Optimization on Extraction Conditions of Flavonoids from Edgeworthia chrysantha Lind1.
Pengfei CAO Huafen WU Yinhua CHEN
Abstract [Objectives] The extraction conditions of flavonoids from flowers of Edgeworthia chrysantha Lind1. were optimized.
[Methods] With ethanol as an extraction agent, firstly by single-factor tests, the best levels of ethanol concentration, material-to-liquid ratio, extraction temperature and extraction time were determined. Then the optimal extraction conditions were determined through the quadratic orthogonal regression rotatable design.
[Results] The optimal extraction conditions for flavonoids from E. chrysantha were∶material-to-liquid ratio 1∶15.4, extraction temperature 75℃, extraction time 120 min and ethanol concentration 75%, with which the highest yield of flavonoids was 6.02 mg/g.
[Conclusions] This study provides a theoretical basis for the further development and utilization of E. chrysantha flowers.
Key words Edgeworthia chrysantha Lind1.; Flavonoid; Extraction process; Optimization
Edgeworthia chrysantha Lind1. was first recorded in Quanfangpu. It is a plant of Thymelaeaceae, also known as Baiyishu and Xuehuashu, mainly distributed in the southern part of the Yangtze River Basin in China. E. chrysantha is an important economic crop in China, with a large planting area[1]. E. chrysantha is usually 1 to 2 m high and is branched in a trigeminal shape. The young branches have light yellow hairs[2]. The flowering season of E. chrysantha is in early spring, and the flowering period is three or four months. E. chrysantha has capitula, which are slightly longer than 1 cm, and have light yellow soft hairs densely distributed on the outer periphery and yellow inside. It is also known as Menghua and Dajiehua. E. chrysantha usually grows in areas with higher altitudes. If suitable growth conditions (including climate, temperature, moisture, sunlight, etc.) are given, there is not much difference in the growth status of E. chrysantha[3].
Previously, some countries have used E. chrysantha as an industrial raw material to carry out research. E. chrysantha is rich in fiber, and its content has reached 50%, so it is widely used in papermaking. Moreover, some people used E. chrysantha to develop pesticides and used it in agricultural production and achieved good results. In recent years, the demand for raw materials in the paper industry has decreased, and the pesticides have been gradually replaced by more superior drugs. Therefore, the current research on E. chrysantha mainly focuses on its pharmacological effects. The roots, stems, and flowers of E. chrysantha can be used as medicine. Its roots and stem bark have the effects of relaxing tendons, reducing swelling and relieving pain, and can be used for treating rheumatism. E. chrysantha also has a positive effect in the treatment of traumatic injuries. E. chrysantha flowers can also treat redness and pain in the eyes, and can nourish yin and regulate spirit[3-6].
At present, there are several types of compounds isolated from E. chrysantha: coumarin compounds, flavonoid compounds, sterol compounds, organic acids and nitrogen-containing compounds[23]. E. chrysantha is particularly rich in flavonoids, which are important medicinal ingredients in E. chrysantha. It is estimated that the plant can convert about 2% of carbon in photosynthesis into flavonoids, and most of flavonoids in E. chrysantha are also synthesized through this route[7].
There are two forms of flavonoids, one of which is the free form of glycosides, and the other is carbohydrate-combined glycosides. They all have a common mother nucleus[8]. Flavonoid compounds include flavonoids, isoflavones, flavonols, etc., of which hundreds are found in plants[9]. At the beginning of the last century, crude flavonoids were only used as dyes in various dyeing industries; and by the middle of the last century, some countries used quercetin and rutin in clinical practice and achieved very amazing effects, and people gradually began to pay attention to flavonoids and their clinical application[10].
Flavonoids have a variety of pharmacological functions, mainly including: resisting bacteria, resisting inflammation, preventing allergies, delaying aging, enhancing cardiovascular function, enhancing immunity, mediating the endocrine system, protecting the liver, resisting virus, resisting tumor, and treating chronic prostatitis[5]. At present, the research prospects of flavonoids are very broad. Its products such as flavonoid chewing gum and toothpaste, various ginkgo leaves and hawthorn leaves rich in flavonoid compounds have huge sales in the market, creating a considerable profit[11].
There are many methods for extracting flavonoids from plants. Common methods include hot water extraction, ethanol extraction, acetone extraction, lye extraction, macroporous resin adsorption, ultrasonic method, microwave extraction, and enzymolysis[12]. The hot water extraction method can reduce the cost of solvents. Because the polarity of water will dissolve other polar impurities, it is mainly used in the crude extraction process. Acetone is mainly used in extraction of fat-soluble flavonoids[5]. Macroporous resin adsorption method has significant reproducibility, and can avoid the difficulty of recycling, so it is widely used in the extraction of flavonoids[9]. Microwave extraction method and ultrasonic method are the latest flavonoid extraction methods, with a higher extraction rate, but both need to be improved due to immature technical aspects[11]. Enzymolysis is an auxiliary method for extracting flavonoids, and the main function is to dissolve the cell wall to dissolve the flavonoids[12]. In order to obtain the highest extraction rate, it is usually necessary to compare the effects of different extraction methods on the extraction rate of flavonoids and select the optimal combination of methods. Zhang[13] extracted total flavonoids in Stellera chamaejasme by cold immersion combined with ultrasonic extraction method and continuous heating-reflux extraction method, as well as subsequent polyamide adsorption method, alkaline extraction-acid precipitation method and solvent extraction method, and obtained different flavonoid contents, respectively. In this study, the ethanol extraction method was adopted. According to the principle of like dissolves like, flavonoids could be dissolved in ethanol well, and because ethanol extraction method is cheaper and safer than other methods, it is also the most common method for extracting flavonoids.
Due to the universality of flavonoids in plants, many scholars have studied the best extraction process of flavonoids in plants such as lotus, citrus, Kalimeris, honeysuckle and bamboo leaves, but the extraction process of flavonoids in E. chrysantha flowers is rarely reported. Therefore, in this study, the extraction process of flavonoids from E. chrysantha flowers was investigated to obtain the best extraction conditions of flavonoids from E. chrysantha flowers. This study provides a theoretical basis for the further development and utilization of E. chrysantha flowers.
Materials and Methods
Materials, instruments and reagents
Materials
E. chrysantha flowers: The flowers were collected from Chashuping Village, Gaoping Township, Suichang County, in March 2018. The samples were dried at 60 ℃, pulverized into particles with a biological pulverizer and sieved through an 80-mesh sieve for use.
Reagents
Rutin, 95% ethanol, aluminum nitrate, sodium nitrite, sodium hydroxide and hydrochloric acid were all analytically pure.
Instruments
scout SL electronic balance (Shanghai Shenan Medical Instrument Factory); HHS electric heating constant temperature water bath (Shanghai Boxun Industry & Commerce Co., Ltd.); TDL240B centrifuge (Taicang Experimental Equipment Factory); 723C visible spectrophotometer (Shanghai Youpu Scientific Instrument Co., Ltd.).
Determination of flavonoid content
The determination of flavonoids in the flowers of E. chrysantha was carried out by sodium nitrite-aluminum nitrate colorimetric method[13].
Solution preparation
Preparation of rutin standard solution: A certain amount of rutin standard product (20 mg) was accurately weighed and placed in 100 ml volumetric flask. It was dissolved with 60% ethanol and diluted to constant weight. A certain amount of the solution (25 ml) was accurately measured and added into a 50 ml volumetric flask, diluted to constant volume, and shaken uniformly, obtaining a standard solution containing rutin at 0.1 mg/1 ml.
Preparation of 1 mol/L sodium hydroxide solution: A certain amount of sodium hydroxide (20 g) was accurately weighed, dissolved in 500 ml of distilled water, and stored in a brown bottle. The solution could be stored at room temperature for one month.
Preparation of 10% aluminum nitrate solution: A certain amount of aluminum nitrate (10 g) was accurately weighed and dissolved in 90 ml of distilled water, and stored in a brown bottle. The solution could be stored at room temperature for one month.
Preparation of 5% sodium nitrite solution: A certain amount of sodium nitrite (5 g) was accurately weighed and dissolved in 95 ml of distilled water. The solution could be stored at room temperature for one month.
Plotting of standard curve
Certain amounts of the standard solution (0.0, 2.5, 5.0, 7.5, 10.0 and 12.5 ml) were accurately measured, and added in 25 ml volumetric flasks, respectively. 30% ethanol was added to each of the volumetric flasks to make up to 12.5 ml, followed by adding 5% sodium nitrite solution (0.75 ml), shaking and standing for 5 min. Then, 0.75 ml of 10% aluminum nitrate was added into the solutions, which were stood for 5 min. Next, the solutions were added with 10 ml of 1 mol/L sodium hydroxide solution accurately and diluted to constant volume with 30% ethanol. With the first volumetric flask as a blank, the absorbance was determined at 510 nm. A standard curve was drawn with absorbance as the ordinate (y) and rutin concentration as the abscissa (x). The regression equation was: y=7.039 8x-0.006 6, R2=0.982 2, wherein x is the concentration of rutin (mg), and y is the absorbance value.
Determination of flavonoids in E. chrysantha
The absorbance value of flavonoids in each sample extract was determined by the same method, and the content of flavonoids in E. chrysantha was calculated according to the regression equation.
Extraction rate of flavonoids (E. chrysantha flower powder mg/g)=(Flavonoid weight mg/E. chrysantha flower weight g)
Effects of single factors on extraction rate of flavonoids
Effect of ethanol concentration on extraction rate of flavonoids
Ethanol concentration was adjusted to 55%, 65%, 75%, 85%, and 95%, respectively. A certain amount of E. chrysantha flower powder (2 g, accurate to 0.001) was weighed, and added in clean conical flasks, respectively. Into the conical flasks, 30 ml of ethanol solutions at different concentrations were added, followed by shaking well. The extraction systems were stood at room temperature for 10 min, extracted at 65 ℃ for 1.5 h, and centrifuged (4 000 r/min, 15 min). The supernatants were collected and determined at 510 nm for flavonoid content.
Effect of material-to-liquid ratio on extraction rate of flavonoids
A certain amount of E. chrysantha flower powder (2 g, accurate to 0.001) was weighed, and added into five conical flasks, respectively. 75% ethanol was added according to 1∶11, 1∶13, 1∶15, 1∶17 and 1∶19, followed by shaking well. The extraction systems were stood at room temperature for 10 min, extracted at 65 ℃ for 1.5 h, and centrifuged (4 000 r/min, 15 min). The supernatants were collected and determined at 510 nm for flavonoid content.
Effect of extraction temperature on extraction rate of flavonoids
A certain amount of E. chrysantha flower powder (2 g, accurate to 0.001) was weighed, and added in clean conical flasks, respectively. Into the conical flasks, 30 ml of ethanol solution was added, followed by shaking well. The extraction system was stood at room temperature for 10 min, extracted in water baths at 60, 65,70, 75 and 80 ℃ for 1.5 h, respectively, and centrifuged (4 000 r/min, 15 min). The supernatants were collected and determined at 510 nm for flavonoid content.
Effect of extraction time on extraction rate of flavonoids
A certain amount of E. chrysantha flower powder (2 g, accurate to 0.001) was weighed, and added in clean conical flasks, respectively. Into the conical flasks, 30 ml of ethanol solution was added, followed by shaking well. The extraction system was stood at room temperature for 10 min, extracted in a water baths at 70 ℃ for 1, 1.5, 2, 2.5 and 3 h, respectively, and centrifuged (4 000 r/min, 15 min). The supernatants were collected and determined at 510 nm for flavonoid content.
Quadratic regression rotatable orthogonal design
On the basis of single factor research, using ethanol concentration, temperature, time, and material-to-liquid ratio as experimental factors, a quadratic regression orthogonal rotatable design test was conducted, and the yield of flavonoids was used as an index to optimize the extraction parameters of flavonoids in E. chrysantha.
Results and Analysis
Effects of single factors on extraction rate of flavonoids
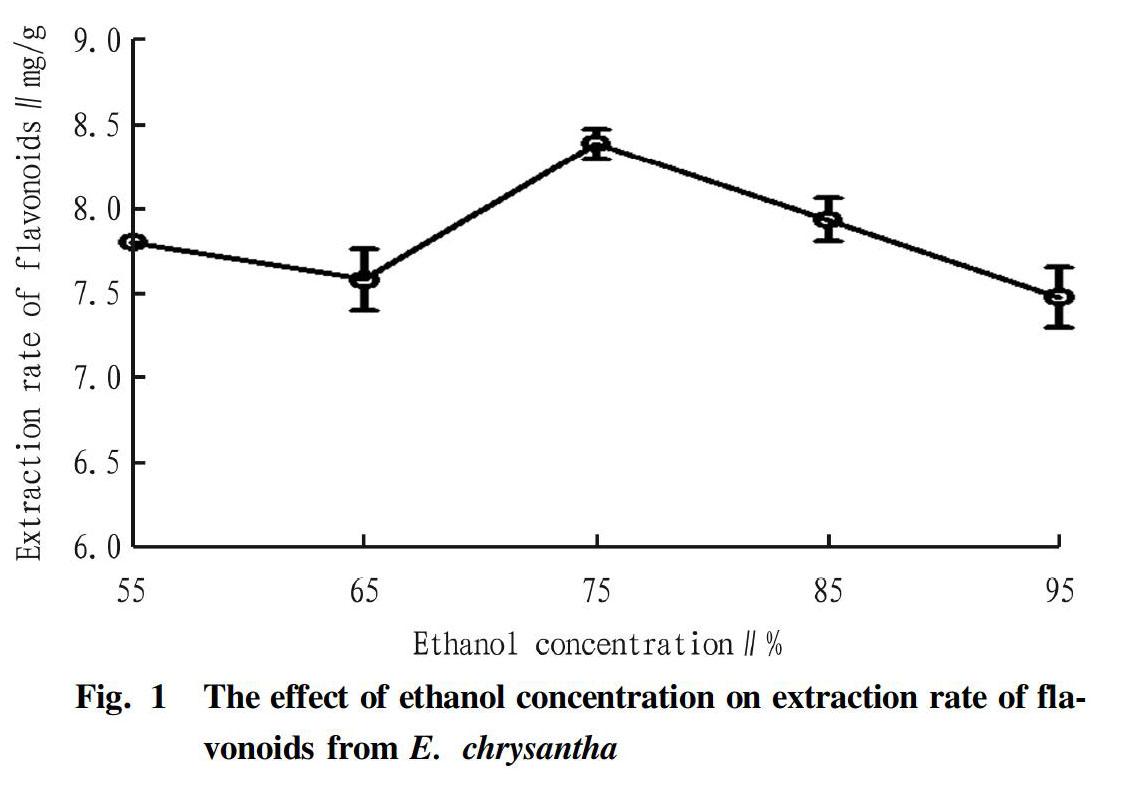
Effect of ethanol concentration on extraction rate of flavonoids
The effect of ethanol concentration on the extraction rate of flavonoids is shown in Fig. 1. When the ethanol concentration was between 55% and 65%, there was no obvious change in the extraction rate of flavonoids; and when the ethanol concentration was between 65% and 75%, the extraction rate of flavonoids gradually increased with the increase of ethanol concentration, and reached a maximum close to 8.5 mg/g at 75%. According to the principle of like dissolves like, flavonoids are easily soluble in ethanol, so within a certain range, increasing the concentration of ethanol will help the dissolution of flavonoids. When the ethanol concentration reached 75%, the extraction rate of flavonoids was maximum; with the further increase of ethanol concentration, the extraction rate of flavonoids from E. chrysantha decreased instead, because too high ethanol concentration caused impurities other than flavonoids to be dissolved out too much, thus hindering the dissolution of flavonoids[14]. In this test, ethanol concentrations of 55%, 65%, 75%, 85% and 95% were finally selected as the five levels of ethanol factor in the orthogonal test.
Pengfei CAO et al. Optimization on Extraction Conditions of Flavonoids from Edgeworthia chrysantha Lind1.
Effect of material-to-liquid ratio on extraction rate of flavonoids
The effect of material-to-liquid ratio on flavonoid extraction rate is shown in Fig. 2. When the material-to-liquid ratio was in the range of 1∶11-1∶15, the extraction rate of flavonoids was relatively stable. When the material-to-liquid ratio increased from 1∶13 to 1∶15, the extraction rate of flavonoids increased. It might be because as the content of the solvent increased, the contact area of ethanol and flavonoids increased. Therefore, increasing the amount of extracting liquid in a certain range helped to increase the yield of flavonoids. When the material-to-liquid ratio was 1∶15, the extraction rate of flavonoids from E. chrysantha flowers was the largest, reaching 10.012 mg/g. However, when the material-to-liquid ratio reached 1∶15, the extraction rate of flavonoids decreased instead, which might be because many impurities were dissolved from the plant, preventing the dissolution of flavonoids[15]. Therefore, the five levels of material-to-liquid ratio in the orthogonal test were 1∶11, 1∶13, 1∶15, 1∶17 and 1∶19.
Effect of extraction temperature on extraction rate of flavonoids
The effect of extraction temperature on the extraction rate of flavonoids in E. chrysantha is shown in Fig. 3. In the range of 50-70 ℃, as the temperature increased, the flavonoid content also continued to increase, which might be because high temperature was conducive to molecular movement and dissolution of flavonoids. When the extraction temperature was 70 ℃, the content of flavonoids in E. chrysantha flower reached a maximum, which was 10.119 mg/g. However, when the temperature continued to increase, the content of flavonoids decreased with the temperature increasing, which might be due to the oxidation of flavonoid compounds[16]. Therefore, 60, 65, 70, 75 and 80 ℃ were set as the five levels of extraction temperature in the orthogonal test.
Effect of extraction time on extraction rate of flavonoids
Extraction time is a very important factor affecting the extraction rate of flavonoids. It could be seen from Fig. 4 that when the extraction time increased from 1 to 2 h, the extraction rate of flavonoids significantly increased, because a sufficient reaction time was required for the full dissolution of flavonoids. The best extraction time was 2 h. When the extraction time continued to increase, the extraction rate decreased, which might be due to some negative reactions caused by the long extraction time[17]. Therefore, the time of the orthogonal test was determined to be 1, 1.5, 2, 2.5 and 3 h.
Quadratic regression rotatable orthogonal design to determine the best extraction conditions
Test scheme and results
On the basis of the above single factor test, with the extraction rate of flavonoids from E. chrysantha as the investigation index, and the four-factor five-level quadratic orthogonal rotatable design (full implementation) was used to optimize the optimal parameters of the flavonoid extraction process. Each factor had five levels, and there were a total of 36 combinations. The factors and coded levels are shown in Table 1, and the test results are shown in Table 2.
Establishment and check of regression equation
The test data in Table 2 was input into a computer, and fit by standard polynomial regression method through the DPS data processing system. The mathematical model of flavonoid extraction rate with such four factors as ethanol concentration, material-to-liquid ratio, extraction temperature and extraction time was:
Y=5.882 00+0.053 54X1+0.244 71X2+0.312 37X3+0.004 04X4-0.036 03X21-0.017 91X22+0.193 84X23+0.212 09X24-0.099 19X1X2+0.073 31X1X3+0.206 31X1X4+0.193 69X2X3-0.079 81X2X4+0.109 19X3X4
Through the analysis of variance of the quadratic regression model (Table 3), it was found that the first terms of the material-to-liquid ratio and temperature reached a very significant level, the second term of temperature reached a significant level, and the second term of time reached a very significant level. The interaction of ethanol concentration and extraction time and the interaction of material-to-liquid ratio and temperature were more obvious. Since the design was orthogonal and there was no correlation between the regression coefficients, the terms not signiciant at the α=0.10 significant level could be eliminated, and the simplified regression equation was:
Y=5.882 00+0.244 71X2+0.312 37X3+0.193 84X23+0.212 09X24+0.206 31X1X4 +0.193 69X2X3
Meanwhile, in order to test the validity of the regression equation, the regression equation should be tested for lack of fit. It could be seen from Table 3 that the lack of fit F1=2.247 00,
Main factor effect analysis
Due to the orthogonal design was adopted, all factors were processed by dimensionless linear coding, and all regression coefficients were irrelevant, so the absolute values of the regression coefficients could be used to directly compare the effects of various factors on the extraction rate of flavonoids. It could be seen from the significance level p of each regression coefficient in the analysis of variance table that the material-to-liquid ratio (X2) and temperature (X3) had a very significant effect on the soluble flavonoid yield at the a=0.01 level, while the ethanol concentration (X1) and time (X4) had a non-significant effect on the yield of flavonoids at the a=0.01 level. The F value can determine the influence of various factors on the extraction rate of flavonoids, and the greater the F value, the stronger the effect. Therefore, the effects of various factors on the extraction rate of flavonoids ranked as temperature>material-to-liquid ratio>ethanol concentration>time.
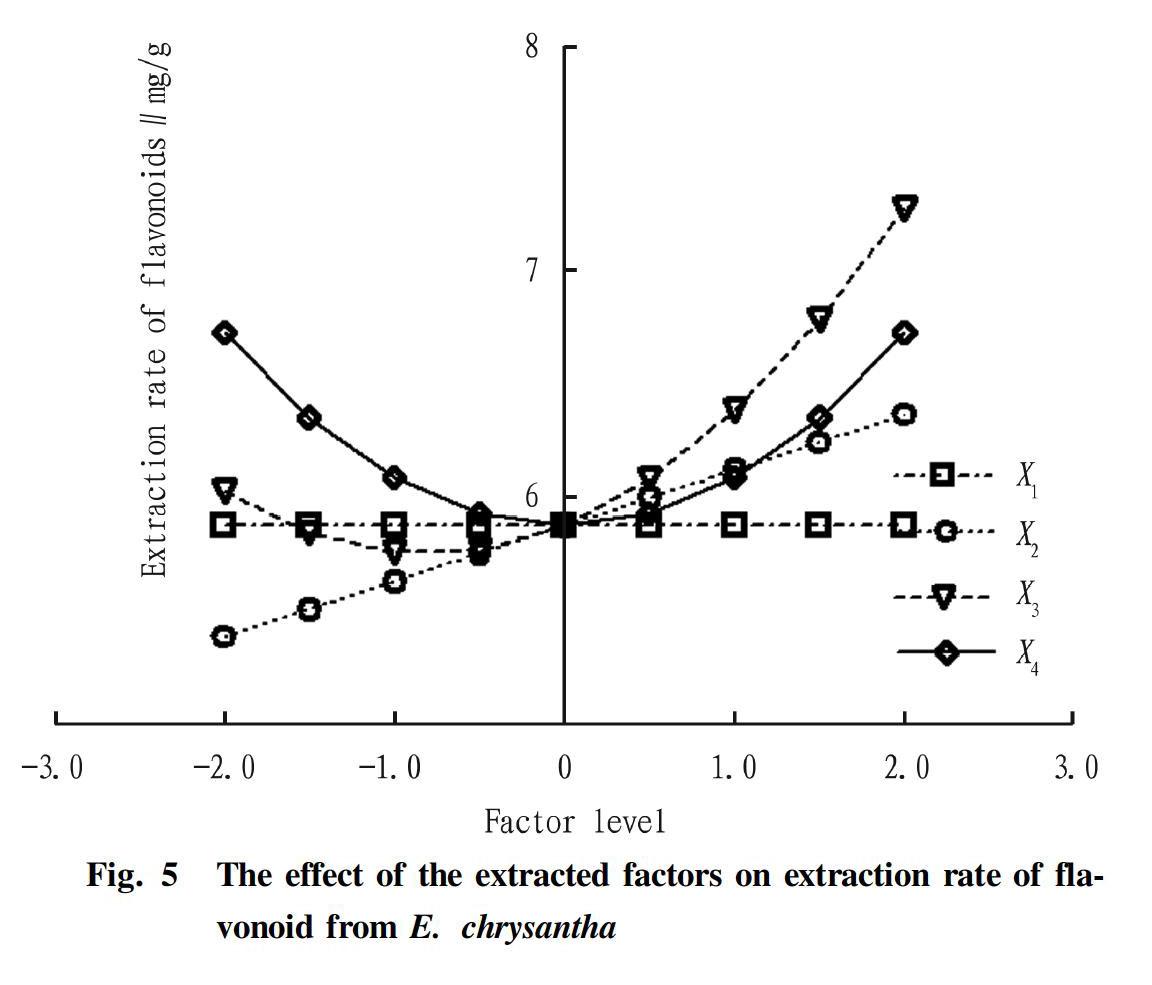
Single factor effect analysis
The single factor effect analysis is shown in Fig. 5. In the range of -2≤Xi≤2, the relationship between temperature and flavonoid yield was a parabola with an upward opening, indicating that there was a reasonable range for the effect of temperature on flavonoid yield, that is, the effect on flavonoid yield had a trend of decreasing first and then increasing. The relationship between material-to-liquid ratio and flavonoid yield was close to linear, indicating that these two factors played a significant role, and as the temperature and the material-to-liquid ratio increased, the increase in flavonoid yield was promoted. The relationship curve between ethanol concentration and flavonoid yield was relatively flat, indicating that the relationship between ethanol concentration and flavonoid yield was less significant than the effects of temperature and material-to-liquid ratio. The relationship between time and flavonoid yield was a parabola with an opening downward, indicating that there was a reasonable range for this factor, that is, the effect on flavonoid yield decreased first and then increased. The temperature curve was the steepest, indicating that temperature had the greatest influence on the extraction rate of flavonoids.
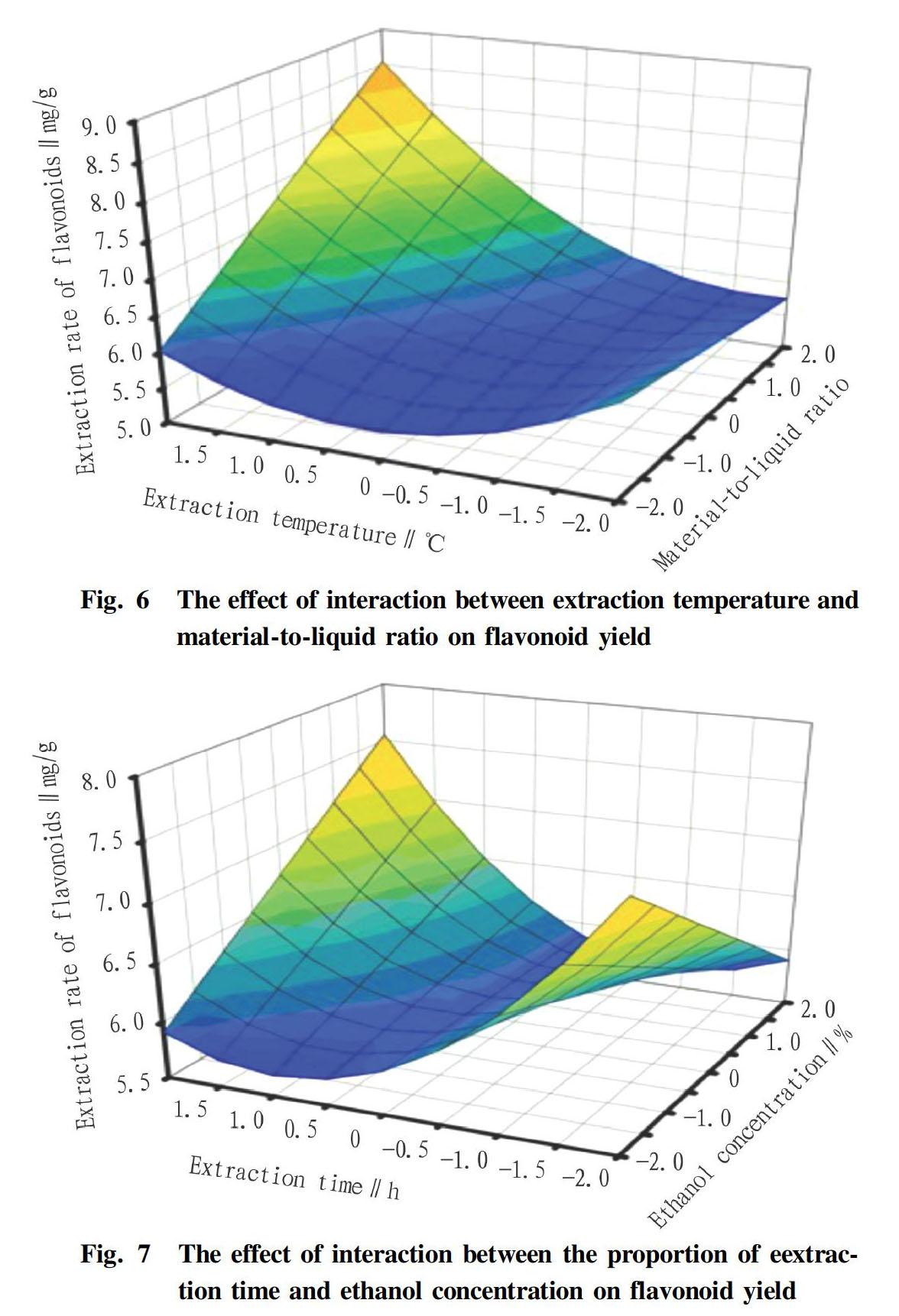
Interaction effect analysis
It could be seen from Fig. 6 that as the extraction temperature and liquid-solid ratio increased, the extraction rate of flavonoids increased first and then decreased. Of them, the effect of extraction temperature on the extraction rate of flavonoids was extremely significant, which was shown by a steep curve, and the extraction temperature had a greater impact when the material-to-liquid ratio was lower. In contrast, the effect of the material-to-liquid ratio on the extraction rate of flavonoids was secondary, which was reflected by a slightly smoother curve and the extraction rate of flavonoids increasing first and then decreasing with the increase of the material-to-liquid ratio, which might be due to the dissolution of other impurities in flowers, which hindered the dissolution of flavonoids. Therefore, if we want to obtain a higher content of flavonoids, we can appropriately reduce the volume of ethanol.
It could be seen from Fig. 7 that the impact surfaces of extraction time and ethanol concentration on the extraction rate of flavonoids were relatively gentle. The curvature of the three-dimensional curved surface can reflect the interaction between the factors, and the smaller the curvature, the weaker the interaction between the two factors[18]. It could be seen that the interaction between extraction time and ethanol concentration was not significant. A too-short extraction time was not conducive to the dissolution of flavonoids. When the time was extended, the yield of flavonoids increased, and the curve was steeper. The effect of ethanol concentration on the yield of flavonoids increased first and then decreased, which showed that too-low or too-high concentrations were not conducive to the extraction of flavonoids. The ethanol concentration had a greater effect on the extraction rate of flavonoids when the extraction time was short or long. Therefore, if we want to obtain a higher content of flavonoids, the conditions should be appropriately controlled to low concentration ethanol and short extraction time or high ethanol concentration and long extraction time.
Optimization and verification of extraction process conditions of flavonoids from E. chrysantha
The optimal ethanol concentration, material-to-liquid ratio, extraction temperature and extraction time were predicted through the model, and the optimal extraction conditions were obtained∶material-to-liquid ratio 1∶15.4, extraction temperature 75 ℃, extraction time 120 min, and ethanol concentration 75%, with which the predicted value of flavonoid extraction rate was 6.02 mg/g. Since the combined condition was not in the orthogonal test, a verification test was required. According to the obtained optimal process parameters, three parallel verification tests were carried out, obtaining a determined value of 6.07 mg/g, which was only different from the predicted value by 0.03 mg/g. The verification test showed that the equation had a good predictability, and the deviation from the actual value was small, indicating that the model was reliable.
Discussion
In this paper, the extraction process of flavonoids from E. chrysantha was studied by quadratic orthogonal rotatable design. Compared with the traditional single factor test and orthogonal test method, the quadratic orthogonal design not only retains the advantages of the two, but also facilitates calculation and reduces the number of times[19]. According to the principle of like dissolves like, flavonoids are easily soluble in ethanol, which is beneficial to the extraction of flavonoids[20]. Compared with other methods, ethanol extraction has the advantages of low cost and high safety, and it is also a more commonly used flavonoid extraction method. The test results showed that the optimal extraction process conditions of E. chrysantha flavonoids were∶material-liquid ratio 1∶15.4, extraction temperature 75 ℃, extraction time 120 min, and ethanol concentration 75%, with which the flavonoid extraction rate was 6.07 mg/g. The effects of various factors on flavonoid yield ranked as: temperature>material-liquid ratio>ethanol concentration>time.
Effect of extraction temperature on extraction rate of flavonoids
If the temperature is too low, it is not conducive to the dissolution of flavonoids, because appropriately increasing the temperature can speed up the movement of molecules, so that the solvent can quickly act on the solute, thereby speeding up the extraction rate of flavonoids in E. chrysantha[21]. However, a too-high temperature will also produce corresponding negative effects. For example, the structure of some flavonoids will be destroyed at high temperature, or a too-high temperature will increase the dissolution of other ester-soluble substances, which hinders the dissolution of flavonoids. These reasons may cause the extraction rate of flavonoids to decrease. According to different materials, the extraction temperature of flavonoids is also different. For example, Wu[22] carried out the determination of the main ingredients in Salsola collina Pall. as a raw material, used an orthogonal test to optimize the extraction process of flavonoids and determined an optimal extraction temperature of S. collina flavonoids at 60 ℃. Dai[23] extracted flavonoids from Lonicera caerulea and determined an optimal extraction temperature at 55 ℃. Zhou[24] used yam as a material to optimize the extraction of flavonoids, and found that the extraction temperature of 50 ℃could give a maximum content of flavonoids. The above-mentioned scholars studied the effect of temperature on the extraction rate of plant flavonoids, and found that with the temperature increasing, the extraction rate of flavonoids all showed a trend of first rising and then decreasing. Because there are very few scholars currently studying the extraction process of E. chrysantha flavonoids, the best temperature for the extraction of E. chrysantha has not yet been documented. In this study, the extraction temperature of flavonoids from E. chrysantha flowers was optimized, and it was found that the effect of temperature on the extraction rate of flavonoids had a similar trend of first rising and then falling, and the optimal extraction temperature was determined to be 75 ℃. Compared with other materials, the extraction temperature of flavonoids in E. chrysantha was higher.
Effect of material-to-liquid ratio on extraction rate of flavonoids
Increasing the volume of ethanol is equivalent to increasing the contact area between the solvent and the solute. Therefore, increasing the volume of ethanol can appropriately increase the extraction rate of flavonoids, and can dissolve flavonoids with higher polarity. However, if the volume of ethanol exceeds a suitable volume, the reaction time will be increased accordingly, and meanwhile, the dissolution of other polar compounds will be increased, which will not only reduce the purity of flavonoids, but may also affect the dissolution of active ingredients. For example, Zhang et al.[15] used celery leaves as a raw material to extract flavonoids, and found that the extraction rate of flavonoids also increased correspondingly when the ratio of material to liquid gradually increased. When it reached 1∶40, the highest content of flavonoids was obtained, but with further increase in the volume of ethanol, it led to a decrease in extraction rate instead. Wu et al.[25] used bamboo leaves as a raw material to extract flavonoids by response surface method, and found that when the ratio of material to liquid increased from 1∶5 to 1∶20, the extraction rate of flavonoids increased significantly, but when the volume of ethanol continued to increase, the change rate of the extraction rate was very small, so the optimal material-to-liquid ratio was finally determined to be 1∶24.3. It could be seen that too high or too low material-liquid ratio is not conducive to the extraction of flavonoids. In this study, using E. chrysantha flowers as a raw material, it was found that increasing the volume of ethanol within a certain range could increase the extraction rate of flavonoids, and after exceeding a certain volume, it also led to a decrease in extraction rate. Compared with other plants, the optimal material-to-liquid ratio for extracting flavonoids from E. chrysantha flowers was relatively lower. The results of this study showed that the material-to-liquid ratio had a very significant effect on the extraction rate of flavonoids from E. chrysantha. From the economic point of view, the final optimum ratio of material to liquid was 1∶15.4.
Effect of ethanol concentration on extraction rate of flavonoids
Flavonoids are easily soluble in polar solvents, and the increase in ethanol volume fraction is conducive to the dissolution of flavonoids[25]. However, excessively high ethanol concentration will cause impurities other than flavonoids to dissolve too much, thus hindering the dissolution of flavonoid compounds, so a too-high ethanol concentration will reduce the extraction rate of flavonoids[14]. Of course, for different materials, different ethanol concentrations should be used. During the process of optimizing the extraction process of total flavonoids in honeysuckle, Li[20] found that when the ethanol concentration increased from 15% to 95%, the extraction rate of flavonoids increased, but with further increase of the ethanol concentration, the extraction rate hardly changed. Liu et al.[19] found in the process of optimizing the extraction process of Rosa banksiae flavonoids that the maximum content of flavonoids could be obtained when the ethanol concentration was 70%. Hou et al.[26] used mint leaves as a raw material to optimize the extraction process of total flavonoids by response surface methodology, and found that when the ethanol concentration increased, the extraction rate of flavonoids also increased accordingly, and 80% ethanol was finally selected. In this study, the material used was E. chrysantha flowers. It was found during the experiment that the concentration of ethanol relative to the temperature and the ratio of material to liquid had no significant effect on the extraction rate of flavonoids. Finally, it was determined that the optimal ethanol concentration for flavonoid extraction was 75%. Mao et al.[5] also used E. chrysantha as a material to extract flavonoids in a constant temperature water bath, and found that 75% ethanol concentration was the best, which is consistent with the research result in this study.
Effect of extraction time on extraction rate of flavonoids
Time is a significant factor in most flavonoid extraction process research. Generally, in the initial stage, sufficient extraction time is conducive to the dissolution of flavonoids, but too long time will lead to the appearance of other compounds[27], so the appropriate time for the extraction of flavonoids should be adjusted according to the corresponding materials. Gao et al.[18] found in the optimization of the extraction process of flavonoids from garlic that in the initial stage, the extraction rate increased with the increase of extraction time, but the extraction rate decreased after a certain time. Finally, 3.98 h was selected as the best time for the extraction of total flavonoids in garlic. Lin et al.[28] used the response surface method to optimize the extraction process of epimedium flavonoids. When the extraction time was 2 h, the flavonoid content was the largest, and there was little change afterwards. When the extraction time reached 2.5 h, the flavonoid extraction rate was found to decrease instead. Zhao et al.[29] optimized the extraction process of total flavonoids from sea buckthorn pomace, and also found that the extraction time had a tendency to increase first and then decrease. In this study, time had no significant effect on the extraction rate of flavonoids from E. chrysantha flowers, and had the least effect on extraction rate.
To sum up, the best extraction parameters of flavonoids in E. chrysantha flowers were∶material-to-liquid ratio 1∶15.4, extraction temperature 75 ℃, extraction time 120 min, and ethanol concentration 75%, with which the flavonoid extraction rate was 6.07 mg/g .
References
[1] LI ZG, LI XP, LIU WH, et al. Study on chemical constituents of fragrance from fresh flowers of Edgeworthia chrysantha[J]. Chemistry and Industry of Forest Products, 2004, 24(1): 83-86. (in Chinese)
[2] XU ZL, CAI ZH. Research progress of chemical composition and pharmacological action of Edgeworthia chrysantha[J]. Journal of Anhui Agricultural Sciences, 2011, 39(31): 19110-19111, 19114. (in Chinese)
[3] TONG SQ, JI Z, YE YJ, et al. Chemical constituents from Edgeworthia gardneri Flos[J]. Lishizhen Medicine and Materia Medica Research, 2006, 17(1): 44-45. (in Chinese)
[4] FENG WS, XIE XH, JI CR, et al. Pharmacological research on Edgeworthia chrysantha[J]. Journal of Henan college of Traditional Chinese Medicine, 1994, 9(5): 9-13. (in Chinese)
[5] MAI T, CHEN G, TONG SQ, et al. Studies on optimal extraction conditions of flavonoid from the flowers of Edgeworthia chrysantha[J]. Journal of Chinese Medicinal Materials, 2007, 30(1): 95-97. (in Chinese)
[6] YAN JZ, TONG SQ, CHU JJ, et al. Progress in chemical composition of Edgeworthia chrysantha[J]. Zhejiang Chemical Industry, 2003(8) : 1-2. (in Chinese)
[7] ZHANG HJ, ZHAO YY, OUYANG L. Study on the chemical composition of Edgeworthia chrysantha[J]. Natural Product Research and Development, 1997, 9(1): 24-27. (in Chinese)
[8] ZUO RH, WANG B, CHEN NF, et al. The effect of flavonoids of Pteridium aquilinum on growth performance of young wanxi white goose [J]. China Poultry, 2006, 27(6): 25-27. (in Chinese)
[9] WENG DB, GU JJ. Study on extraction and determination of flavonoids and polysaccharides from yellow skin seeds[J]. Journal of Jiangsu Institute of Education: Social Science, 2010. 2(26): 29-32. (in Chinese)
[10] ZHU Z, LENG JS. Optimization of extraction process of cactus flavonoids by response surface methodology[J]. Good Science, 2010, 31(12): 185-189. (in Chinese)
[11] WENG DB, ZHOU LN. Extraction separation of polysaccharides and flavonoids from Chrysanthemum mankingense[J]. Journal of Traditional Chinese Medicine University of Hunan, 2007, (27): 349-351. (in Chinese)
[12] CAI J, JING Q, WANG W, et al. Research progress of flavonoid extraction process[J]. Journal of Huaiyin Institute of Technology, 2003, 12(5): 83-85. (in Chinese)
[13] ZHANG RG, LIU XS, SISURONG HA. The influence of different extractions on total flavonoids in Stellera chamaejasme L[J]. Heilongjiang Animal Science and Veterinary Medicine, 2010(1): 24-27. (in Chinese)
[14] HU JL. Study on optimum extracting conditions of flavonoids from Myrica Ruba leaves [J]. Food Science, 2003, 24(1): 96-99. (in Chinese)
[15] ZHANG G, YANG TS, LIU JG, et al. Study on extracting flavonoids from celery[J]. Food Science, 2002, 23(8): 121-125. (in Chinese)
[16] WU HY, GAN H, GU ZY, et al. Optimization of total flavonoids extraction from bamboo leaves by response surface methodology[J]. Food Science, 2008, 29(11): 196-200. (in Chinese)
[17] YANG GN, XU YW, SUN YL. Optimization of the adsorption technology of the total flavones from Hypericum Japonicum by response surface methodology[J]. Journal of Jishou University, 2012, 33(6): 89-93. (in Chinese)
[18] GAO SY, XU TT. Optimization of extraction technique of flavonoids from garlic by response surface methodology[J]. China Condiment 2013, 3(38): 44-49. (in Chinese)
[19] LIU YF, XIA HT, CHEN L, et al. Determination of total flavonoids in Rosa banksiae Ait by fluorescence spectrophotometry[J]. Journal of Anhui Agricultural Sciences, 2011, 39(11): 6384-6385. (in Chinese)
[20] LI XJ, PAN HL, CHEN H, et al. Optimization of ultrasonic-assisted extraction of total flavonoids from honeysuckle by response surface methodology[J]. Journal of Shaanxi Normal University, 2009, 37 (2): 81-90. (in Chinese)
[21] HUANG AG. Industrial research on extracting flavonoids from lotus leaf[J]. Science and Technology of Food Industry, 2000, 79(5): 14-15. (in Chinese)
[22] WU SG, CHEN G. Optimization of extraction process of flavonoids from Salsola collina[J]. Hubei Agricultural Sciences, 2012, 51(13): 2815-2817. (in Chinese)
[23] DAI XP. Study on extracting technology of total flavones from Lonicera edulis Turcz[J]. Journal of Anhui Agricultural Sciences, 2011, 29(2): 759-760, 763. (in Chinese)
[24] ZHOU HL, WANG LJ, XIE LL, et al. Study on the extraction technology of flavonoids from Chinese yam[J]. Hubei Agricultural Sciences, 2012, 51(17): 3824-3826. (in Chinese)
[25] WU HY, GAN H, GU ZY, et al. Optimization of total flavonoids extraction from bamboo leaves by response surface methodology[J]. Food Science, 2008, 29(11):196-200. (in Chinese)
[26] HOU XM, LI LX, ZHANG ZF, et al. Total flavonoids from Mentha haplocalyx Briq. leaves: Optimization of extraction process by response surface methodology and antioxidant activity[J]. Food Science, 2013, 34(6): 124-127. (in Chinese)
[27] HU JL. Study on optimum extracting conditions of flavonoids from Myrica ruba leaves[J]. Food Science, 2003, 24(1): 96-99. (in Chinese)
[28] LIN M, ZHANG F, WU DQ. Optimization of the extraction process for flavonoids in Herba Epimedii by response surface method[J]. Journal of Traditional Chinese Veterinary Medicine, 2012(6): 16-19. (in Chinese)
[29] ZHAO WJ, JIN SQ, RANA. MAMAT, et al. Optimization of extraction of total flavones from marc of sea buckthorn[J]. The Food Industry, 2013, 34(3): 86-89. (in Chinese)
- 农业生物技术(英文版)的其它文章
- Investigation on Agronomic Characters of Dwarf Mutant 778 in Broomcorn Millet (Panicum miliaceum L.) and Analysis of Its Sensitivity to GA
- Construction of Technology System on Development and Repropagation in Vitro of Several Cultivars in Pear
- Construction of Camellia oleifera Cultivation Standardization System
- Nutrients Determination in Nuts from Different Torreya grandis Cultivars
- Photosynthetic Physiological Response to Drought Stress of Populus euphratica at Different Ages in Minqin
- Effects of UV-B Radiation on the Activity of Antioxidants in Flue-cured Tobacco Leaves

