Effects of UV-B Radiation on the Activity of Antioxidants in Flue-cured Tobacco Leaves
Jiye QIANG Yunfei CHEN
Abstract [Objectives] This study was conducted to investigate the effects of UV-B radiation on the activity of main antioxidants in flue-cured tobacco.
[Methods] The flue-cured tobacco variety Yunyan 87 was used as the test material for pot planting. Xuanwei County (1 997 m) in Qujing Tobacco District, Yunnan was selected as the test point. Three levels of UV-B radiation was set to investigate the activity of PPO, SOD, POD and CAT and the contents of flavonoids and malondialdehyde during the development of the middle leaves of flue-cured tobacco in the same planting area by reducing the natural conditions by 75.04% (A1), 70.01% (A2) and 30.02% (A3), respectively.
[Results] Reducing UV-B radiation significantly increased the activity of tobacco SOD, POD and CAT, which showed that flue-cured tobacco in the low-latitude plateau area of Yunnan has adapted to the higher local UV-B radiation, and the flue-cured tobacco has formed a unique antioxidant enzyme system. The weakening of UV-B radiation treatment might partially change the antioxidant enzyme system of flue-cured tobacco, but there were no significant differences in the activity of antioxidant enzymes between different degrees of weakening UV-B radiation. The change trends of flavonoids and malondialdehyde in leaves at two different leaf positions were similar. The malondialdehyde content of treatment A1 was significantly higher than those of the CK, A2 and A3, and 70.01%-75.04% of UV-B radiation intensity might be the lower limit of Yunyan 87s adaptability to UV-B radiation. With the decrease of UV-B radiation, the sensitivity of flue-cured tobacco gradually decreased.
[Conclusions] This study made a preliminary exploration for explaining the correlation of UV-B radiation and Yunnan flue-cured tobacco.
Key words UV-B; Flue-cured tobacco; Antioxidant enzyme; Flavonoid; MDA
Plants in their natural environment must be affected by solar UV-B radiation during their growth and development. Studies have shown that enhanced UV-B radiation can induce an increase in the generation rate of reactive oxygen species (ROS) in plants, which intensifies the oxidative stress that plants are exposed to, thus affecting a series of physiological metabolic processes[1-2]. An important way for plants to defend against UV-B damage is improved antioxidant activity. A large number of studies have shown that under the influence of UV-B radiation, plant superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) show different changing characteristics, and there are inter-species and intra-variety differences[3-7].
The cell membrane is one of the main damaged parts by UV-B radiation. The effect of UV-B radiation on the plants antioxidant system and enzyme activity is firstly to cause the destruction of the membrane structure, which further causes a large amount of exosmosis of intracellular Cl-, K+ and Na+, a reduction of the fluidity of the membrane, loss of polar lipids and decreases in unsaturated fatty acid index and production of ethylene, and causes increases in the activity of LOX fatty acid oxidase and the oxidation rate of membrane lipids and the production of active oxygen radicals, which makes the free radical scavenging system lose balance and leads to membrane lipid peroxidation, which ultimately leads to increased accumulation of membrane lipid peroxidation product MDA and improved activity of antioxidant enzymes. The current understanding of the effect of UV-B radiation on plant protection enzyme systems is mostly obtained by artificial irradiation in a greenhouse, growth room or natural environment to enhance UV-B radiation, while the current UV-B radiation under natural conditions may already pose a threat to the plant itself[8]. In the early stage, the characteristics of the temporal and spatial distribution of ultraviolet radiation intensity in the Yunnan region belonging to the low-latitude plateau were studied[9], and the physiological and ecological effects of UV-B radiation on Yunnan primroses with changes in altitude and latitude were analyzed in detail. Gaberǒi et al.[10] studied Pulmonaria officinalis in Ljubljana (46°04′ N, 14°31′ E) and showed that weakening UV-B radiation had no significant effects on the photosynthetic pigments and PSII photochemical efficiency, but reduced the flavonoid content. He et al.[8] also obtained consistent results on the study of wheat flavonoids. Under the condition of weakening UV-B radiation intensity near the ground by 15.3% in Nanjing, they found that the content of wheat flavonoids decreased significantly, which was the most sensitive during the jointing-booting period; the content of flavonoids in the upper leaves of wheat was significantly greater than that of the middle and lower leaves; and in a single leaf, the content of flavonoids at the base of the leaf was significantly lower than that at the tip and middle of the leaf. It has been found that the current UV-B radiation level has already caused a great impact on primroses[12]. Under the three-dimensional climatic conditions formed by the special and complicated terrain in Yunnan, the altitude of the flue-cured tobacco planting area has an important influence on the composition of flue-cured tobacco quality[13-15]. Under the special UV-B radiation environment in Yunnan, there are few studies on the resistance of flue-cured tobacco to the current UV-B radiation in Yunnan. In this study, Yunyan 87, a flue-cured tobacco variety with a wide planting area in Yunnan, was used as the research material. The Qujing tobacco area mainly producing Yunnan tobacco leaves was selected, and Banqiao Town of Xuanwei County in the the tobacco area was used as the test point. The effects of UV-B radiation on the activity of main antioxidants in flue-cured tobacco were studied by reducing the intensity of solar ultraviolet radiation to different degrees under natural conditions. This study made a preliminary exploration for explaining the correlation of UV-B radiation and Yunnan flue-cured tobacco.
Materials and Methods
Experimental materials and design
The experiment was conducted in 2018 in Banqiao Town (103°45′ E, 26°18′ N, altitude 1 997 m), Xuanwei County, Qujing City, Yunnan Province. The flue-cured tobacco variety was Yunyan 87. The pot was 40 cm in diameter, 35 cm in diameter at the bottom and 40 cm in height. Each pot was filled with 15 kg of soil, which had a pH value of 7.79, and contained organic matter of 5 415 g/kg, and alkali-hydrolyzale nitrogen, available phosphorus and available potassium of 0.138, 14.912 and 41.611 g/kg, respectively. The seedlings were transplanted on May 9, 2008, by one plant per pot. The bottom application involved special fertilizer for flue-cured tobacco (N∶P2O5∶K2O=2∶1∶4) 47 g and calcium-magnesium-phosphorus fertilizer 47 g, and the topdressing used special fertilizer 20 g and potassium sulfate 8 g. Moreover, seedling-promoting fertilizer (N∶P2O5∶K2O=2∶1∶4) was applied 3 times, at 15, 30 and 45 d after transplanting, respectively. According to the cultivation and management method in the field, the plant row spacing was 45 cm × 100 cm, and the UV-B weakening treatment was started on May 25.
The experiment was set with four treatments, A1, A2 and A3 (to facilitate the penetration of rainwater, the top of each film was ironed to form small holes in a uniform distribution with a hole distance of 20 cm × 20 cm and a diameter of about 0.5 cm) which simulated different degrees of UV-B reduction, and natural environment treatment (without weakening UV-B) as a control (CK). Each treatment had 20 pots. A rectangular shelf with a height of 1.8 m, a length of 5 m and a width of 3.5 m was placed on the top of corresponding treatment for film covering. During the experiment, for the UV-B radiation intensity (mW/m2, RADIOMETER ultraviolet radiation instrument produced in France, wavelength range 295-395 nm, center wavelength 312 nm) and light intensity (lx, ZDS 210 automatic range illuminance meter produced by Shanghai Jiading Xuelian Instrument Factory) at a height of 150 cm, the average UV-B reduction rates of A1, A2 and A3 were about 25%, 50% and 65%, respectively, and the visible light was about 70%-80%. The change of UV-B intensity in each treatment was within 3%-10%, and that of visible light was within 2%-7%.
Determination of SOD, POD and CAT activity
After 25 d of treatment, 10 pieces of the 7th leaf were taken and punched to form six leaf discs with an area of 3 cm2 uniformly at the leaf stalk, middle and tip parts of the leaf, which were put into plastic bags, quickly frozen with liquid nitrogen, and stored at -20 ℃. During the determination, about 0.5 g (fresh weight) was weighed and added with 5 ml of enzyme extracting solution (50 mmol/L PBS (pH7.0)+0.4% PVP), followed by grinding in an ice bath. The mixture was subjected to refrigerated centrifugation at 10 000 g for 20-30 min, obtaining a supernatant as the crude enzyme extract. The activity of polyphenol oxidase (PPO) was determined by colorimetry[16]. The enzyme reaction system included 3.9 ml of 0.05 mol/L phosphate buffer (pH 5.5), 1.0 ml of 0.1 mol/L catechol and 0.0 ml of the enzyme extract. It was incubated at 37 ℃ for 10 min, quickly placed in an ice bath, immediately added with 2 ml of 20% trichloroacetic acid, and centrifuged at 5 000 g for 10 min. The supernatant was collected and diluted appropriately, and its optical density was determined at a wavelength of 525 nm. The activity of superoxide dismutase (SOD) was determined by the nitro-blue tetrazolium method[16], in which the solutions were added in the following order: PBS (pH 7.8) 2.3 ml, MET 0.2 ml, EDTA 0.1 ml, enzyme extract 0.1 ml, NBT 0.2 ml, riboflavin 0.1 ml. The mixture was placed in a transparent test tube rack, and illuminated in a lighted incubator for 15 min, and with the largest tube free of illumination as the CK, OD560 was quickly measured. The activity of peroxidase (POD) was determined using the guaiacol method[16]. The enzyme reaction solution included: acetate buffer (0.1 M, pH 5.4) 2.0 ml, guaiacol (0.25%) 0.8 ml, enzyme extract 0.1 ml, and H2O2 (0.75%) 0.1 ml, and the change of the OD460 absorption value was continuously recorded. The activity of catalase (CAT) was determined by ultraviolet absorption method[6]. Specifically, a 3 ml reaction system contained PBS (50 mmol/L, pH 7.0) 1.9 ml, H2O2 (0.75%) 1.0 ml and enzyme extract 0.1 ml, and the change of the OD460 absorption value was continuously recorded.
Determination of flavonoid content
The determination was carried out referring to the method of Nogués et al.[11] with some modifications. A certain area of the leaf discs was taken and cut into pieces, which were put into a 10 ml graduated test tube, which was then added with 5 ml of acidified methanol solution (methanol∶hydrochloric acid=99∶1, v/v), sealed, and extracted in a dark place at 4 ℃ for 24 h. A certain amount of the extract (0.25 ml) was diluted 100 times and measured for the absorbance at 300 nm on a UV200 ultraviolet spectrophotometer. The content of flavonoids (A300/cm2) was expressed as the absorption value of unit area of the leaf in 5 ml of extract at this wavelength.
Determination of malondialdehyde
The content of malondialdehyde was determined by the thiobarbituric acid (TBA) colorimetric method[17]. A certain area of the leaves was added with 10 ml of 5% trichloroacetic acid (TCA) solution. After grinding, the resulting homogenate was centrifuged at 3 000 r/min for 10 min, obtaining a supernatant for later use. A certain amount of MDA extract (1.5 ml) was added in a 10 ml graduated test tube, which was then added with 2.5 ml of 5% trichloroacetic acid solution of 0.5% thiobarbituric acid solution. After mixing well, the reaction system was heated in a boiling water bath for 10 min, cooled rapidly and transferred into a centrifuge tube. After centrifuging at 3 000 r/min for 15 min, the absorbance of the supernatant was determined at 532, 600 and 450 nm, respectively.
MDA concentration (μmol/L)=6.45×(A532-A600)0.155×L-0.65×A450(1)
MDA content (nmol/cm2)=MDA concentration in extract×Dilution factor×4A(2)
Wherein A532, A600 and A450 are the absorbance values at the corresponding wavelengths; 0.155 is the μmol extinction coefficient of MDA at the wavelength of 532 nm; 4 is the volume of the reaction system solution (ml); L is the optical path of the cuvette (1 cm); A is the leaf area (cm2); and the dilution factor is calculated according to Dilution factor=Total extract volume÷Solution volume transferred.
Statistical analysis of data
The data was sorted by Microsoft Excel 2003 (LSD method) and analyzed by SPSS 16.0. Values were expressed as mean±standard error (S.D.).
Results and Analysis
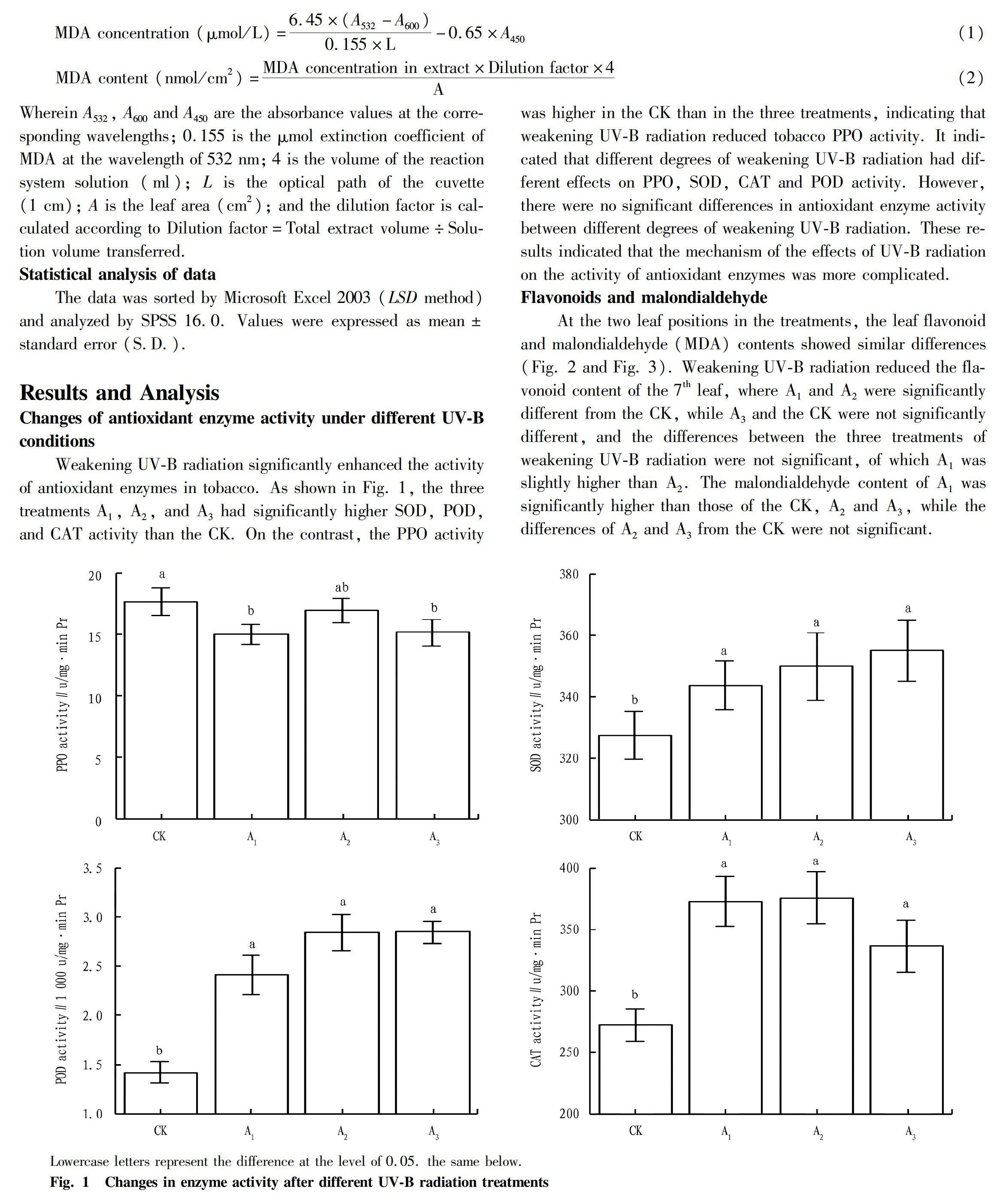
Changes of antioxidant enzyme activity under different UV-B conditions
Weakening UV-B radiation significantly enhanced the activity of antioxidant enzymes in tobacco. As shown in Fig. 1, the three treatments A1, A2, and A3 had significantly higher SOD, POD, and CAT activity than the CK. On the contrast, the PPO activity was higher in the CK than in the three treatments, indicating that weakening UV-B radiation reduced tobacco PPO activity. It indicated that different degrees of weakening UV-B radiation had different effects on PPO, SOD, CAT and POD activity. However, there were no significant differences in antioxidant enzyme activity between different degrees of weakening UV-B radiation. These results indicated that the mechanism of the effects of UV-B radiation on the activity of antioxidant enzymes was more complicated.
Flavonoids and malondialdehyde
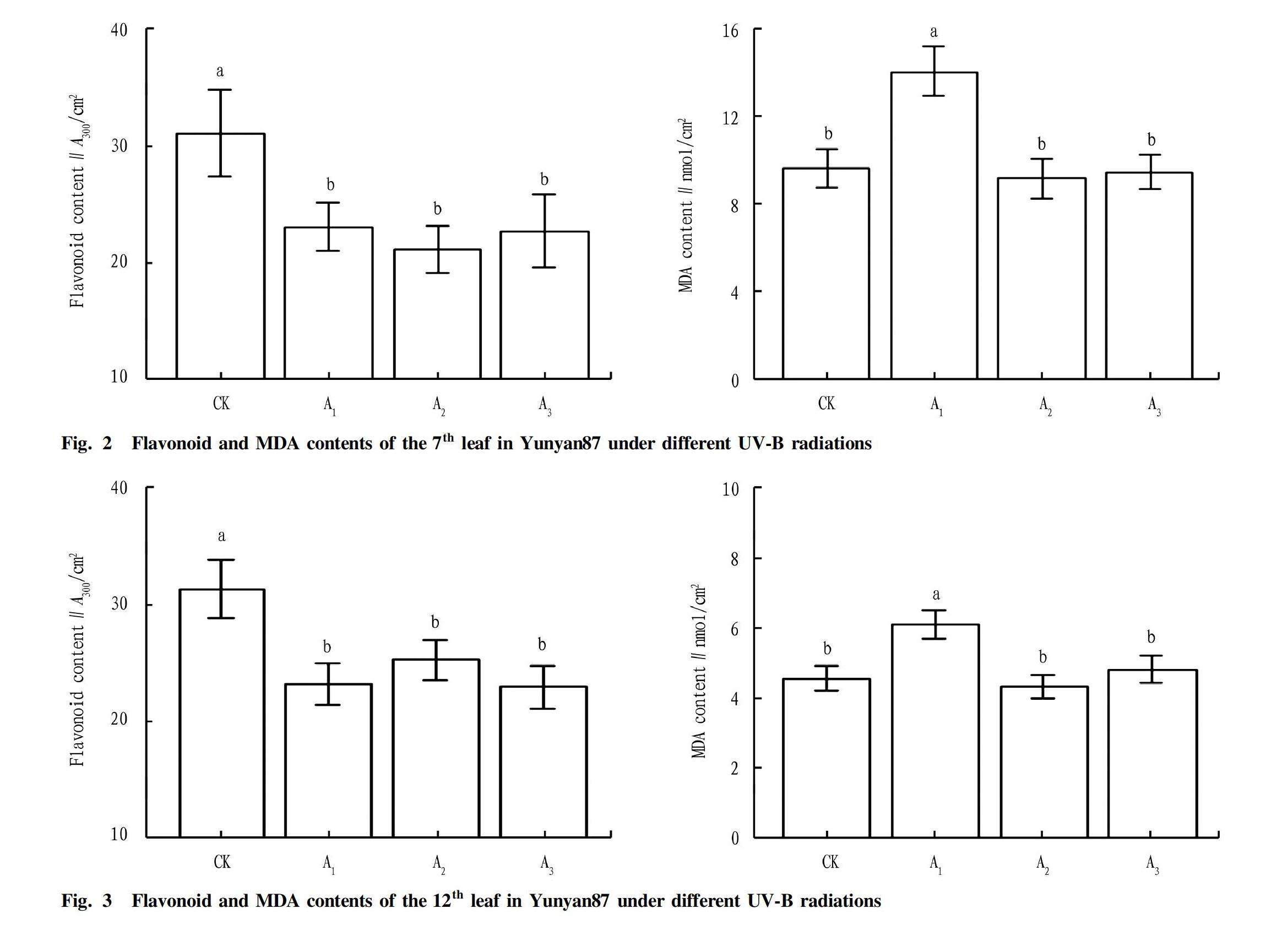
At the two leaf positions in the treatments, the leaf flavonoid and malondialdehyde (MDA) contents showed similar differences (Fig. 2 and Fig. 3). Weakening UV-B radiation reduced the flavonoid content of the 7th leaf, where A1 and A2 were significantly different from the CK, while A3 and the CK were not significantly different, and the differences between the three treatments of weakening UV-B radiation were not significant, of which A1 was slightly higher than A2. The malondialdehyde content of A1 was significantly higher than those of the CK, A2 and A3, while the differences of A2 and A3 from the CK were not significant.
The weakening of UV-B radiation significantly reduced the content of flavonoids in the 12th leaf. With the weakening of UV-B radiation, the content of flavonoids gradually decreased, but the differences between the three treatments with weakened UV-B radiation were not significant. Treatment A1 also increased the malondialdehyde content of tobacco leaves, and had a significant difference from the CK, while the malondialdehyde contents of A2 and A3 were not significantly different from the CK, but were significantly different from A1.
Conclusions and Discussion
Plants in their natural environment must be affected by solar UV-B radiation during their growth and development. Studies have shown that enhanced UV-B radiation can induce an increase in the production rate of reactive oxygen species (ROS) in plants, which intensifies the oxidative stress that plants are exposed to, thus affecting a series of physiological metabolic processes. An important way for plants to defend against UV-B damage is improved antioxidant enzyme activity. A large number of studies have shown that under the influence of UV-B radiation, plant superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) show different changing characteristics, and there are inter-species and intra-variety differences[3-7]. The results of this study showed that weakening UV-B radiation significantly increased the activity of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) in flue-cured tobacco, indicating that flue-cured tobacco in the low-latitude plateau area of Yunnan has adapted to the local higher UV-B radiation, and grown under high UV-B radiation conditions for a long time, flue-cured tobacco has formed a unique antioxidant enzyme system. The treatments of weakening UV-B radiation might partially change the antioxidant enzyme system of flue-cured tobacco, but there were no significant differences in the activity of antioxidant enzymes between different degrees of weakening UV-B radiation. Flavonoids are involved in the photoprotection effect on plant damage caused by UV-B radiation, but the role of flavonoids in protecting plant photosynthesis is limited[19]. The results of this study showed that when the UV-B radiation was weakened, the flavonoid content of tobacco leaves also decreased, indicating that there is a positive correlation between UV-B radiation and the flavonoid content of tobacco leaves. The cell membrane system is one of the main targets of UV-B radiation damage. After the membrane system is damaged by UV-B radiation-induced peroxidation, the content of malondialdehyde (MDA) is mainly increased[20-21]. In this study, under the condition of A1, the MDA content of the leaves was significantly higher than those of other treatments, which might be that the self-defense of the cell membrane system changed due to the change of UV-B radiation. Presumably, the reason might be that under the conditions of A2 and A3, the tobacco leaves received less UV-B radiation stress which was not in their sensitive range, so the differences of A2 and A3 from the CK were not significant.
The results of this study show that Yunyan 87 is very sensitive to changes in UV-B radiation intensity. Is there a range near A1 that is sensitive to changes in UV-B radiation? This study shows that 70.01%-75.04% of UV-B radiation intensity might be the lower limit of the UV-B radiation intensity suitable for flue-cured tobacco growth.
Flue-cured tobacco in Yunnan has been adapted to high UV-B radiation during long-term evolution and growth, and partially reduced UV-B radiation has a greater impact on antioxidant contents. However, with the decrease of UV-B radiation, the sensitivity of flue-cured tobacco gradually decreased. The weakening of UV-B radiation increased the content of most antioxidants. These results also indicate that the mechanism of flue-cured tobaccos effect on the antioxidant system needs further study.
References
[1] HE JM, SHE XP, MENG ZN, et al. Reduction of rubisco amount by UV-B radiation is related to increased H2O2 content in leaves of mung bean seedlings[J]. Journal of plant physiology and molecular botany, 2004, 30(1): 291-296.
[2] WU XH, LUO XY. Influence of UV-B radiation on contents of rubisco and H2O2 and proteolytic activity in leaves of alfalfa[J]. Chinese Journal of Grassland, 2008, 30(1): 27-32. (in Chinese)
[3] YAN B, DAI QJ. Effects of ultraviolet B on active oxygen metabolism and membrane system in rice leaf tissue[J]. Plant Physiology Communications, 1996, 22(4): 373-378. (in Chinese)
[4] CHEN T, WANG XL. The effect of enhanced UV-B radiation on the activities of CAT, POD and SOD in wheat leaves[J]. Journal of Wuhan Botanical Research, 1999, 17(2): 101-104. (in Chinese)
[5] LIU Y, ZHONG ZC, WERGER MJA, et al. Effects of α-NAA and UV-B radiation on photosynthetic pigments and protective enzyme activities in the seedlings[J]. Acta Ecologica Sinica, 2003, 23(1): 8-13. (in Chinese)
[6] YU J, TANG XX, ZHANG PY, et al. Effects of CO2 enrichment on photosynthesis, lipid peroxidation and activities of antioxidativeenzymes of Platymonas subcordi formis subjected to UV-B radiation stress [J]. Journal of Integrative Plant Biology, 2004, 46(6): 682-690.
[7] WU XC, LIN WX, GUO YC, et al. Effect of enhancing ultraviolet-B radiation on antioxidant systems in rice seedling leaves[J]. Fujian Journal of Agricultural Sciences, 2001, 16(3): 51-55. (in Chinese)
[8] HE DL, WANG CH, HE YH, et al. The effect of reduction of ultraviolet-B irradiance on the content of flavonoid in leaves of wheat[J]. Chinese Journal of Agrometeorology, 2003, 24(4): 32-34. (in Chinese)
[9] GU J, CHANG YL, ZHOU P, et al. Monthly changes of SOD and POD activities in Primula and their relations[J]. Acta Botanica Boreali-Occidentalia Sinica, 2006, 26(4): 766-771. (in Chinese)
[10] GABERIK A, NOVAK M, TROT T, et al. The influence of enhanced UV-B radiation on the spring geophyte Pulmonaria officinalis[J]. Plant Ecology, 2001(154): 51-56. (in Chinese)
[11] NOGUS S, ALLEN DJ, MORISONJIL, et al. Ultraviolet-B radiation effects on water relations, leaf development, and photosynthesis in droughted pea plants[J]. Plant Physiology, 1998(117): 173-181.
[12] WANG SY, LU H, YANG J. Effects of different planting altitudes on main chemical components of flue-cured tobacco in Qujing area[J]. Southwest China Journal of Agricultural Sciences, 2007, 20(1): 45-48.
[13] ZHOU P, CHEN ZY. Analysis of the spatio-temporal characteristics of UV-B strength change over the Yunnan Plateau[J]. Journal of Natural Resources, 2008, 23(3): 487-493. (in Chinese)
[14] ZI XN, QIANG JY, CHEN ZY, et al. Influence of UV-B radiation on the color chlorophyll change of Primula henryi[J]. Journal of Agro-Environment Science, 2006, 25(3): 587-591. (in Chinese)
[15] LUO LQ, CHEN ZY, DING JL, et al. The influence of low latitude and high elevation regions UV-B radiation on flavonoids content of Primula[J]. Journal of Yunnan Agricultural University, 2007, 22(2) : 229-233
[16] ZOU Q. Plant physiological and biochemical experiment technology and guidance[M]. Beijing: China Agricuture Press,1995: 36-39. (in Chinese)
[17] SUN Q, HU JJ. Plant physiology research techniques[M]. Yangling: Northwest Agriculture & Forestry University press, 2006: 165-172. (in Chinese)
[17] GU J, CHEN ZY, ZI XN, et al. Response mechanism of plant enzymatic system to UV-B radiation[J]. Chinese Journal of Ecology, 2006, 25(10): 1269-1274. (in Chinese)
[18] LIU M, LI RG, FAN H, et al. Effects of UV-B radiation on photosynthetic pigments and several enzymes in flue-cured tobacco[J]. Acta Botanica Boreali-Occidentalia Sinica, 2007, 27(2): 291-296. (in Chinese)
[19] SHANGGUAN ZP, ZHENG SX. Plant water physiology ecology and climate environment change on the Loess Plateau[M]. Beijing: Science Press, 2008. (in Chinese)
[20] PANDELOVA I, HEEWITT SR, ROLLINS-SMITH LA, et al. UV-B dose-toxicity thresholds and steady-state DNA-photoproduct levels during chronic irradiation of inbred Xenopus laevis tadpoles[J]. Photochemistry and Photobiology, 2006(82): 1080-1087.
[21] HILAL M, RODRGUUEZ-MONTELONGO L, ROSA M, et al. Solar and supplemental UV-B radiation effects in lemon peel UV-B-absorbing compound content-seasonal variation[J]. Photochemistry and Photobiology, 2008(84): 1480-1486.
- 农业生物技术(英文版)的其它文章
- Investigation on Agronomic Characters of Dwarf Mutant 778 in Broomcorn Millet (Panicum miliaceum L.) and Analysis of Its Sensitivity to GA
- Construction of Technology System on Development and Repropagation in Vitro of Several Cultivars in Pear
- Construction of Camellia oleifera Cultivation Standardization System
- Nutrients Determination in Nuts from Different Torreya grandis Cultivars
- Photosynthetic Physiological Response to Drought Stress of Populus euphratica at Different Ages in Minqin
- Analysis of Differences in Biochemical Components Between Yunnan and Kenya Tea Tree Varieties

