Construction of Technology System on Development and Repropagation in Vitro of Several Cultivars in Pear
Yu WANG Baofeng HAO Jicheng HAN Lijuan GAO Longfei LI Shujun ZHAO Jintao XU Minghui JI Jie LI Haie ZHANG
Abstract It is the most available way to obtain self-rooted seedlings by tissue culture, which could breed de-virus seedlings with uniform traits and contribute to improve seedling quality. To construct a complete and efficient technology system, this test used stem with buds of 16 cultivars and super lines including Yali, Jingbaili, 03-04-034, 03-08-080 and so on as materials to study the best way for explant sampling time and position, sterilization time using HgCl2 and hormone concentrations. The result indicated that the optimum technology system was that the middle parts of longest shoots before 20 d after full-blossom were sterilized for 8 min by 0.1% HgCl2 and inoculated on the development medium MS+6-BA 0.5 mg/L+IBA 0.2 mg/L+saccharose 3%+agar 0.6% (pH=5.8), and then trans-inoculated into propagation medium MS+6-BA 1.0 mg/L+IBA 0.3 mg/L+saccharose 3%+agar 0.6% (pH=5.8).
Key words Pear; Tissue culture; Development and propagation technology system
Pear is the second fruit tree in North China, its cultivated area and production are both over 60 percent of the worlds[1]. Pear is a perennial woody plant, most the seedlings of which are cloning lines obtained by grafting and cannot be propagated by seeds, but grafting can change the traits of scion varieties to some extent[2-4] while the self-rooted stock can certainly maintain them[5]. Propagation of self-rooted stock can also solve the problem of application of dwarf rootstock, which is now applied in pear cultivation with the method that dwarf rootstocks are grafted on arbor pear trees such as Pyrus betulaefolia and varieties then are grafted on the dwarf rootstocks, this method cannot apply dwarf rootstock as the base stock which greatly weakens the effect of dwarf rootstock. Now self-rooted seedlings are hardly propagated by the methods of cottage and layering while can be multipropagated by tissue culture. It is the best way to propagate cloning seedlings to obtain self-rooted ones, which also can breed de-virus seedlings with uniform traits and contribute to improve seedling quality[6].
In recent years, tissue culture has been widely applied in fruit trees, especially the application in strawberry has achieved the factory production[7-13]. And it has also more and more been used to propagate apple[14-20], grape[21-22], kiwi fruit[23-25], cherry[26-27] and so on. For pear, the reports indicated that sterilizing for 5 min by 0.1% HgCl2 is the best way for Kuerlexiangli whose optimum propagation medium is MS+1.5 mg/L 6-BA+0.1 mg/L IBA+1.5 mg/L GA3[28], for Douli the optimum propagation medium is MS+0.6 mg/L 6-BA+0.2 mg/L NAA[29], and development and propagation medium of Eli are WPM+2.0 mg/L 6-BA+0.5 mg/L NAA+1.0 mg/L ZT+2% saccharose+0.2% active carbon+0.65% agar and WPM+3.0 mg/L 6-BA+0.3 mg/L NAA+1.0 mg/L ZT+2% saccharose+0.2% active carbon+0.65% agar[30]. Golden pear[31], Nanguoli[32], Xueqing, Zhongliyihao, BA29[33] also have their own optimum media. All these reports told us that different varieties have different sterilization time, development and propagation medium and different hormone types and concentrations, and until now there is not a technology system for tissue culture that broadly suit to most of the varieties. In this study, we used 16 varieties including Yali, Jingbaili, 03-04-034 and 03-08-080 as materials to study the best way for explant sampling time and position, sterilization time using HgCl2 and hormone concentrations, aiming to find a technology system for de-virus of explants, seedling development and propagation and provide theoretical reference and technical support for practice.
Materials and methods
Materials
9 of super lines and varieties highly resistant to pear scab (03-04-034, 03-08-080, 10-02-052, 10-02-030, 10-01-078, 10-02-066, 10-02-074, Xianghongli, Wanyuli) and 7 of ones highly susceptible to pear scab (10-01-101, 10-01-123, 10-05-163, 10-04-159, 10-05-100, Yali, Jingbaili) were used as materials, and the tests were performed from 2017 to 2019.
Methods
Effect of sterilization time and sampling position on inoculation
Yali, Jingbaili, 03-04-034 and 03-08-080 were used as materials to study the optimum sterilization time and sampling position. In spring, shoots of the varieties and super lines were sampled from trees and removed leaves, and then were cut into three parts: base, middle and upper, and then every part were cut into 1.5-2.0 cm stem pieces with one bud. Every part was repeated 3 times and each repetition included 30 stem pieces. The materials were rinsed thoroughly and then sterilized by 0.1% HgCl2 for 4, 6, 8, 10, 12 and 14 min, respectively. The stem pieces were removed the dead parts killed by HgCl2 and then were inoculated on the medium MS+6-BA 0.5 mg/L+IBA 0.2 mg/L+sucrose 3%+agar 0.6% (pH=5.8), and 1 stem piece was placed in a separate conical flask. Stems that were contaminated and dead should be surveyed continuously during the process of culture.
Effect of different sampling time on inoculation
The sterilization and sampling methods from "Effect of sterilization time and sampling position on inoculation" were used to further study the effect of different sampling time on inoculation. In 2017-2019, 9 of super lines and varieties highly resistant to pear scab (03-04-034, 03-08-080, 10-02-052, 10-02-030, 10-01-078, 10-02-066, 10-02-074, Xianghongli, Wanyuli) and 7 of ones highly susceptible to pear scab (10-01-101, 10-01-123, 10-05-163, 10-04-159, 10-05-100, Yali, Jingbaili) were used as materials and sampled at days 10, 15, 20, 25 and 30 d after full-blossom. The middle parts of the shoots of these super lines and varieties were also cut into 1.5-2.0 cm stem pieces, sterilized for 8 min and inoculated on the medium. Every super lines and varieties were repeated 3 times, and every repetition included 30 stem pieces. Pollution should be surveyed and recorded continuously.
In another test, the middle parts of the shoots of 5, 10, 15 and 20 cm at 15 d after full-blossom from 16 super lines and varieties were also cut into 1.5-2.0 cm stem pieces, sterilized for 8 min and inoculated on the medium. Every super lines and varieties were repeated 3 times, and every repetition included 30 stem pieces. Germination should be surveyed and recorded continuously.
Effect of hormone concentrations on seedlings development in tissue culture
In propagation culture, the optimum IBA and 6-BA concentrations were tested, IBA was set with 3 concentration levels: 0.20, 0.25 and 0.30 mg/L, and 6-BA was set with 6 concentration levels: 0.2, 0.40, 0.60, 0.80, 1.0 and 1.20 mg/L. This test was designed according to complete experimental design. The middle parts of seedlings with one bud without leaves from tissue culture were used as materials, and every conical flask was inoculated with 3 stem pieces. Each treatment was repeated 3 times and every 5 conical flask was as one repetition. The growth and vitrifaction should be observed and recorded 45 d after inoculation.
Data analysis
Data was analyzed using Excel 2013 and SPSS.
Results and Analysis
Effect of sterilization time and sampling position on inoculation
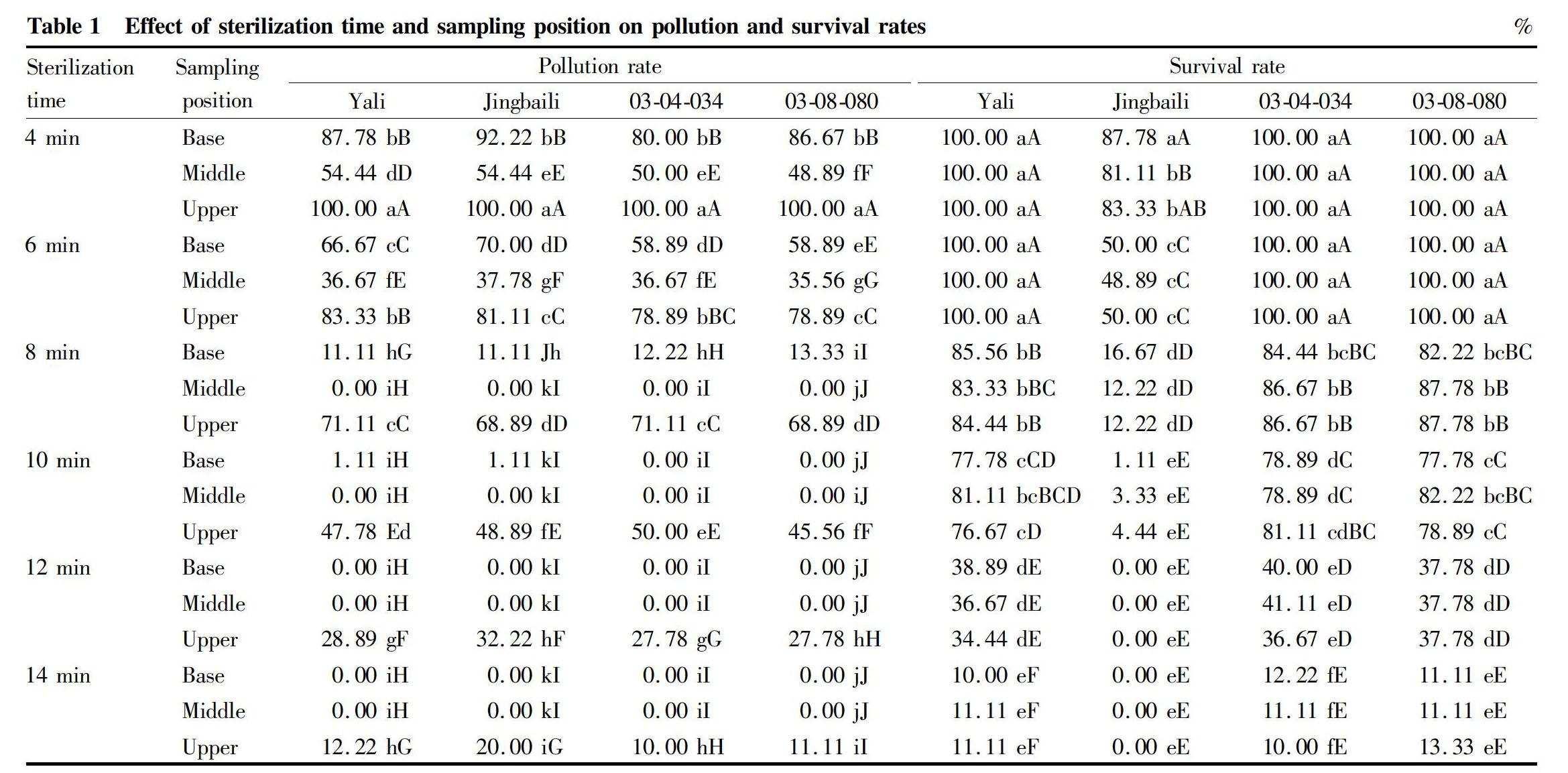
The effect of different sterilization time on the rates of pollution and survival is shown in Table 1. The result indicated that the pollution and survival rates of base, middle and upper of shoots of different super lines and varieties were all decreasing with the increase of the sterilization time. For one super line or variety, the pollution rate of the upper of the shoots was the highest, followed by the base, and the last was the middle. And the survival rates of different positions had no significant differences.
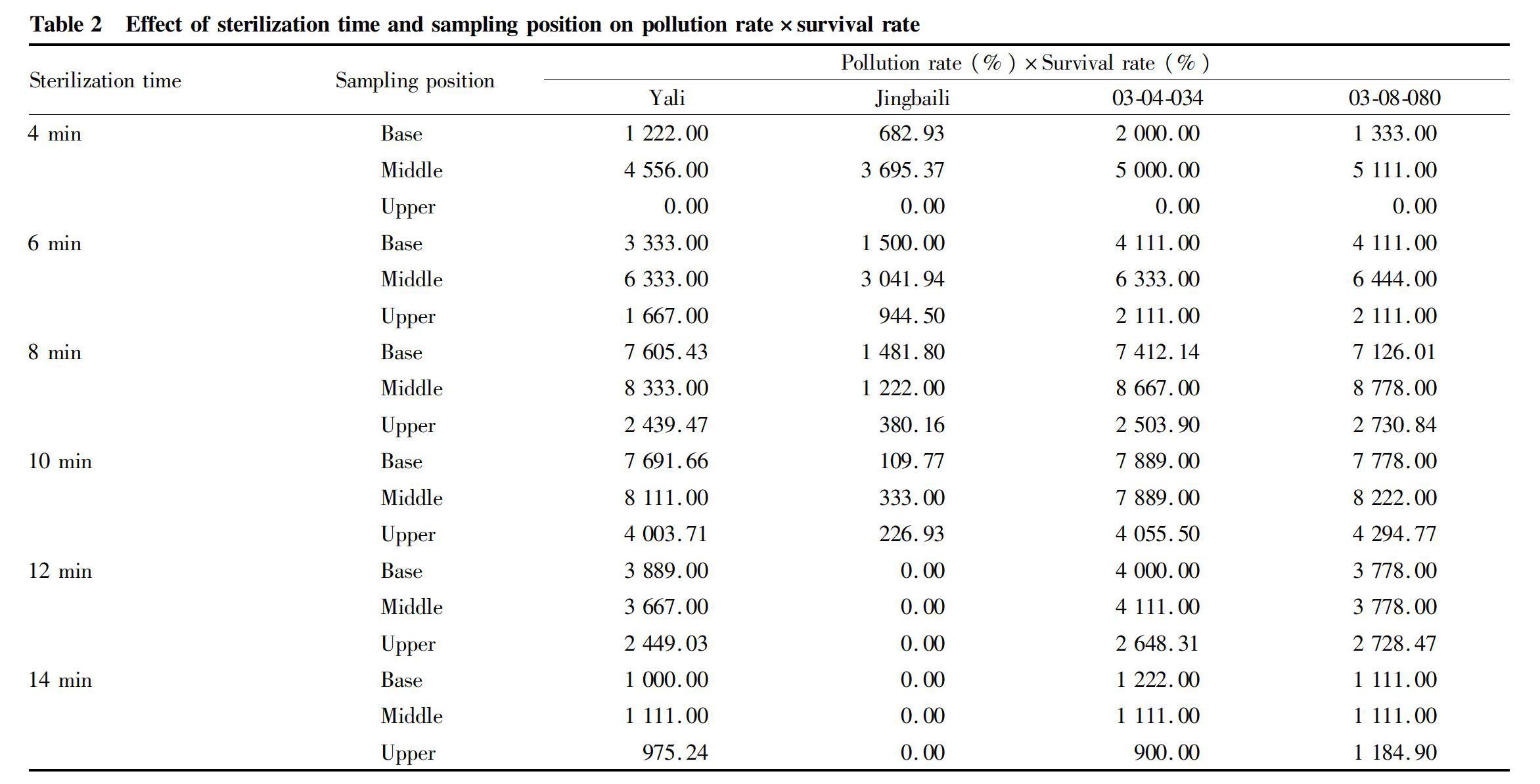
To evaluate the effect of sterilization, the pollution and survival rate were needed to be evaluated overall, and the overall effect of sterilization time and sampling position on pollution and survival rates is shown in Table 2. The result indicated that when sampling the middle parts and sterilizing for 8 min, the pollution rate×survival rate of Yali, 03-04-034 and 03-08-080 were all the highest, respectively were 83.33%, 86.68% and 87.78%. When sampling the middle parts and sterilizing for 6 min, the pollution rate×survival rate of Jingbaili was the highest, was 36.95%, and for 8 min, the pollution rate×survival rate of Jingbaili was 12.22%.
So, for Yali, 03-04-034 and 03-08-080, the best sterilization method was sampling the middle of shoots and sterilizing for 8 min. Jingbaili was specific and was too susceptible to HgCl2 to survive and needed shorter sterilization time.
Effect of sampling time on inoculation
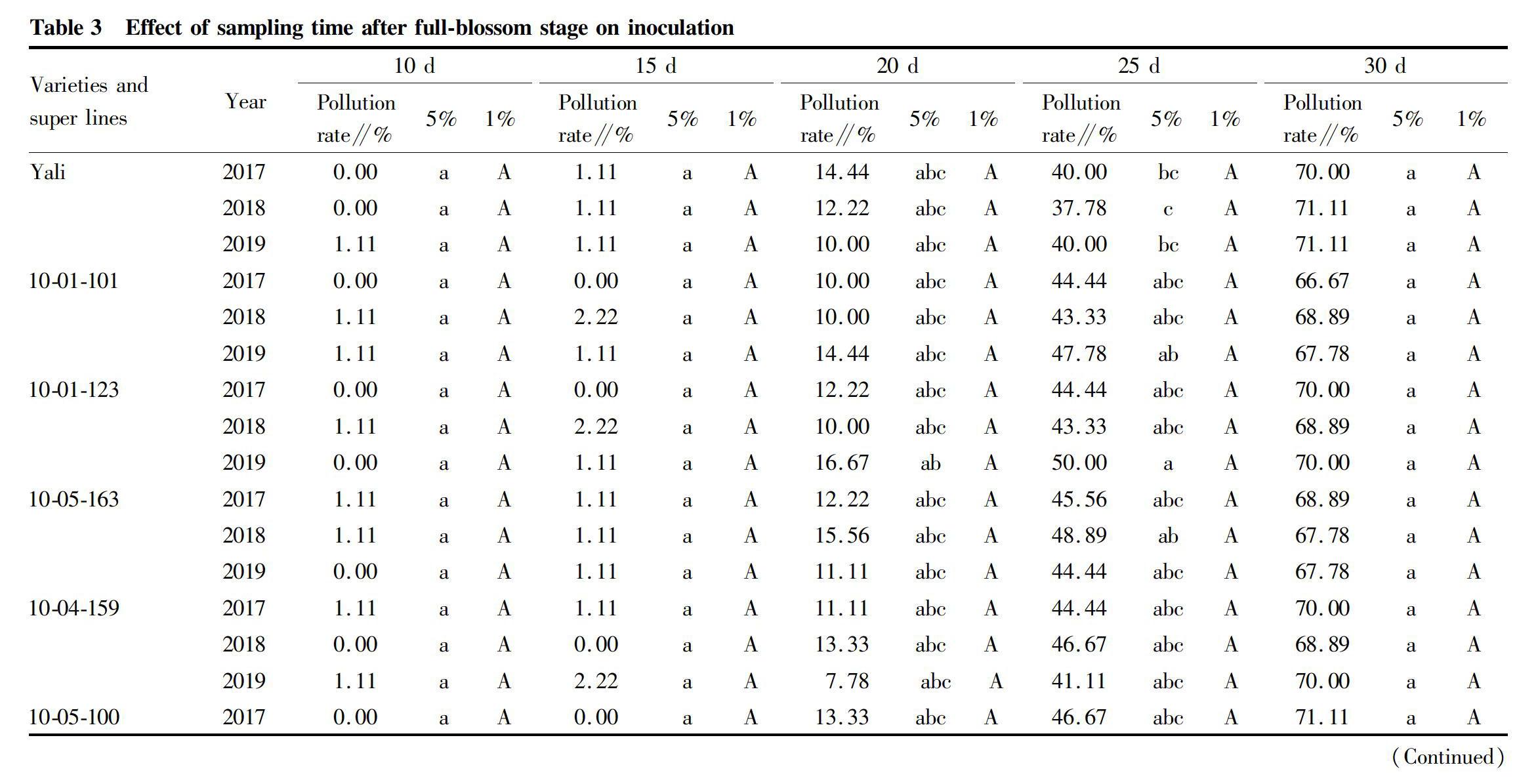
The effect of sampling time on inoculation is shown in Table 3. The result indicated that the pollution rate of the samples 10-15 d after full-blossom were low and no more than 2.22%, from 20 d after full-blossom, the pollution rates began to increase and were between 5.56% and 17.78%, then after 25 d, the pollution rates were between 37.78% and 48.89%, and after 30 d, the pollution rates reached over 66.67%. The pollution rates of different varieties and super lines at the same sampling time had no significant differences.
The effect of sample length on inoculation is shown in Table 4. The result indicated that the germination rate of the middle parts of all 16 varieties and super lines increased with the shoot age growing. The germination rate of 5 cm shoots was between 20.00% and 27.78%; the germination rate of 10 cm shoots was between 42.22% and 52.22%; the germination rate of 15 cm shoots was between 53.33% and 65.56%; the germination rate of 20 cm shoots was between 52.22% and 63.33%; and the germination rate of 15 cm shoots was not significantly different from that of 20 cm. And the germination rates of all 16 varieties and super lines also had no significant differences.
Considering the overall result of Table 3 and Table 4, the best sampling method was sampling the longest shoots no more than 20 d after full-blossom, then using the middle parts of these shoots to inoculate.
Effect of hormone concentrations on seedling development in tissue culture
The effect of hormone concentrations on seedling growth in tissue culture is shown in Table 5. The results indicated that when the IBA concentration was kept constant, the seedling growth of Yali and 03-04-034 all increased with the 6-BA concentration increasing, while when the 6-BA concentration was kept constant, the seedling growth of Yali and 03-04-034 also increased with the IBA concentration increasing. However, vitrifaction began to appear when the seedlings propagated for 5 times under the 6-BA 1.0 mg/L, the content of no vitrifaction was 0.800-0.933, and the content of no vitrifaction was even just 0.133-0.267 under the 6-BA 1.2mg/L. Considering the overall result of seedling growth and no vitrifaction, the best hormone concentrations were IBA 0.3 mg/L and 6-BA 1.0 mg/L, under which seedling growth of Yali was 13.423 cm and 03-04-034 was 13.485 cm.
Conclusions and Discussion
HgCl2 is a highly poisonous heavy metal disinfectant, and when the sterilization time is too long plant tissues will be poisoned and the growing point will be killed which certainly affect survival rate. However, on the contrary, a too short sterilization time will lead to the condition that the fungi are not completely killed which immediately impact pollution rate, so optimum sterilization time is very important for inoculation[34]. In this paper, the effects of sterilization time, sampling position and sampling time on inoculation were studied and the results indicated that the best way was sampling the longest shoots no more than 20 d after full-blossom, then sterilizing the middle parts of these shoots for 8 min using 0.1% HgCl2, and then inoculating them on medium.
In this study, vitrifaction began to appear when the seedlings propagated for 5 times under the 6-BA 1.0 mg/L, which is consistent with the research of Li et al.[29]. Considering the overall result of seedling growth and no vitrifaction, the best hormone concentrations were IBA 0.3 mg/L and 6-BA 1.0 mg/L, and this optimum 6-BA concentration was just in the range of 0.5-1.0 mg/L studied by Ran et al.[35] and Shibli et al.[36] in wild pear.
References
[1] ZHANG SL, XIE ZH. Current status, trends, main problems and the suggestions on development of pear industry in China[J]. Journal of Fruit Science, 2019, 36(8): 1067-1072.
[2] KOEPKE T, DHINGRA A. Rootstock scion somatogenetic interactions in perennial composite plants[J]. Plant Cell Reports, 2013, 32(9): 119-126.
[3] JENSEN PJ, RYTTER J, DETWILER EA, et al. Rootstock effects on gene expression patterns in apple tree scions[J]. Plant Molecular Biology, 2003, 53 (4): 493-511.
[4] LI HL, ZHANG H, YU C, et al. Possible roles of auxin and zeatin for initiating the dwarfing effect of M9 used as apple rootstock or interstock[J]. Acta Physiologae Plantarum, 2012, 34(1): 235-244.
[5] ZHANG J, LI YB, YANG YL, et al. Propagation technology of big seedlings on dwarf self-rooted stock in apple[J]. Northwest Horticulture, 2019(43): 32-33.
[6] HU GJ, HONG N, WANG LP, et al. Efficacy of virus elimination from in vitro-cultured sand pear (Pyrus pyrifolia) by chemotherapy combined with thermotherapy[J]. Crop Prot 2012(37): 20-25.
[7] LIAO JJ, LI Y, LI YH. Several methods of decreasing cost in factory tissue production in strawberry[J]. Beijing Agriculture, 2006(1): 18-18.
[8] LI XL, ZHANG JY, ZHANG Z, et al. Construction of system of tissue culture and rapid propagation in strawberry stem tip[J]. Crop Journal, 2016(4): 68-74.
[9] LI HL, WU SW, WANG QK, et al. Study on technology of rapid propagation by tissue culture in strawberry ‘Zijinsiji[J]. South China Fruits, 2017, 46(4): 131-135.
[10] JIN Z, WANG KL, LIANG JH, et al. Study on effect on stem tip culture of medium and exogenous hormones in strawberry ‘Zhangji[J]. Journal of Agriculture, 2015, 5(8): 73-77.
[11] HE HL, YANG J, CAI R, et al. Study on effect of detoxification of stem tip in strawberry[J]. Northern Horticulture, 2005(5): 79-81.
[12] BAI XF, JIANG XM, ZHAO JP, et al. Improvement research on technology of rooting in strawberry tissue seedlings[J]. Ludong University Journal (Natural Science Edition), 2001, 17(1): 34-36.
[13] CHAO HJ, LIU M, JI QL, et al. Tissue culture of stem tip and rapid propagation in strawberry ‘Hongyan[J]. Journal of Beijing University of Agriculture, 2009, 24(4): 14-16.
[14] YANG YZ, ZHOU BB, LI MJ, et al. Optimization of macro-element composition in the medium for in vitro propagation of apple dwarfing rootstock ‘SH6[J]. Journal of Fruit Science, 2020, 37(1): 40-49.
[15] XIE X, XU K, XIE MX, et al. Optimization of rapid micropropagation system of apple meristem-tip culture[J]. Plant Physiology Journal, 2015, 51(12): 2152-2156.
[16] TABART J, FRANCK T, KEVERS C, DOMMES J. Effect of polyamines and polyamine precursors on hyperhydricity in micropropagated apple shoots[J]. Plant Cell Tissue and Organ Culture, 2015, 120(1): 11-18.
[19] AMIRI EM, ELAHINIA A. Optimization of medium composition for apple rootstocks[J]. African Journal of Biotechnology, 2011, 10(18): 3594-3601.
[20] KEPENEK K, KAROGLU Z. The effects of paclobutrazol and daminozide on in vitro micropropagation of some apple (Malus domestica) cultivars and M9-rootstock[J]. African Journal of Biotechnology, 2011, 10(24): 4851-4859.
[21] CAI WB, DUAN H, WANG J, et al. Establishment of in vitro rapid micropropagation of four table grape cultivars[J]. Journal of Nuclear Agricultural Sciences, 2019, 33 (2): 0248-0254.
[22] YANG ZY, GUO WY, LI WY, et al. Effect of different light quality on the growth of tissue culture seedling of ‘Qiuhongbao grape[J]. Journal of Fruit Science, https:∥doi.org/10.13925/j.cnki.gsxb.20200007.
[23] CHEN YQ, ZHAN JX, HU C, et al. A rapid clonal propagation protocol of kiwifruit cultivar Jinkui (Actinidia chinensis var. deliciosa)[J]. Journal of Hubei University (Natural Science Edition), 2018, 40(3): 301-305.
[24] LI W. Kiwi fruit varieties tissue culture to establishment the system of rapid propagation[D]. Yangling: Northwest Agricultural and Forestry University, 2018.
[25] ZHAN JX. Rapid clonal propagation and production of endophytic bacteria-free plants of ‘Jinkui kiwifruit[D]. Wuhan: Hubei University, 2018.
[26] DONG MM. Quality survey of sweet cherry in Zhejiang Province, establishment of rootstock rapid propagation system and study on cherry embryo rescue technology, Hangzhou[D]. Hangzhou: Zhejiang Agricultural and Forestry University, 2018.
[27] XU XY, WU XK, WU YJ. Advances in the research of tissue culture and genetic transformation in cherry[J]. Journal of Fruit Science, 2018, 35(10): 1277-1285.
[28] LIU XF, FENG JR, LIANG XT, et al. Research on tissue culture of Korla fragrant pear[J]. Shandong Agricultural Sciences, 2016, 48(5): 9-13.
[29] LI XG, WANG HW, YANG QS, et al. Low vitrifaction technology of rapid propagation in Douli[J]. Jiangsu Agricultural Sciences, 2012, 40(12): 54-56.
[30] HE BZ, WU SS, LAN SR. Regeneration system establishment of Persea americana[J]. Journal of Forest and Environment, 2016, 36(4): 409-415.
[31] HAN WP, YUAN ML. Study on tissue culture and rapid propagation in Golden pear[J]. Deciduous, 2001(2): 7-8.
[32] WANG JZ, CAI ZM. Stem tip culture of Nanguoli[J]. Northern Fruits, 2004(3): 9-10.
[33] LIU Y. Studies on tissue culture of Xueqing, Lvbaoshi, BA29 rootstock and shoot regeneration from leaves of BA29[D]. Baoding: Hebei Agricultural University.
[34] YAN YQ, JIANG HT, GU C. Construction of system of propagation and regeneration in Xuehuali leaves[J]. Journal of Nanjing Agricultural University, 2017, 40(1): 68-75.
[35] RAN K, WANG HW, WANG SM. Research progress of tissue culture and genetic transformation[J]. Chinese Agricultural Science Bulletin, 2017, 33(4): 74-79.
[36] SHIBLI RA, AJLOUNI MM, JARADAT A, et al. Micropropagation in wild pear (Pyrus syrica)[J]. Scientia Horticulturae, 1997(68): 237-242.
- 农业生物技术(英文版)的其它文章
- Investigation on Agronomic Characters of Dwarf Mutant 778 in Broomcorn Millet (Panicum miliaceum L.) and Analysis of Its Sensitivity to GA
- Construction of Camellia oleifera Cultivation Standardization System
- Nutrients Determination in Nuts from Different Torreya grandis Cultivars
- Photosynthetic Physiological Response to Drought Stress of Populus euphratica at Different Ages in Minqin
- Effects of UV-B Radiation on the Activity of Antioxidants in Flue-cured Tobacco Leaves
- Analysis of Differences in Biochemical Components Between Yunnan and Kenya Tea Tree Varieties

