Coronavirus disease 2019 (COVID-19), MERS and SARS: Similarity and difference
Zeinab Mohseni Afshar, Soheil Ebrahimpour, Mostafa Javanian, Veerendra Koppolu, Veneela Krishna Rekha Vasigala, Amir Hossein Hasanpour, Arefeh Babazadeh✉
1Clinical Research Development Center, Imam Reza Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran
2Infectious Diseases and Tropical Medicine Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, I.R. Iran
3Scientist Biopharmaceutical Development Medimmune Gaithersburg, MD, 20878 USA
4Rangaraya Medical College, NTR University of Health Sciences, Kakinada, India
5Student Research Committee, Babol University of Medical Sciences, Babol, Iran
ABSTRACT SARS-CoV-2 is the causative agent of COVID-19. Since its outbreak in December 2019, COVID-19 has swept the globe.By 17 July 2020, the World Health Organization (WHO) had confirmed 13 119 239 cases and 573 752 deaths, and the numbers are still rising. Current evidence shows that COVID-19 is lower than severe acute respiratory syndrome and Middle East respiratory syndrome in terms of severity and mortality risk,although the infections are particularly more severe in patients with underlying medical conditions. The bulk of COVID-19 patients had close contact with confirmed cases, but an exact origin and specific transmission for COVID-19 are still unknown. As there is no approved antiviral treatment for COVID-19 infection,proper prevention and control practices are essential to control the infection. To have an insight view of COVID-19, we summarized and compared the etiology, clinical manifestations, diagnosis,treatment, and prevention measures of COVID-19, severe acute respiratory syndrome, and Middle East respiratory syndrome.
KEYWORDS: COVID-19; Middle East respiratory syndrome;Severe acute respiratory syndrome; Coronavirus
1. Introduction
Coronaviruses (CoVs), belonging to RNA viruses, are widely distributed among humans, other mammals, and birds, resulting in respiratory, enteric, hepatic, and neurologic diseases. It is known that there are 7 identified human pathogenic coronavirus strains worldwide, among which, two strains,i.e.severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) can cause severe diseases.In December 2019, a new coronavirus strain (SARS-CoV-2) was identified and rapidly spread across the globe[1]. Similar to SARS and MERS, COVID-19 is thought to be a zoonotic infection transmitted from animals to humans, then followed by a rapid human to the human transmission that causes severe respiratory infections and substantial mortality (Figure 1)[2].
SARS-CoV was identified to be the cause of SARS outbreak in 2002 and 2003 in Guangdong Province, China[3]. As the first pandemic of the 21st century, SARS is originally transmitted from animals (bats) to humans. The major routes of viral transmission are through respiratory droplets, fecal-oral mode, or fomites. The virus killed about 1 out of 10 infected people[4]. SARS infection shows flu-like symptoms that usually begin 2-10 d after infection.The most common clinical manifestations of SARS infections include fever, myalgia, occasional cough, shortness of breath,tachypnea, or pleurisy, and watery diarrhea. SARS patients would show lymphocytopenia (abnormally low levels of lymphocytes in the blood), and higher levels of D-dimers, activated partial thromboplastin time, creatine kinase, alanine aminotransferase, and lactate dehydrogenase[5]. Radiographic abnormalities mostly include ground-glass opacifications, but rarely mediastinal lymphadenopathy,cavitation, and pleural effusions. Complications of SARS include spontaneous pneumomediastinum and acute respiratory distress syndrome requiring mechanical ventilation.
MERS-CoV was first identified in the outbreak of the severe respiratory disease in 2012. Another outbreak outside the Arabian Peninsula was reported in South Korea in May 2015, which ended in 2018[6]. It was believed that dromedary camels were the main animal reservoirs of this virus, and human infections occurred through the respiratory droplets or saliva, milk, or raw and undercooked meat of dromedary camels[7]. MERS-CoV infection manifests with a wide spectrum of illnesses and severity, from asymptomatic to mild, moderate, and severe diseases. The incubation period ranges from 2-14 d with a median of 5.2 d[8]. Patients with mild infections may present symptoms such as low-grade fever, dry cough, runny nose, sore throat, and myalgia. Patients with severe infections often suffer from pneumonia those progresses to acute respiratory distress syndrome, multisystem infections, and organ failure. In some cases,pneumonia progresses abruptly after about 7 d after infection[9].The severity of the symptoms peaks after approximately 14 d of infection. Acute kidney injury and gastrointestinal symptoms, such as diarrhea, nausea, and vomiting can also occur in severe infections.For MERS-CoV, human to human transmission occurs through close contact with infected individuals, and the transmission channels include airborne, droplet, or ingestion routes. It was believed that viral transmission in hospitals and other healthcare settings was the main reason for the outbreaks in Saudi Arabia, the United Arab Emirates, and South Korea.
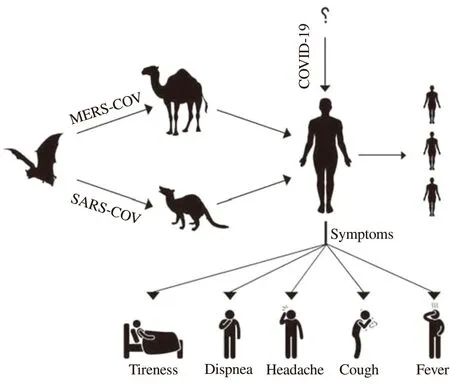
Figure1. Possible hosts and transmission of SARS-CoV, MERS-CoV, and SARS-CoV-2.
With respect to SARS-CoV-2, an outbreak of pneumonia of unknown etiology was reported in Wuhan City, China in December 2019[10]. Laboratory tests ruled out SARS-CoV, MERS-CoV,influenza, avian influenza, and other common respiratory pathogens,and then on the 9th January 2020, the World Health Organization(WHO) announced that a novel type of coronavirus are responsible for the viral pneumonia, which is different from all the known human coronaviruses (MERS and SARS). This newly-discovered virus became the seventh member of coronavirus family. Initially,the virus was called 2019 novel coronavirus but subsequently named as coronavirus disease 2019 (COVID-19)[11]. Many of the patients that are infected early in this outbreak had some association with a large seafood and live animal market while the subsequently infected patients reported no exposure to animal markets, indicating an initial animal-to-person spread and subsequent person-to-person transmission. The current knowledge of COVID-19 transmission is largely based on other coronaviruses. Therefore, we believed and later verified that human to human spread happenedviarespiratory droplets released during coughing and sneezing of an infected person[12]. Also in a very short time, the total number of deaths due to COVID-19 infection has surpassed that of SARS and MERS. The rapid spread suggests that COVID-19 virus is highly contagious and can easily spread between humans. The COVID-19 virus infections are also confirmed in the United States of America, Thailand, Japan,South Korea, Vietnam, Singapore, Canada, and France, indicating the international spread of this potentially life-threatening infection[13].Given the global threats of COVID-19, the present study aimed at summarizing and comparing the etiology, clinical manifestations,diagnosis, possible treatment, and prevention choices of SARS,MERS, and COVID-19.
2. Etiology
Genome sequencing and phylogenetic analysis suggested that SARS-CoV-2 belongs to beta coronavirus in subfamily Coronavirinae, family Coronaviridae, and order Nidovirales.Coronavirus is an enveloped single-stranded RNA virus that presents 9-12 nm surface spikes[14,15]. Coronavirus encodes four major structural proteins in the envelope including a spike protein that interacts with angiotensin-converting enzyme-2 receptors to mediate viral entry into the host cells. COVID-19 has about 89% nucleotide identity with bat SARS-like-CoVZXC21 and 82% identity to human SARS-CoV[16].
3. Clinical manifestation and diagnosis
COVID-19 is an emerging disease, and there is much to learn in terms of its symptoms, severity, diagnosis, and transmissibility. With respect to symptoms, fever, cough, headache, productive cough,hemoptysis, diarrhea, breathing difficulty, and chest discomfort
were reported[1,17]. Symptoms may develop 2-14 d after the first exposure in the majority of the cases, while a few patients developed severe respiratory distress soon after the presentation and hospital admission. The median duration from symptoms onset to dyspnea requiring mechanical ventilation is about 10 d[18]. In fatal cases,the symptoms included acute respiratory distress syndrome, severe pneumonia, septic shock, and multi-organ failure. Although COVID-19 infection appears to be clinically milder than MERS or SARS,in terms of severity and percent mortality[19], the virus is more contagious and spread much more rapidly than SARS and MERS.
The laboratory evaluation of the blood counts on admission showed leukopenia and lymphopenia[20]. Occasionally, some ICU patients presented with elevated prothrombin time, D-dimers, alanine aminotransferase, and aspartate aminotransferase. Procalcitonin level on admission was found to be normal except in patients of bacterial super infection[21]. The typical findings of CT scans of ICU patients on admission were bilateral multiple lobular, subsegmental areas of consolidation, bilateral ground-glass opacity, and subsegmental areas of consolidation in non-ICU patients[22].
Like the epidemiologic situation, the diagnostic landscape is also changing rapidly. WHO published an interim laboratory guide for the detection of COVID-19 virus which includes guidance on patient sampling, pathogen detection, and characterization. The first cases of infection were detected using metagenomics sequencing. Within days of obtaining the viral sequences, polymerase chain reaction(PCR) based assays were developed for diagnostic use[23]. The center for Disease Control and Prevention (CDC) of the United States has developed a new laboratory test kit for the detection of COVID-19 virus. The kit [CDC 2019-novel coronavirus (SARS-CoV-2)real-time reverse transcriptase-PCR diagnostic panel] uses reverse transcriptase-PCR based on the detection of coronavirus RNA and is intended to detect the virus from upper and lower respiratory tract samples from patients that meet the CDC criteria[23].
In addition to testing lower respiratory (bronchoalveolar lavage,sputum, tracheal aspirate), upper respiratory tract specimen(nasopharyngeal swab, oropharyngeal swab or nasal aspirate), CDC also recommends serum, stool, and urine samples for testing[24,25].Specimen’s collection should be done as soon as possible once a patient is identified regardless of symptom onset.
Special attention should be attached to the patient with signs and symptoms of pneumonia, travel history to involved areas, or close contact with people with confirmed infection[26]. Suspected cases of COVID-19 infection should be reported to local health organizations.Then, it will be determined if the case meets the criteria for a patient under investigation for COVID-19. Samples should be obtained from these cases for routine testing of respiratory pathogens along with COVID-19 virus. WHO has taken a three-pronged approach to enhance the diagnostic capacity of the virus, including (1) Forming a network of referral laboratories with expertise in molecular testing of coronaviruses; (2) Strengthening the capacity for rapid detection of the virus; (3) Ensuring test availability through working with commercial and non-commercial agencies that can manufacture the kits.
4. Treatment
COVID-19 infection management must be started as quickly as possible when a suspected patient is identified. There is no specific antiviral treatment recommended currently for COVID-19[27].However, it is recommended supportive care to relieve the symptoms and to ensure appropriate infection control. General measures that are recommended for other severe acute pulmonary infections must be followed including proper infection control measures (airborne,droplet and contact precautions), supportive therapies [hydration,analgesics, antipyretic, intubation, respiratory support, empirical antibiotics (in case of bacterial infection)], managing sepsis if present, and also close monitoring[27,28]. Home management may be appropriate for patients with a mild infection, and adequate measures should be taken to keep the patients in isolation. Management of such patients should be focused on preventing the spread of infection, and monitoring for clinical symptoms and promptly visiting a hospital if the condition deteriorates. In severe cases,treatment should be given to the care of vital organ functioning.
Though no antiviral treatment for any coronavirus infection has been validated to be effective, a combination of lopinavir and ritonavir for SARS patients, a trial of interferon α 2B, lopinavir, and ritonavir among non-human primate models infected with MERS,and a broad-spectrum antiviral nucleotide analog or remdesivir for both MERS and SARS infections inin-vitroand animal models,were proved to be effective, which have been approved for use by Food and Drug Administration for the ongoing COVID-19 pandemic[29,30]. Another drug that has been used widely is chloroquine or hydroxy chloroquine, as some reports showed its efficacy in suppression of viral replication[31]. Moreover, in the outbreak of SARS infection, pegylated interferon-α suggested restraining effect against viral replication[32]. Research studies with a combination of corticosteroids and interferon against the SARS virus suggested that these agents can be used without any recognizable adverse effects.
Investigational agents have been explored for the antiviral treatment of COVID-19.In vitrostudies showed that remdesivir is active against the COVID-19 virus, and the manufacturers of remdesivir are conducting a randomized clinical trial in China to further evaluate its efficacy[33]. A new investigational drug application for compassionate use of remdesivir was mentioned in a case report about coronavirus in the United States[34]. However, there is no published evidence that remdesivir or any other antiviral drugs investigated for SARS and MERS can result in effective outcomes for treating COVID-19 virus. WHO and CDC suggested not using glucocorticoids in patients with COVID-19 pneumonia unless there are other indications such as chronic obstructive pulmonary disease because of persuasive evidence of short and long-term adverse effects of glucocorticoids[34].
It is reported of the application of hydroxychloroquine in pre or post-exposure prophylaxis, due to its function to reduce viral shedding duration or restraining viral replication. but further controlled clinical trials should be conducted before wide promotion[35].
Finally, a (human) monoclonal antibody has been developed recently. 47D11 binds a conserved epitope on the spike RBD explaining its ability to cross-neutralize SARS-CoV and SARSCoV-2, by a mechanism that is independent of receptor-binding inhibition[36]. This will be useful for the development of antigen detection tests and serological assays targeting SARS-CoV-2. These antibodies can alter the course of infection by virus clearance or protection of an uninfected host from the virus.
5. Prevention
There is currently no approved vaccine available to prevent COVID-19 infection but some attempts are in progress such as in the United States an experimental vaccine against COVID-19 was tested on monkeys[35]. Before the invention of an effective vaccine,the best practice to prevent COVID-19 is by avoiding exposure to the virus. For COVID-19 prevention, CDC recommends the same approaches that are designed to help prevent the spread of respiratory viruses[36], which include washing hands with soap and water for at least 20 seconds; applying alcohol-based sanitizer that has at least 60% alcohol; avoiding touching the eyes, nose, and mouth with unwashed hands; strictly control visitor access to the facility treating coronavirus patients; training and educating the public and healthcare personnel; putting on a surgical mask and hand hygiene;avoiding close contact with anyone who has fever and cough; staying home when you are sick; covering the nose or mouth when coughing or sneezing with a tissue; throwing the coughed or sneezed tissue in the trash; disinfecting the contaminated surfaces repeatedly; avoiding close contact with live or dead animals or their droppings; avoiding visiting wet markets and live poultry markets; tightening regulation of raw milk, meat or animal organs with special care and avoiding consuming undercooking meat and raw milk. Based on WHO recommendations, any non-essential travel to high-risk areas should be restricted, and travelers should adhere to typical precautions while traveling to or from affected areas. Extreme care must be taken by travelers who have medical conditions and at high risk for developing coronavirus complications including adults ≥50 years old,pregnant women, persons with cardiopulmonary diseases, diabetes,liver, blood, kidney disorders, and weakened immune system due to HIV, cancer or other diseases[37].
Screening health statuses of travelers, health care workers, and other people with close contacts with confirmed patients is an efficient measure to contain the spread of infection. These measures are being actively pursued in several countries, including China,the UK, Singapore, Thailand, Malaysia, South Korea, Japan, Italy,Australia, Iran, and the USA[38-40].
6. Discussion and conclusion
Both SARS and COVID-19 outbreaks are supposedly originated from wild animals. Scientists believe that bats are the reservoirs for the SARS coronavirus and then spread to the civet cat before transmission to humans. It is suggested that COVID-19 shares more than 70% genomic similarity with SARS-CoV, and there is speculation that both viruses use the same receptors for viral entry into the host cells[41]. MERS virus, another beta coronavirus, appears to be distantly related to COVID-19 virus.
SARS spread to 30 countries infecting 8 000 people and causing 774 deaths (with a fatality rate of 9.6%)[42]. SARS ended up infecting 5 237 people in mainland China, and the number has been surpassed by COVID-19 on January 29, 2020, when Chinese officials confirmed 5 974 cases of the COVID-19[43]. One day later,on January 30, 2020, the novel coronavirus cases surpassed 8 096 worldwide, the total SARS cases in 2003. MERS infected 2 494 people (2012) and killed 858 people with a fatality rate of 34.4%[44].Until 16 May 2020, COVID-19 has infected more than 4 425 485 people and killed 302 059 people and thus surpassing the total numbers of SARS and MERS[45]. Every year an estimated more than 300 000 people died in the world due to complications from seasonal influenza viruses, implying 1 781 confirmed cases and 795 deaths per day.
One of the biggest differences between SARS and COVID-19 is the speed at which COVID-19 was reported and identified. This led to a quick implementation of screening measurements to restrict the spread of the infection. However, this novel coronavirus induced syndrome is more asymptomatic in the early stage compared to SARS. COVID-19 is now considered as an alarming threat to global health and requires a prompt response from the public, scientific and healthcare communities for timely detection, management, and prevention of the infection.
Conflict of interest statement
The authors report no conflict of interest.
Acknowledgments
The authors gratefully thank the Department of Infectious Diseases of Babol University of Medical Sciences, Iran.
Authors’ contributions
Z.M.A. and S.E. conceived the study; M.M.A., M.J., V.K.,V.K.R.V, A.H.H., and A.B. collected all data; S.E. and A.B. drafted the manuscript; and all authors commented on the drafts of the manuscript and approved the final draft of the paper.
 Journal of Acute Disease2020年5期
Journal of Acute Disease2020年5期
- Journal of Acute Disease的其它文章
- Using point-of-care ultrasound in ocular emergencies: A mini review
- An epidemiological report on the burden and trend of injuries in the Philippines from 2011 to 2018
- Epidemiological profile and management of acute pyelonephritis in the emergency department of a tertiary hospital: A retrospective observational study
- Evaluation of the neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and monocyte lymphocyte ratio for diagnosis of testicular torsion
- Effect of proximal femur nail anti-rotation on unstable intertrochanteric fractures: A prospective observational study
- Antibacterial activity of plant extracts in different solvents against pathogenic bacteria: An in vitro experiment
