Investigation on Agronomic Characters of Dwarf Mutant 778 in Broomcorn Millet (Panicum miliaceum L.) and Analysis of Its Sensitivity to GA
Bo ZHANG Xiaojie LIU Yingjie GUO Xiaoping JIA Dezhi YANG Yuan ZHAO Lingfeng DAI Shujun KOU Xiaomei ZHANG Dianyun HOU Xuehai ZHU
Abstract In order to investigate the differences between agronomic traits of dwarf mutant and original material 260 and whether the cause of dwarf is related to GA synthesis or signaling pathway, this experiment used dwarf mutant 778 and its original material 260 as experimental materials. Morphological observation and determination were performed for agronomic traits on plant height, ear length, internode length, internode number, seed length, seed width and number of seeds in different growth periods and different concentrations. The plants were treated by GA spraying, and the changes of plant height, root length, stem width, leaf length and leaf width were measured. The results are as follows: ① The plant height of the dwarf mutant material was significantly different from that of the original high material, which was mainly caused by the difference between above-ground basal part and the length of the first and second elongation joints. ② Comparing and analyzing the differences of traits between dwarf mutant material 778 and original high material 260, it was found that the plant height, ear length, internode number, grain number per ear and internode length of dwarf mutant 778 were significantly lower than that of high stalk 260 (P<0.01), and the seed length of dwarf mutant 778 was significantly higher than that of high stalk 260 (P<0.05). ③ Different concentrations of gibberellin (0, 50, 100, 200 mg/L) had no significant effect on plant height and root length of dwarf mutant 778 (P>0.05). Different concentrations of gibberellin had significant effects on plant height, root length and sensitivity coefficient of high stalk 260 (P<0.05). And compared with the control group, all high materials 260 treated with different concentrations of gibberellin performed differently in plant morphology and growth potential. ④ Under the conditions of 100 and 200 mg/L GA, the difference of plant height between the dwarf mutant and the high stalk control decreased with time, and there was no difference at the end. There were no differences in sensitivity coefficient GRI between different concentrations of gibberellin treatment groups, indicating that the external gibberellin could restore the scorpion dwarf mutant to the original high stalk, and the gene that causes the mutation might be related with the gibberellin synthesis pathway.
Key words Panicum miliaceum L.; Dwarf mutant; Agronomic traits; GA; Dwarfing approach
In the practice of crop breeding, crop height is closely related to lodging resistance, yield and other important agronomic traits. Improving plant height has become the main technical route for creating ideal plant types. Obtaining new and excellent germplasm resources is an essential step in plant height improvement. The dwarf trait, as a common trait, once promoted the first "green revolution", which led to a leap in wheat and rice production. High-quality dwarf resources can be used to breed dwarf, high-yielding new varieties, and can effectively solve the lodging problem[1-5]. Therefore, in-depth study of the differences between different agronomic traits of tall and short stalks and the exploration of the inherent molecular mechanism of controlling plant height are crucial to further increase crop yields.
At present, there are many studies on dwarf mutants. For example, Wang et al.[6] conducted genetic analysis on a dominant dwarf mutant of maize, and the research showed that the typical mutation phenotype of the dominant dwarf mutants D8 and D9 of maize was plant dwarfing. The leaves are narrow and thick green, the tillers increase, the flowering period is delayed, anthers are attached to the female ear florets, and the application of exogenous gibberellin is not sensitive. The dwarf gene D-10 is located on the 2nd chromosome of maize. Chen[7] compared the agronomic economic traits of maize dwarf mutant K125d with the homologous inbred line K211. Studies have shown that compared with the homologous inbred line K211, the growth period of K125d was significantly extended, and the ear height was significantly reduced. The average plant height was reduced by 53.07%, the leaf overlap was dense, the number of leaves and leaf width increased significantly, but the leaf angle was significantly reduced, which was conducive to the establishment of an ideal plant type. The yield of per plant decreased by 34.41%, but the difference in ear length and number of ear rows did not differ significantly, thus it has the potential to increase yield. The reason for its dwarfing is the shortening of the internode length caused by the shortening of the stem cell length. Wang et al.[8] conducted research and evaluation on the dwarfing effects of several winter wheat dwarf germplasm materials as male parents, and identified three types of excellent dwarf germplasm of Shan 160, Northwest Dwarf and Lin 5064/Yannong 15, and the use of these excellent germplasms is proposed.
There are been many reports on the in-depth study of dwarf genes. For example, the wheat Rht gene, which encodes a protein that inhibits the gibberellin signal transduction pathway, resulting in plant dwarfing[9]; rice sd1 gene also has many related reports, because it encodes a defective GA20 oxidase, which affects the biosynthetic pathway of gibberellin to affect the height of the plant[10]; and a semi-dominant dwarf mutant of Brassica napus was found to have the DS-1 gene located on the A6 chromosome, encoding the DELLA protein acted as the gibberellin receptor[11]. Studies on dwarf genes indicate that dwarf traits are related to gibberellin biosynthesis and transduction pathways.
As a relatively drought-resistant cereal crop, broomcorn millet has stable productivity and good economic benefits, thus it is irreplaceable by other crops in dry farming and disaster relief[12-17]. Most of the research on broomcorn millet is focused on the analysis of resistance to diseases and pests as well as yield components, and there have been no reports on the induction of dwarf mutants and related gene[18-23]. Therefore, this study started with a mutant material 778 of broomcorn millet dwarf stalk, according to morphological observations and determination of 7 field agronomic traits at the seedling stage of broomcorn millet, and gibberellin-spraying was applied to further explore whether the cause of dwarfing is related to gibberellin biosynthesis and the transduction pathway, laying the foundation for gene cloning and functional analysis to control mutant traits.
Materials and Methods
Plant material
The experiment was conducted on the farm of Henan University of Science and Technology in 2017. The material was broomcorn millet original high stalk material 260 and dwarf mutant material 778 (mutated by high stalk 260 through EMS). The seeds of the material were all provided by Researcher Zhu Xuehai of Zhangjiakou Academy of Agricultural Sciences.
Experimental drugs and instruments
KH2PO4, KNO3, Ca(NO3)·4H2O, MgSO4·7H2O, H3BO3, MnCl2·4H2O, ZnSO4·7H2O, CuSO4·5H2O, NaMoO4·2H2O, FeSO4·7H2O, Na-EDTA and anhydrous ethanol were all analytically pure made in China. GA was an analytical reagent produced by Sanland Corporation in the United States. The light incubator GZX-150B was produced by Shanghai Kuntian Instrument Co., Ltd., and the micropipette was produced by Eppendorf.
Material cultivation and processing
Field planting and management
Broomcorn millet dwarf mutant 778 and original tall stalk 260 were sown on the experimental field of Kaiyuan Campus of Henan University of Science and Technology on May 18, 2017. The plant spacing was 5 cm and the row spacing was 40 cm. Each variety was planted with 3 rows with a row length of 2 m. Standardized management was carried out, and the phenotypes were recorded by taking pictures during the growth period of broomcorn millet.
Indoor plant materials and growth status
The experimental material seeds were placed in a refrigerator at 4 ℃ for 48 h. Then, four plastic small pots (10 cm×10 cm) were fill with soil to the half level, and each small pot was planted with 150 seeds according to two pots of raw materials and two pots of dwarf mutant materials. The seeds were cultured in a constant temperature incubator, and watered daily to wait for germination. Hydroponic transplantation was not performed until the materials grow to the four-leaf stage. The hydroponic method was carried out according to the Hoagland method, and the culture medium was changed every 3 d.
GA spray treatment
50, 100 and 200 mg/L GA solutions were sprayed separately to different treatment groups, and the control group was sprayed with water. The above treatments were sprayed once every 6 d.
Determination of phenotypic data
Determination of field phenotypic data
After the broomcorn millet was matured, 10 uniformly selected mutants 778 and 260 of the original high-stalk material of the broomcorn millet were randomly selected in the test field for field agronomic traits determination of plant height, ear length, internode length, internode number, seed length, seed width and spike number. If there were tillers, the agronomic traits of the main stem should be measured.
Measurement of GA treatment group data
Starting from the time when plants were treated by GA, the plant height and root length of the GA experimental and control groups were measured every 6 d and the data was recorded.
Statistical analysis
The recorded data was processed with EXCEL, and the GA sensitivity coefficients of high-stalk material 260 and short-stalk mutation material 778 at various concentrations were calculated. SPSS 17.0 was used for single-factor variance analysis, and Duncans method was used for multiple comparative analysis.
GA sensitivity coefficient (GRI)=Seedling height of GA treatment group/Seedling height of GA control group.
SPSS 17.0 was used for single-factor variance analysis, and Duncans method was used for multiple comparative analysis.
Results and Analysis
Morphological analysis of dwarf mutants
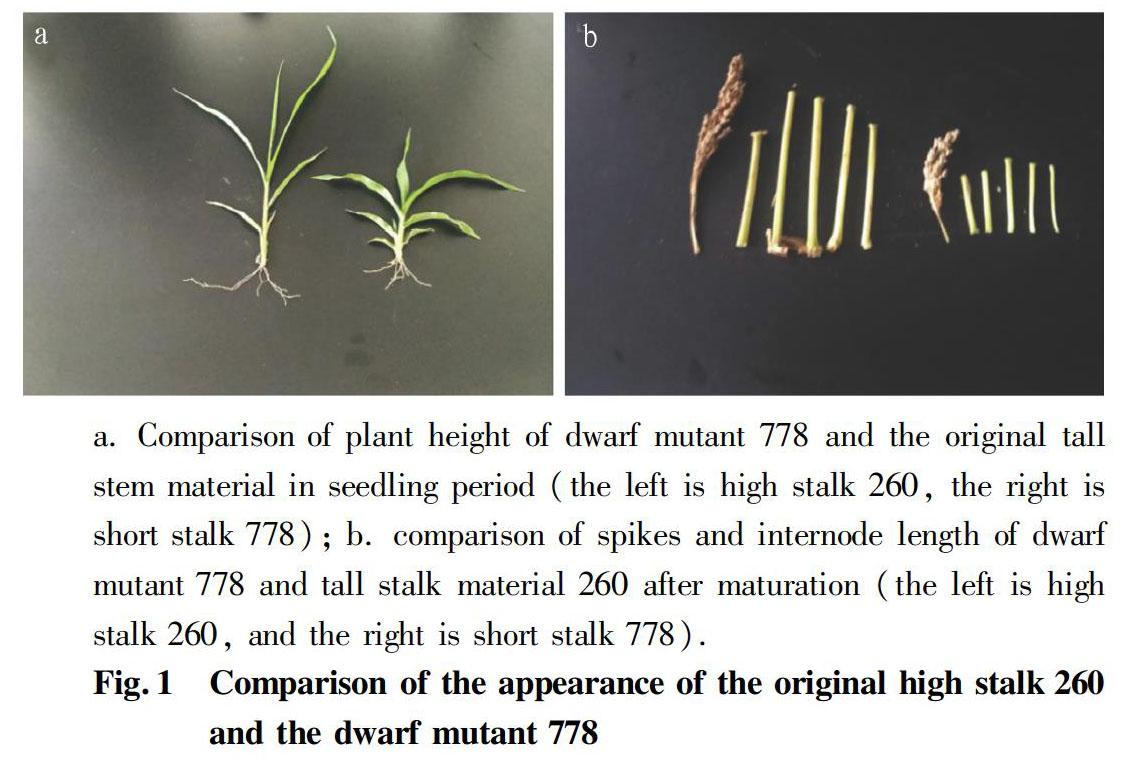
As shown in Fig. 1a and Fig. 1b, there was a clear difference between the dwarf mutant 778 and the original high stalk material 260 in plant height at the seedling stage. The ground base nodes as well as the first and the second elongated nodes of the short stalk mutant 778 were shorter than the original high stalk material 260. In addition, the leaves of short stalk mutants were short and wide, and the leaf color was dark green, while the leaves of tall stalk material were slender and the leaf color was lighter. After maturation, the spikes of the high-stalk material were longer and loose, while the short stalk material was shorter and tighter; and starting from the first node under the ear, the length of the 5 internodes of the high stalk material was longer than that of the dwarf mutant material.
Analysis of agronomic characters of dwarf mutants
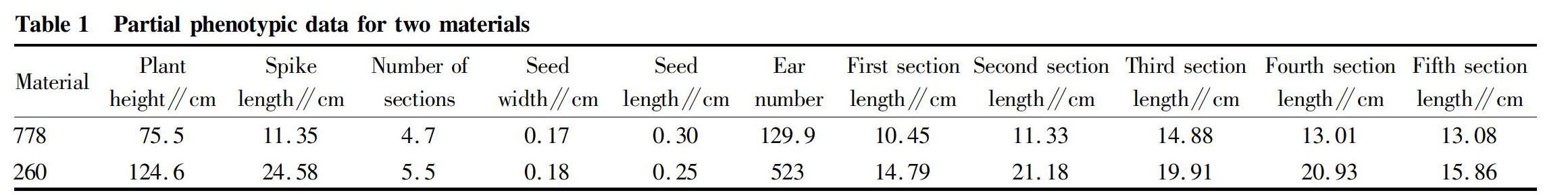
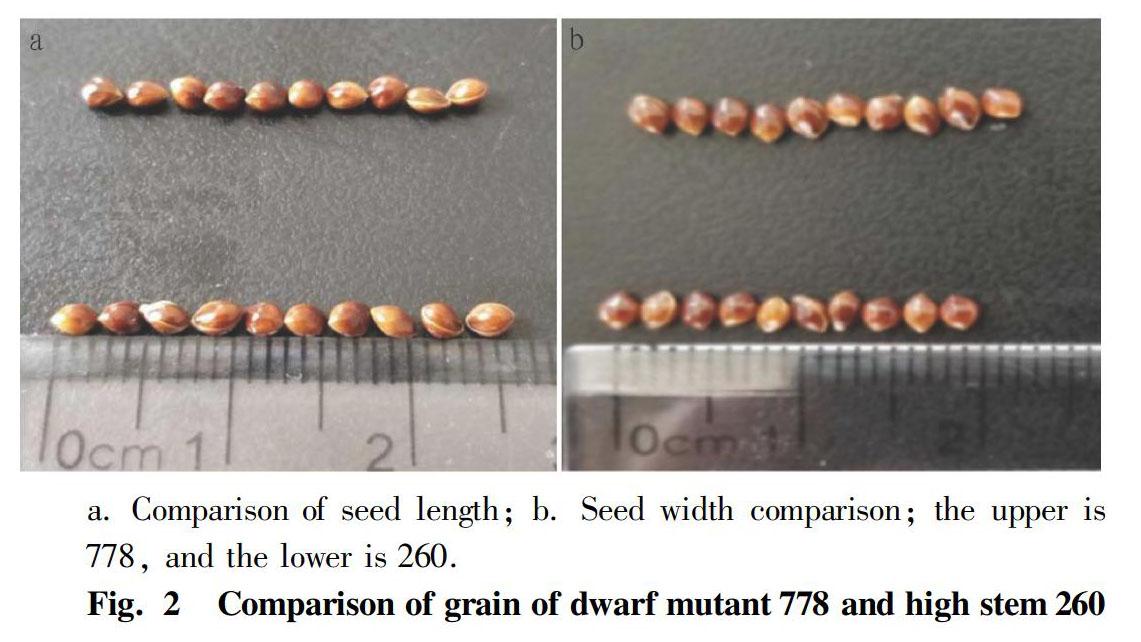
It can be seen from Table 1 that the ear length of dwarf mutant 778 was 11.35 cm, the ear length of tall stem 260 was 24.58 cm, and the ear length of dwarf mutant 778 was significantly lower than that of tall stem 260 (P<0.01). The number of sections of dwarf mutant 778 was 4.7, and the number of sections of tall stem 260 was 5.5. The number of sections of dwarf mutant 778 was significantly lower than that of tall stem 260 (P<0.01). In Fig. 2, the seed length of dwarf mutant 778 was 0.30 cm, the seed length of tall stem 260 was 0.25 cm, which was significantly lower than that of the dwarf mutant 778 (P<0.05). The difference in seed width between dwarf mutant 778 and tall stem 260 was not significant (P>0.05). The seed width of tall stem 260 was slightly larger than that of dwarf mutant 778.
The results of comparative analysis of spike number and plant height of dwarf mutant 778 and tall stem 260 are shown in Table 1. As can be seen from Table 1, the number of spike grains and plant height of dwarf mutant 778 were 129.9 grains and 75.5 cm, respectively. The grain number and plant height of tall stem 260 were 523 grains and 124.6 cm, respectively. The grain number and plant height of dwarf mutant 778 were significantly lower than that of tall stem 260 (P<0.01).
The first section length, the second section length, the third section length, the fourth section length, and the fifth section length of dwarf mutant 778 were: 10.45, 11.33, 14.88, 13.01 and 13.08 cm, respectively. The first section length, the second section length, the third section length, the fourth section length, and the fifth section length of tall stem 260 were: 14.79, 21.18, 19.91, 20.93, and 15.86 cm, respectively. The first section length, the second section length, the third section length, the fourth section length, and the fifth section length of the dwarf mutant 778 were significantly lower than that of the high stalk 260 (P<0.01).
GA sensitivity analysis
It can be seen from Table 1 that the plant height of the dwarf material at the three GA treatment concentrations was higher than that of the control, and with the extension of time, the difference in plant height between the treatment group and the control group became greater, and the plant height of the treatment group was significantly higher than the plant height of the control group. From the 12th d (second measurement), with the increase of GA concentration, the plant height of dwarf mutants also showed an increasing trend. The GRI value of the dwarf material 100 (GRI=1.26, 1.23, 1.36) (the first data is the GRI obtained from the first measurement, the second data is the GRI value obtained from the second measurement, and the third data is GRI value obtained from the third measurement. the same below) was higher than the GRI value of the dwarf 50 (GRI=1.15, 1.14, 1.21) and slightly greater than the GRI value of dwarf 200 (GRI=1.08, 1.25, 1.43), which indicated that the dwarf mutant was more sensitive to GA concentration of 100 mg/L. According to the data in the table, it could be seen that after three GA sprays, the dwarf mutants plant height could be restored to its normal plant height, indicating that the cause of dwarf mutant might be related to the GA synthesis pathway.
Effect of GA of different concentrations on individual traits
The effect of different concentrations of gibberellin on plant height is shown in Fig. 3. From Fig. 3, it can be seen that the differences in plant height of dwarf material on the 6th and the 12thd in the gibberellin of 0, 50, 100 and 200 mg/L groups was not significant (P>0.05). On the 7thd, the plant height of the high stalk material in the 200 mg/L gibberellin group was significantly higher than that in the 50 mg/L gibberellin group (P<0.05), and there was no significant difference between other groups (P>0.05). On the 12thd, the plant height of the high-stalk material in the 200 mg/L gibberellin group was significantly higher than that in the 50 mg/L gibberellin group (P<0.05), and significantly higher than those of other groups (P<0.05).
The effect of different concentrations of gibberellin on root length is shown in Fig. 4. As can be seen from Fig. 4, the differences in the root length of dwarf material on the 6thand the 12thd in the gibberellin groups of 0, 50, 100 and 200 mg/L were not significant (P>0.05). On the 6thd, the root length of the high stalk material in the gibberellin group of 0 mg/L was significantly higher than that in the gibberellin groups of 50 mg/L and gibberellin of 200 mg/L (P<0.05), and compared with the gibberellin group of 100 mg/L, there were no significant differences (P>0.05). On the 12thd, the root length of the tall stalk material in the gibberellin groups of 0 and 200 mg/L gibberellin was significantly higher than that in the 50 mg/L gibberellin group (P<0.05).
The effect of different concentrations of gibberellin on GRI is shown in Fig. 5. From Fig. 5, it can be seen that on the 6thand the 12thd, there were no significant differences in the GRI of dwarf materials in different gibberellin concentration groups (P>0.05). On the 6thand 12thd, the GRI of high-stalk material was significantly different among the different concentrations of gibberellin (P<0.05), and showed an increasing trend with the increase of gibberellin concentration.
Conclusions and Discussion
At present, there are no reports on dwarf mutants of broomcorn millet, but more than 80 dwarf mutants have been reported on rice. According to different phenotypic traits, they are divided into multiple types. This study reported the dwarf mutant of broomcorn millet for the first time, through morphological observation and the determination of field agronomic traits of broomcorn millet dwarf mutant 778, and the results showed that the panicle length and plant height of the dwarf mutant 778 were significantly lower than those of the height stalk 260 (P<0.01), which is consistent with Shulin Chens dwarf mutant NAUH164 of wheat variety Sumai 3 produced by the ethyl methylsulfonate treatment[24]. The seed length of dwarf mutant 778 was also significantly longer than that of tall stem, which is consistent with the research results of Lu et al.[25] from the new wheat dwarf mutant NM9. This study revealed some typical characteristics shared by dwarf mutants, which laid a foundation for the utilization and development of dwarf stalk resources of broomcorn millet.
There have been many reports on the molecular mechanism of plant dwarfing. More than 30 dwarfing-related genes have been cloned from rice, and most of them are involved in gibberellin synthesis or transduction pathways. Ueguchitanaka et al.[26] reported a new GA-insensitive rice dwarf mutant gid1 whose GID1 gene encodes an unknown protein similar to hormone-sensitive lipase, recombinant glutathione S-transferase (GST)-GID1 only has high affinity for biologically active GAs, while the mutant GST-GID1 corresponding to the three gid1 alleles does not have GA binding affinity. In addition, GID1 binds to rice DELLA protein SLR1 in a GA-dependent manner in yeast cells. Over expression of GID1 leads to a GA-allergic phenotype. These results indicate that GID1 is a soluble receptor that mediates GA signal transduction in rice. Spielmeyer et al.[27] combined sequence data from the rice genome with previous mapping studies to locate the putative GA 20-oxidase gene (Os20ox2) on the predicted map position of sd-1 on chromosome 1 and proposed semi-dwarfing (sd-1) phenotype is the result of a lack of active GAs in the elongation node caused by the defective 20-oxidase GA biosynthesis enzyme. Wang et al.[28] mutagenized Lijiang Xintuan Black Millet with EMS (ethyl-methane sulfonate), and confirmed that the mutant is a new D1 allelic mutant by map-based cloning and functional gene complementation verification. The D1 gene at the 2 522th base of junction of the 6thexon and intron was mutated from G to A, resulting in the exon 6 being cut off and unable to translate the functional Gα subunit.
In summary, plant dwarfing is related to gibberellin synthesis or transduction pathways. For dwarf mutants, the gibberellin sensitivity test can be used to test it. If the mutant returned to normal plant height after gibberellin treatment, it would mean that the dwarf mutation was related to the gibberellin synthesis pathway. If it was not sensitive to the treatment, it might be the dwarfing was caused by gibberellin conduction pathway or other pathway changes. Therefore, in this study, we used GA to treat dwarf mutants, and found that after spraying gibberellin, the dwarf mutants can return to normal plant height, thereby we further judge that the dwarfing reason is related to the gibberellin synthesis pathway.
References
[1] SPIELMEYER W, ELLIS MH, CHANDLER PM. Semidwarf (sd-1), green revolution" rice, contains a defective gibberellin 20-oxidase gene[J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(13): 9043-9048.
[2] YANG EN, LI J,YANG WY, et al. Agronomic traits and utilization of large-spike and high-yield dwarf wheat breeding parent SW3243[J].Agricultural Science & Technology, 2011, 26(2): 114-117.
[3] PENG LJ, WEN W, LI Y, et al. Cloning and preliminary functional analysis of GhGAI4b gene encoding DELLA protein from Gossypium hirsutum[J]. Xinjiang Agricultural Sciences, 2012, 49(3): 405-413.
[4] ZOU J, CHEN Z, ZHANG S, et al. Characterizations and fine mapping of a mutant gene for high tillering and dwarf in rice (Oryza sativa L.)[J]. Planta, 2005, 222(4): 604.
[5] BRENNAN J. Spillover effects of international agricultural research: CIMMYT-based semi-dwarf wheats in Australia[J]. Agricultural Economics, 2006, 3(4): 323-332.
[6] WANG YJ, MIAO N, SHI Y, et al. Genetic analysis of a dominant dwarf mutant in maize[J]. Acta Agriculturae Boreali-Sinica, 2010, 25(5): 90-93.
[7] CHEN L. Genetic Study of maize dwarf mutant K1 25d and its sensitivity to the exogenous GA3[D]. Yaan: Sichuan Agricultural University, 2016.
[8] WANG SB, LI SP, YANG YJ, et al. Study and assessment on shortening[J]. Journal of Henan Vocation-Technical Teachers College, 2003, 31(1): 4-6.
[9] HEDDEN P. The genes of the green revolution[J]. Trends Genet, 2003, 19(1): 5-9.
[10] SPIELMEYER W, ELLIS MH, CHANDLER PM. Semidwarf (sd-1), green revolution rice, contains a defective giberellin 20-oxidase gene[J]. Proc Natl Acad Sci USA, 2002, 99(13): 9043-9048.
[11] LIU C, WANG JL, HUANG TD, et al. A missense mutation in the VHYNP motif of a DELLA protein causes a semi-dwarf mutant phenotype in Brassica napus[J]. Tag. theoretical & Applied Genetics. theoretische Und Angewandte Genetik, 2010, 121(2): 249.
[12] CHAI Y. Nutrition and production survey of com millet (glutinous millet)[J]. Grain processing, 2009, 34(4): 90-91.
[13] CHAI Y, FENG BL. Present situation and developing strategies of minor grain crops in China[J]. Agricultural Research In The Arid Areas, 2003, 21(3): 145-151.
[14] CHEN H X, Talk about the development of food grains other than wheat and rice in China[J]. Shaanxi Journal of Agricultural Sciences, 2004(2): 36, 39.
[15] XIANG JY, LI HQ, XIANG YM, et al. Analysis on high and stable yield and adaptability of proso millet varieties[J]. Journal of Hebei Agricultural Sciences, 2017, 21(4): 5-7.
[16] KAHNOVA J, MOUDRY J. Content and quality of protein in proso millet (Panzcum mzlzaceum L.) varieties[J]. Plant Foods for Human Nutri, 2006, 61(1): 43-47.
[17] ZHENG BD. The present and future of coarse cereals and the public nutrition in China[J]. Food and Nutrition in China, 2009(3): 58-60.
[18] ZHAO M, LI ST, YU ZB, et al. Broomcorn millet resources in Inner Mongolia and its integrated utilization[J]. Inner Mongolia Agricultural science and technology, 2007(6): 101-102.
[19] DIAO XM, CHENG RH. Current breeding situation of foxtail millet and common millet in china as revealed by exploitation of 15 years regional adaptation test data[J]. Scientia Agricultura Sinica, 2017, 50(23): 4469-4474.
[20] HU XY, LU P, HE JB, et al. Principal components and cluster analysis of agronomic traits of proso millet (Panicum miliaceum)[J]. Journal of Plant Genetic Resources, 2008, 9(4): 492-496.
[21] ZHOU Y, LIU JJ, ZHANG PP, et al. Response of leaf defensive enzymes and antioxidant to smut fungus stress in broomcorn millet[J]. Scientia Agricultura Sinica, 2016, 49(17): 3298-3307.
[22] WANG L, WANG XY, WEN QF, et al. Identification and evaluation of resistance to dustbrand in Chinese proso millet germplasm resources[J]. Journal of Plant Genetic Resources, 2008, 9(4): 497-501.
[23] ZHANG JRF, ZHOU Y, YANG P, et al. Study on physiological changes and correlation with resistance level to the head smut of broomcorn millet after an infection with Sphacelotheca destruen[J]. Journal of China Agricultural University, 2015, 20(3): 108-113.
[24] CHEN S, GAO R, WANG H, et al. Characterization of a novel reduced height gene (Rht23) regulating panicle morphology and plant architecture in bread wheat[J]. Euphytica, 2015, 203(3): 1-12.
[25] LU Y, XING L, XING S, et al. Characterization of a putative new semi-dominant reduced height gene, Rht_NM9, in wheat (Triticum aestivum L.)[J]. Journal of Genetics & Genomics, 2015, 42(12): 685-698.
[26] UEGUCHITANAKA M, ASHIKARI M, NAKAJIMA M, et al. Gibberellin insensitive dwarf1 encodes a soluble receptor for gibberellin[J]. Nature, 2005, 437(7059): 693-8.
[27] PENG J, RICHARDS DE, HARTLEY NM, et al. ‘Green revolution genes encode mutant gibberellin response modulators[J]. Nature, 1999, 400(6741): 256.
[28] WANG CH, MA J, WANG S, et al. Genetic identification of a new D1-allelic mutant and analysis of its gene function in rice[J]. Acta Agronomica Sinica, 2016, 42(9): 1261-1272.
- 农业生物技术(英文版)的其它文章
- Construction of Technology System on Development and Repropagation in Vitro of Several Cultivars in Pear
- Construction of Camellia oleifera Cultivation Standardization System
- Nutrients Determination in Nuts from Different Torreya grandis Cultivars
- Photosynthetic Physiological Response to Drought Stress of Populus euphratica at Different Ages in Minqin
- Effects of UV-B Radiation on the Activity of Antioxidants in Flue-cured Tobacco Leaves
- Analysis of Differences in Biochemical Components Between Yunnan and Kenya Tea Tree Varieties

