Retrievable puncture anchor traction method for endoscopic ultrasound-guided gastroenterostomy: A porcine study
Guo-Xin Wang, Kai Zhang, Si-Yu Sun
Abstract
Key words: Retrievable puncture anchor; Endoscopic ultrasound; Endoscopic ultrasoundguided gastroenterostomy; Gastric outlet obstruction; Gastroenterostomy; Electrocauteryenhanced delivery of lumen-apposing metal stents
INTRODUCTION
Endoscopic ultrasound-guided gastroenterostomy (EUS-GE) has become an alternative to the surgical or standard endoscopic treatment methods for gastric outlet obstruction (GOO) when endoscopic stents cannot be placed. However, EUS-GE presents certain challenges and the current procedural methods have limitations. One source of difficulty during EUS-GE is the high mobility of the intestine, which can tent away during puncture with a needle or electrocautery-enhanced delivery of lumenapposing metal stents (ECE-LAMS). In order to improve EUS-GE, we developed a retrieval puncture anchor traction (RPAT) method for EUS-GE. We evaluated the feasibility of the RPAT method using a pig model.
MATERIALS AND METHODS
Animals
Six Bama mini pigs (15-20 kg) were selected to undergo the RPAT method of EUS-GE, which consisted of placing fully covered metal stents between the animals’ stomach and bowel walls. This pig model study was approved by the Ethics Review Committee and Ethics Committee of Shengjing Hospital of China Medical University (No. 2018PS482K).
Preoperative preparation
Each pig received a full liquid diet two days before the operation, and drinking water was forbidden the day before surgery. Based on the weight of each animal, propofol was intramuscularly injected for the induction of anesthesia. The pigs were placed on their left side. Venous access was established through the ear vein. The animals’ breathing was maintained by the insertion of a tracheal tube. The animals were monitored with continuous cardiopulmonary monitoring.
RPA
The RPA (Vedkang Inc., Changzhou, Jiangsu, China) is made up of two wires (Figure 1A). The direction of the anchor head can be altered by pulling the retrieval wire (Figure 1B). The anchor can be sent through the needle (Figure 1C and D)[1].
Operative procedure
RPAT method of EUS-GE: First, a gastroscope was advanced into the duodenum. A guidewire (0.035 inch/480 mm; Cook Medical Inc., Bloomington, IN, United States) was sent through the working channel of the gastroscope. A nasal biliary drainage catheter (NBDC) (7Fr; Cook Medical Inc., Limerick, Ireland) was then inserted along the guidewire. Next, the gastroscope and guidewire were removed, leaving the NBDC in place. Approximately 200 mL of saline solution containing methylene blue was injected into the bowel through the NBDC to optimally dilate the bowel and facilitate the subsequent EUS-guided puncture. Methylene blue was added to confirm bowel placement by aspirating the blue saline solution with a needle (described below) before the ECE-LAMS insertion. A longitudinal (linear) ultrasound endoscope (EG-3830-UT; Pentax Japan) was inserted into the stomach and the puncture area (i.e., the closest area between the intestine and the stomach) was marked. We used color Doppler to prevent vascular damage during the puncture. A needle (19-G, Boston Scientific Corp., Massachusetts, United States) was then passed through the working channel, and the intestine was punctured under EUS guidance (Figure 2A and B). After removal of the stylet, saline solution with methylene blue was drawn out and a contrast agent was injected for endoscopy. The RPAT was then passed through the needle (Figure 2C and D) and inserted into the small intestine. The needle and the echoendoscope were removed over the wire of the RPAT. The small bowel was pulled with the anchor (Figure 2E and F). RPAT only pulls the intestinal canal during stent implantation to prevent free action, so the pulling action is gentle rather than violent. During the procedure, we did not observe the angle of the digestive tract wall due to stretching. The echoendoscope was reinserted into the stomach and the 19-G needle was advanced through the working channel. Additional saline solution with methylene blue was injected through the NBDC using multiple 50-mL syringes if the bowel expansion was insufficient. The identified small-bowel loop was punctured under EUS guidance followed by aspiration of the methylene blue solution to confirm the correct puncture site when the small bowel was pulled with the anchor. The guidewire was inserted through the needle. Next, the needle was removed, and ECELAMS (16 mm/30 mm; Micro-Tech/Nan Jing Co., Ltd. Nanjing, Jiangsu, China) was inserted over the guidewire and released into the small intestine until the distal flares were fully open under EUS guidance (Figure 2G and Figure H). Through endoscope monitoring, while keeping the proximal section in the field of vision, the rest of the stent was released. Finally, the stent was confirmed and leakages were excluded by EUS (Figure 3A and B). After the operation, the retrieval cord was pulled using a pair of forceps (Figure 2I and J). This changed the direction of the anchor and made it easy to remove (Figure 2K and L).
RESULTS
All six operations were successfully performed. Each metal stent was placed successfully, and the EUS-GEs selected the body of the stomach as the puncture site in each case. All RPAs were able to be removed with no complications. No animal showed signs of peritonitis or any other complications after the procedure. Within a few hours after anesthesia, the pigs were able to tolerate a regular diet. No clinically significant adverse reactions were observed in the following weeks. Four weeks after EUS-GE, it was confirmed that the stents remained intact, in their original positions, with no hyperplastic tissue overgrowth or ingrowth in any animal. The fistulas were mature in all six pigs and all the stents were easily removed using forceps without significant bleeding. On autopsy, there were no signs of bleeding, organ damage, or peritonitis, and complete adhesions of the intestines to the stomach wall were seen.
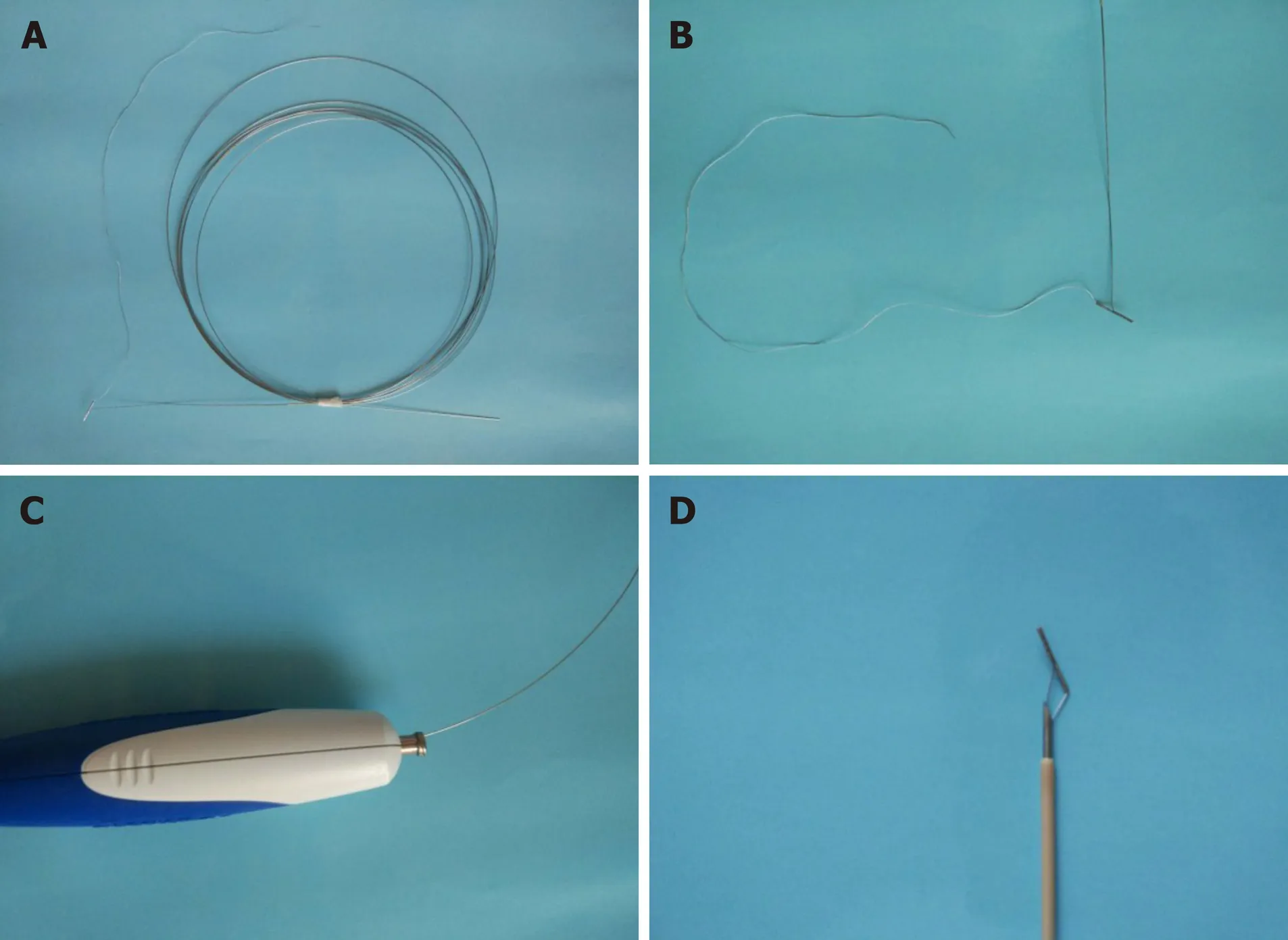
Figure 1 The retrievable puncture anchor. A: The retrievable puncture anchor (Vedkang Inc., Changzhou, Jiangsu, China) in its entirety; B: The head of the anchor; C and D: A view of the passage of the anchor along the shaft of the puncture needle.
DISCUSSION
Surgical GE is a traditional choice for patients with malignant GOO. Intraluminal selfexpanding metal stents (SEMS) placed through the endoscope is another method to relieve GOO[2-4]. Surgical GE is superior to endoscopic SEMS placement in terms of lumen patency[4]; however, adverse events including delayed gastric emptying, extended hospital stay, increased cost of hospitalization, and delayed treatment of cancer are not uncommon[4,5]. The main limitation of placing SEMS endoscopically is repeated occlusion of the lumen, which is mainly due to tumor ingrowth, overgrowth, or both[4]. In general, EUS-GE can provide durable lumen patency, avoid the risk of tumor overgrowth and ingrowth, and avoids the need for surgery in patients with end-stage disease[6]. In 2002, Fritscher-Ravenset al[7]reported the results of EUS-GE using special compression buttons in pigs. In 2012, Binmoelleret al[8]described the use of a new intraluminal metal stent in a porcine model that was used to invent an EUSGE anastomosis. Recently, Itoiet al[9]used another endoluminal stent for EUS-GE.
The emergence of stents allows easier creation of anastomoses between the small intestine and the stomach wall. These stents are fully covered with flanges at either end to prevent leakage and migration. The EUS-GE technique using an echoendoscope is an excellent means of providing endoscopic access to the duodenal or jejunal lumen distal to obstructed intestinal segments. Various techniques have been used with EUS-GE[10], including antegrade EUS-GE (the “traditional/downstream” method and the “rendezvous method), retrograde EUS-GE, EUS-guided double-balloon-occluded GE, and direct EUS-GE[9,11-13].
One of the challenges associated with direct EUS-GE is the inability to puncture the small bowel, despite the use of an ECE-LAMS, resulting in it being pushed away rather than punctured[11]. Inadequate expansion of the small bowel or rapid dissipation of the infused contrast and methylene blue (due to peristalsis) can also be limiting factors[11]. The RPAT method for EUS-GE did not use glucagon or other antispasmodic drugs to reduce the mobility of the bowel. The RPA can pull the intestine closer to the stomach wall to reduce intestinal mobility. Compared with traditional EUS-GE, RPAT reduces the distance between the stomach and the bowel, and provides sufficient reverse tension during ECE-LAMS implantation. With sufficient opposite pullingforce, the ECE-LAMS is relatively easy to implant. The stomach wall and intestine of six experimental animals were clearly visible in the ultrasound field of view during the entire surgical procedure. We used an NBDC in the RPAT method to reduce the procedure time for EUS-GE, while avoiding the infusion of large amounts of saline into the intestine to prevent colon swelling. Prior to puncturing and guidewire insertion, a saline solution containing methylene blue was added in order to maintain the expansion of the bowel, provide a better field of view, and improve the safety of the operation.
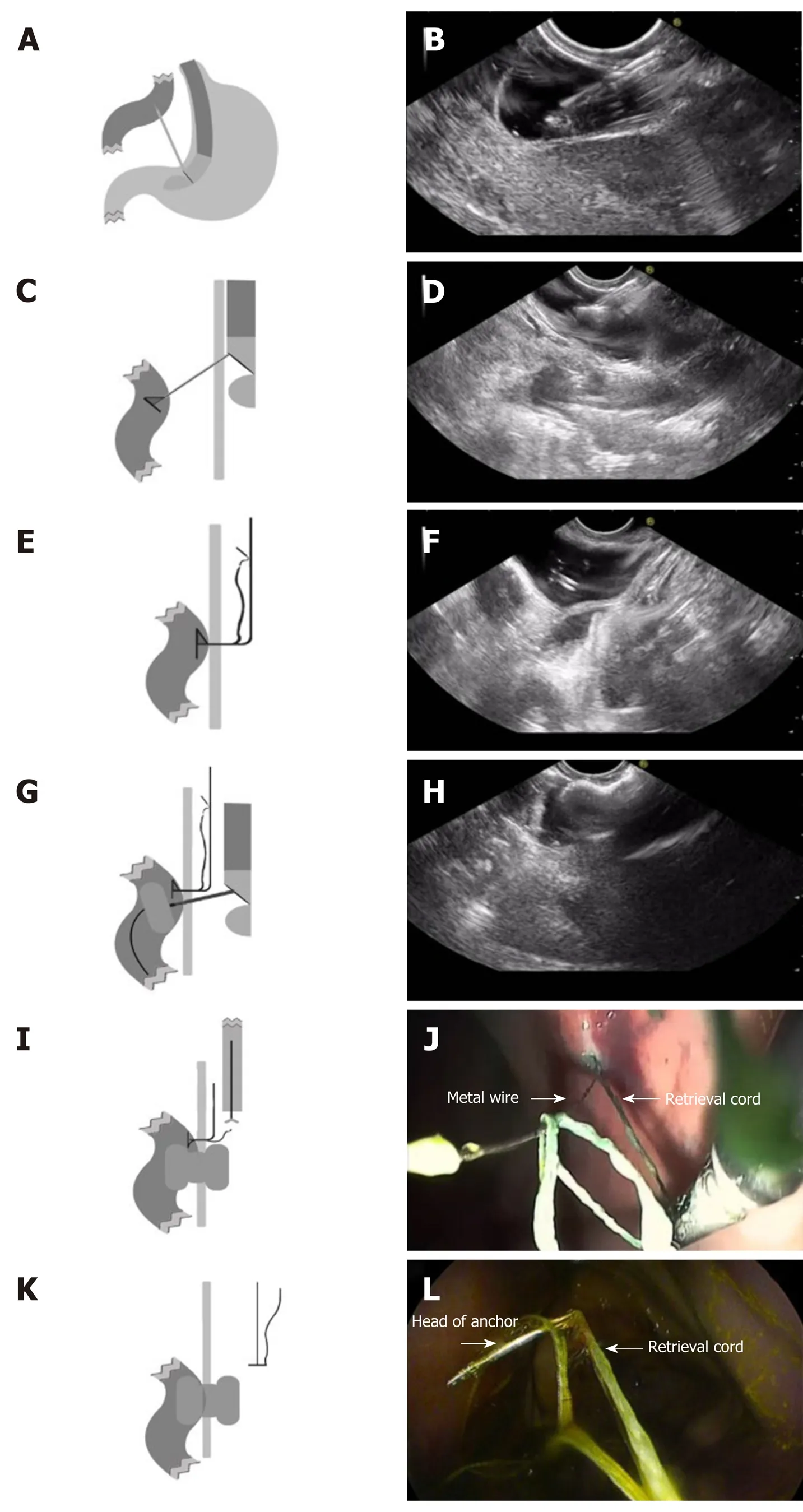

Figure 3 Endoscopic imaging of stent placement and anastomoses. A and B: Endoscopic imaging shows the well-reflected proximal flange of the stent immediately after deployment in the initial session; C: The stents were still in the same position 4 wk later and no traumatic changes were observed; D: A standard gastroscope was easily advanced through the anastomosis to observe the afferent and efferent bowel loops.
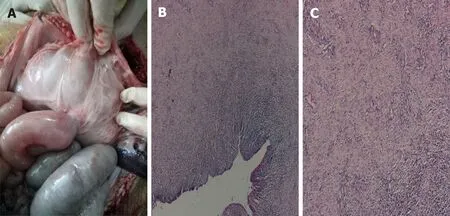
Figure 4 Autopsy and histological findings. A-C: Necropsy and histological evidence shows complete adhesion between the bowel and the stomach wall (20 ×).
In contrast to other techniques used with EUS-GE, the RPAT method for EUS-GE does not use a balloon, which reduces the difficulty of performing the operation as the advancement of the dilated balloon over the guidewire under fluoroscopic guidance can be quite challenging. Moreover, the RPAT method for EUS-GE is suitable for cases where the digestive tract is severely stenotic, allowing only the passage of liquid solution. The RPAT method for EUS-GE can help pull the intestine by pulling the anchor and can inflate the intestine by allowing the injection of saline solution through the NBDC at any time.
Although advanced EUS techniques and the availability of the proper equipment allow EUS-GE to be performed, the procedure is still in its early stages of development. This new technique is accompanied by technical issues requiring instrument modification that must be addressed prior to its clinical application[10,14-21]. Interventional EUS treatment is a newly developed method for the treatment of many diseases[9,19-22]. The small bowel has variable motility and thus tents away from the puncturing needle or the cautery tip of the ECE-LAMS. Hence, we have developed the RPAT method to improve the current EUS-GE method.
The success rate of EUS-GE using the RPAT method was 100% in our study. Remarkably, the retrievable puncture devices anchor to the bowel without causing tissue injury, and the RPA can also be removed without complications. Four weeks after the EUS-GE, it was found that all the stents remained in their original positions. Necropsy revealed complete adhesion between the intestinal wall and the stomach wall.
There are a few limitations in this study. First, the study consisted of a small number of animals. Second, the follow-up period was four weeks, limiting the evaluation of long-term observations and results. Lastly, the procedure outcomes and findings were not compared to those of other non-EUS-guided gastrojejunostomy techniques[8]. A larger study with a longer follow-up period that compares several gastrojejunostomy techniques is needed.
In general, we proved the technical success of the RPAT method used with EUS-GE in a pig model. The RPAT method for EUS-GE is promising as a minimally invasive treatment. Clinical prospective trials are warranted to verify the efficacy of this treatment method. We are convinced that this treatment method can be widely used in EUS-GE in the future.
ARTICLE HIGHLIGHTS
 World Journal of Gastroenterology2020年25期
World Journal of Gastroenterology2020年25期
- World Journal of Gastroenterology的其它文章
- Chinese expert consensus and practice guideline of totally implantable access port for digestive tract carcinomas
- Monoacylglycerol lipase reprograms lipid precursors signaling in liver disease
- Type I and type II Helicobacter pylori infection status and their impact on gastrin and pepsinogen level in a gastric cancer prevalent area
- Predictors of irreversible intestinal resection in patients with acute mesenteric venous thrombosis
- Multiphase convolutional dense network for the classification of focal liver lesions on dynamic contrastenhanced computed tomography
- Chronic atrophic gastritis detection with a convolutional neural network considering stomach regions
