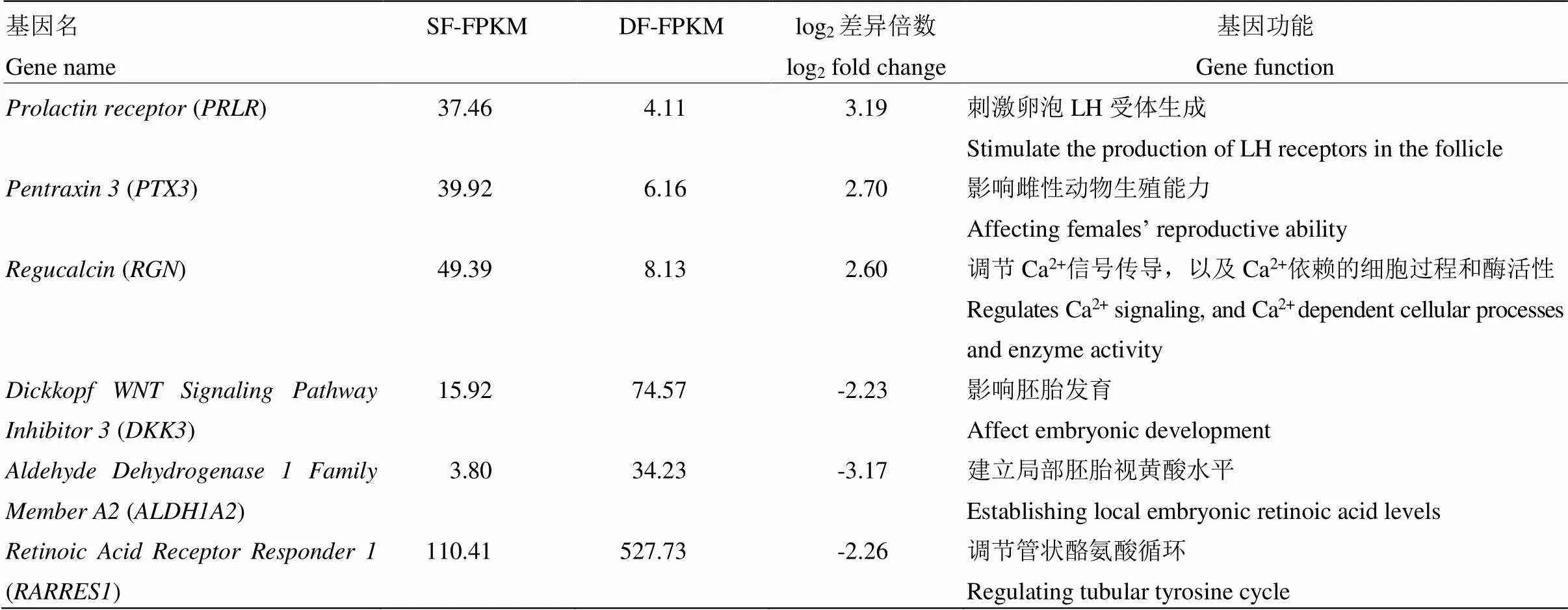
表3 可能与山羊卵泡发育相关的候选基因
3 讨论
雌性哺乳动物卵巢上的DF持续生长、成熟并排出卵子是依赖于颗粒细胞中FSH通过激活cAMP-蛋白激酶A通路,进而诱导芳香化酶将膜细胞分泌的雄激素转化为E2,而SF由于生长速率和E2分泌降低,从而走向闭锁[11]。HUSSEIN等[10]研究发现,颗粒细胞的凋亡是引起卵泡闭锁的关键因素;颗粒细胞受到凋亡蛋白酶激活坏死因子(TNF)、Fas等刺激后,使促凋亡蛋白的构象发生变化,从而由细胞液转移到线粒体外膜上,并与膜上及膜内的抗凋亡蛋白相互作用,最终导致其凋亡[12-13]。LI等[14]通过对牛发生偏差前的最大卵泡(PDF1)和发生偏差后的最大卵泡(ODF1)颗粒细胞进行转录组测序,获得83个差异表达基因,其中、和在卵泡发育过程中可能起抑制作用。本研究通过对贵州白山羊DF和SF颗粒细胞进行高通量测序筛选出695个差异表达基因,其中233个在SF颗粒细胞中表达显著上调,462个表达下调。结合功能分析,筛选出具有代表性的6个可能与山羊卵泡发育密切相关的基因,qRT-PCR验证结果表明,、、、和在DF和SF颗粒细胞中的表达趋势与高通量测序结果完全一致,在DF和SF颗粒细胞中的表达趋势与高通量测序结果虽相反,但差异不显著(>0.05)。
在哺乳动物中,促乳素受体(PRLR)对维持黄体以及孕酮的分泌起着重要的作用[15]。PRL可能通过对子宫内膜的直接作用在妊娠中发挥作用[16]。PRL能够促进排卵、着床及胎盘发育的过程[17], 敲除小鼠卵泡中的,使得卵母细胞释放延迟,成熟受损进而导致排卵减少[18]。颗粒细胞中正五聚蛋白3 (PTX3) 基因的表达也与卵母细胞及胚胎的发育能力有关,是预测胚胎发育能力的可靠指标[19-20]。在小鼠排卵前卵泡腔内,mRNA表达主要位于卵丘细胞中,而在颗粒细胞中表达很少[21],进一步证实了笔者的数据准确性。钙调素(Regucalcin,RGN)在牛大卵泡(直径>10 mm)中的表达量是小卵泡(直径<5 mm)中的9.8倍,被认为是参与了优势卵泡的形成及提高颗粒细胞的存活率[22]。这与本研究的结果产生分歧,可能是物种之间表达的差异所致。
Dickkopf WNT信号通路抑制剂3(dickkopf WNT signaling pathway inhibitor 3,DKK3)是一种旁分泌蛋白,它可以通过Wnt信号通路参与胚胎发育[23]。表观遗传沉默,会破坏正常Wnt/β-catenin 信号传导和细胞凋亡调控[24]。醛脱氢酶1家族成员A2 (Aldehyde Dehydrogenase 1 Family Member A2,ALDH1A2)启动子中存在雌激素反应元件位点[25],在切除大鼠卵巢的子宫内,E2可以促进表达,但却抑制表达[26]。是调节性腺中肾RA合成的主要酶,通过释放全反维甲酸(RA)的酶来启动细胞的减数分裂。构建和双敲除小鼠与全反维甲酸反应元件(RARE)报告小鼠杂交表明,在缺乏这两种酶产生RA情况下,雌性小鼠可发生减数分裂,雄性小鼠不可发生减数分裂[27-30]。目前对全反维甲酸受体应答器1(retinoic acid receptor responder 1,RARRES1)的报道较少,作为RA释放的下游基因,可能在细胞的减数分裂过程中起重要作用。
4 结论
本研究在贵州白山羊DF与SF颗粒细胞中共获得695个差异表达mRNA,其中233个在SF颗粒细胞中表达显著上调,462个则表达下调。通过功能分析,筛选出6个可能与山羊卵泡发育密切相关的基因,qRT-PCR结果发现,、、和在DF和SF颗粒细胞中表达量存在极显著差异,推测在山羊卵泡发育过程中可能促进卵泡的优势化或导致闭锁,对深入探究调控卵泡发育机制具有重要意义。
[1] QUAN Q, ZHENG Q, LING Y H, FANG F G, CHU M X, ZHANG X R, LIU Y, LI W Y. Comparative analysis of differentially expressed genes between the ovaries from pregnant and nonpregnant goats using RNA-Seq., 2019, 26(3): 1-12.
[2] RO S, SONG R, PARK C, ZHENG H, SANDERS K M, YAN W. Cloning and expression profiling of small RNAs expressed in the mouse ovary., 2007, 13(12):2366-2380.
[3] LI P F, MENG J Z, ZHU Z W, FOLGER J K, LYU L H. Detection of genes associated with follicle development through transcriptome analysis of bovine ovarian follicles GCs., 2018,13:127-140.
[4] BAO B, GARVERICK H A, SMITH G W, SMITH M F, SALFEN B E, YOUNGQUIST R S. Changes in messenger ribonucleic acid encoding luteinizing hormone receptor, cytochrome P450-side chain cleavage, and aromatase are associated with recruitment and selection of bovine ovarian follicles., 1997, 56(5): 1158-1168.
[5] REVERCHON M, CORNUAU M, RAMÉ C, GUERIF F, ROYÈRE D, DUPONT J. Resistin decreases insulin-like growth factor I-induced steroid production and insulin-like growth factor I receptor signaling in human granulosa cells., 2013, 100(1): 247-255.
[6] KHARITONENKOV A, DIMARCHI R. Fibroblast growth factor 21 night watch: advances and uncertainties in the field., 2016, 281(3):233-246.
[7] DE CASTRO T, RUBIANES E, MENCHACA A, RIVERO A. Ovarian dynamics, serum estradiol and progesterone concentrations during the interovulatory interval in goats., 1999, 52(3):399-411.
[8] LI J, LI Z, LIU S, ZIA R, LIANG A, YANG L. Transcriptome studies of granulosa cells at different stages of ovarian follicular development in buffalo., 2017,187:181-192.
[9] TERENINA E, FABRE S, BONNET A, MONNIAUX D, ROBERT- GRANIÉ C, SANCRISTOBAL M, TOSSER-KLOPP G. Differentially expressed genes and gene networks involved in pig ovarian follicular atresia.2017, 49(2):67-80.
[10] HUSSEIN M R. Apoptosis in the ovary: Molecular mechanisms., 2005,11(2):162-178.
[11] FOLGER J K, JIMENEZ-KRASSEL F, IRELAND J J, LV L L, SMITH G W. Regulation of granulosa cell cocaine and amphetamine regulated transcript (CART) binding and effect of CART signaling inhibitor on granulosa cell estradiol production during dominant follicle selection in cattle., 2013, 137: 1-8.
[12] BROMFIELD J J, IACOVIDES S M. Evaluating lipopolysaccharide- induced oxidative stress in bovine granulosa cells., 2017, 34(12): 1619-1626.
[13] HAN P, XIN H Y, PENG J X, ZHANG L, SONG Y X, LI G, CAO B Y, AN X P. Identification and expression of X-linked inhibitor of apoptosis protein during follicular development in goat ovary., 2017, 98:30-35.
[14] LI P F, MENG J Z, LIU W Z, SMITH G W, YAO J B, LYU L H. Transcriptome analysis of bovine ovarian follicles at predeviation and onset of deviation stages of a follicular wave., 2016, 2016:1-9.
[15] ZI X D, CHEN D W, WANG H M. Molecular characterization, mRNA expression of prolactin receptor () gene during pregnancy, nonpregnancy in the yak ()., 2012, 175(3):384-388.
[16] RUAN W, CATANESE V, WIECZOREK R, FELDMAN M, KLEINBERG D L. Estradiol enhances the stimulatory effect of insulin-like growth factor-I (IGF-I) on mammary development and growth hormone-induced IGF-I messenger ribonucleic acid., 1995, 136(3):1296-1302.
[17] PERKS C M, NEWCOMB P V, GROHMANN M, WRIGHT R J, MASON H D, HOLLY J M. Prolactin acts as a potent survival factor against C2-ceramide-induced apoptosis in human granulosa cells., 2003, 18(12):2672-2677.
[18] TISSIER P R, HODSON D J, MARTIN A O, RomanòN,Mollard P. Plasticity of the prolactin (PRL) axis: Mechanisms underlying regulation of output in female mice., 2015, 846:139-162.
[19] LI S H, LIN M H, HWU Y M, LU C H, YEH L Y, CHEN Y J, LEE R K. Correlation of cumulus gene expression of,,, andwith oocyte maturation, fertilization, and embryo development., 2015, 13(1): 93.
[20] ZHANG X Q, JAFARI N, BARNES R B, CONFINO E, MILAD M, KAZER R R. Studies of gene expression in human cumulus cells indicate pentraxin 3 as a possible marker for oocyte quality., 2005, 83(4):1169-1179.
[21] GARLANDA C, BOTTAZZI B, BASTONE A, MANTOVANI A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility., 2005, 23(1):337-366.
[22] HATZIRODOS N, IRVING-RODGERS H F, HUMMITZSCH K, HARLAND M L, MORRIS S E, RODGERS R J. Transcriptome profiling of granulosa cells of bovine ovarian follicles during growth from small to large antral sizes., 2014, 15(1):24.
[23] VOORHAM Q J, JANSSEN J, TIJSSEN M, SNELLENBERG S, MONGERA S, GRIEKEN N C, GRABSCH H, KLIMENT M, REMBACKEN B J, MULDER C J, ENGELAND M V, MEIJER G A,STEENBERGEN R D, CARVALHO B. Promoter methylation of Wnt-antagonists in polypoid and nonpolypoid colorectal adenomas., 2013, 13(1): 603.
[24] BHATTACHARYYA S, FEFERMAN L, TOBACMAN J K. Chondroitin sulfatases differentially regulate Wnt signaling in prostate stem cells through effects on SHP2, phospho-ERK1/2, and Dickkopf Wnt signaling pathway inhibitor (DKK3)., 2017, 59(8): 100242-100260.
[25] WANG X S, SPERKOVA Z, NAPOLI J L. Analysis of mouse retinal dehydrogenase type 2 promoter and expression., 2001, 74(2): 245-250.
[26] LI X H, KAKKAD B, ONG D E. Estrogen directly induces expression of retinoic acid biosynthetic enzymes, compartmentalized between the epithelium and underlying stromal cells in rat uterus., 2004, 145(10):4756-4762.
[27] KUMAR S, CUNNINGHAM T J, DUESTER G. Resolving molecular events in the regulation of meiosis in male and female germ cells., 2013, 6(288): pe25.
[28] KRENTZ A D, MURPHY M W, SARVER A L, GRISWOLD M D, BARDWELL V J, ZARKOWER D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary., 2011, 356(1):63-70.
[29] NAILLAT F, PRUNSKAITE-HYYRYLAINEN R, PIETILA I, SORMUNEN R, JOKELA T, SHAN J D, VAINIO S J. Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development., 2010, 19(8):1539-1550.
[30] BOWLES J, FENG C W, SPILLER C, DAVIDSON T L, JACKSON A, KOOPMAN P. FGF9 suppresses meiosis and promotes male germ cell fate in mice., 2010, 19(3):440-449.
Screening and Analysis of Follicular Development Related Genes in Goat
ZHAO YuanYuan1, LI PengFei2, XU QinZhi1, AN QingMing1, MENG JinZhu1
(1Tongren University, Tongren 554300, Guizhou;2College of Life Science, Shanxi Agricultural University, Taigu 030801, Shanxi)
【】 Dominant follicles (DF) and subordinate follicles (SF) were the most important two stages in the follicle development in the first follicle wave of goat. With further development of follicles, DF may eventually develop into mature follicles until ovulation, and SF will move towards atresia, while apoptosis of granulosa cells is the key factor leading to follicular atresia. However, the molecular mechanisms of promoting follicle dominance or causing its atresia are still unclear. 【】 This study was aimed to screen the key genes affecting follicular development and provide a theoretical basis for further exploring the regulation mechanism of follicular development by high-throughput sequencing of DF and SF granulosa cells in the first follicular wave of goat.【】Ten healthy Guizhou white goats were selected (1 year old), and the prostaglandin F2αwere injected respectively for estrus synchronization. B-type ultrasonography was used to detect the follicle growth situation, and then all of the goats were slaughtered when estrus had appeared for three days. DF (4.5-6 mm in diameter) and SF (3-4.5 mm in diameter) were obtained, granulosa cells were separated in the first follicle wave, respectively. Total RNA were extracted, and libraries were constructed and sequenced by Illumina Hiseq 2500 platform. FastQC was used to evaluate the quality of raw reads sequenced and filter them to obtain clean reads with high quality. Trinity was used to assemble clean reads from scratch to obtain unigenes. mRNA was obtained by comparing unigenes with goat RefSeq database using CLC Genomics Workbench. DESeq2 software was used to analyze the differential expression of the obtained mRNA. The goseq and kobas software were used for GO analysis and KEGG signal pathway analysis. Finally, qRT-PCR was used to verify the selected key genes that might affect the follicle development. 【】After the raw reads which obtained by sequencing were filtered, 43 217 934 clean reads were obtained from DF granulosa cells, accounting for 95.19% of raw reads. 40 766 348 clean reads were obtained from SF granulosa cells, accounting for 95.35% of raw reads. When the unigenes were compared with the RefSeq database of goat, a total of 33 896 annotated transcripts were obtained. Setting FPKM>1 and q value<0.05, a total of 13 644 genes were obtained in two types of follicle granulosa cells. By setting parameters: FPKM≥1, SF-FPKM/DF-FPKM>1,<0.05, 695 differentially expressed mRNAs were obtained, of which 233 were significantly up-regulated and 462 were down-regulated in SF granulosa cells. GO functional enrichment analysis was performed on 695 differentially expressed mRNAs and concentrated in 42 groups of three major categories: biological processes accounted for 47.6%, cellular components 47.6%, and molecular functions 4.8%. KEGG signaling pathway analysis revealed 20 pathways, among which ribosome pathway related genes were most significantly enriched. Six genes that might be closely related to follicle development in goats were screened out, among which,andwere up-regulated in subordinate follicles granulosa cells.,andwere down-regulated. qRT-PCR showed that the expression trend of,,,andwas consistent with high-throughput sequencing results, and the expression level ofin SF granulosa cells was significantly higher than that of dominant follicles (<0.01). The expression levels of,and<0.01).【】,,andhad extremely significant differences in the expression levels of dominant follicles and subordinate follicles. It was speculated that those genes might promote the predominance of follicles or lead to atresia during the follicle development of goat, which was of great significance to further explore the regulation mechanism of follicular development.
goat; high-throughput sequencing; follicle development; granulosa cells

10.3864/j.issn.0578-1752.2020.17.016
2019-06-24;
2020-07-10
贵州省教育厅青年科技人才成长项目(黔教合KY字[2018]348)、贵州省普通高等学校科技拔尖人才支持计划(黔教合KY字[2017]089)、铜仁学院博士启动基金项目(trxyDH1601)、铜仁学院生态畜牧创新团队项目(CXTD[2020-19])、贵州省农业科技示范园区项目(黔科合农园字[2014]5007号)、沿河土家族自治县科技合作计划项目“良种山羊与沿河白山羊杂交组合试验与示范推广”
赵园园,E-mail:84840293@163.com。通信作者孟金柱,E-mail:mjz122021@126.com
(责任编辑 林鉴非)

