In-vitro Antibacterial Effect of Hedyotis hedyotidea Ethanol Extract
Fengyan SONG Chanxin CHEN
Abstract [Objectives] This study was conducted to investigate the antibacterial effect of the extract from Hedyotis hedyotidea (DC) Merr.
[Methods] Through in-vitro antibacterial tests, the antibacterial effects of H. hedyotidea ethanol extract on Escherichia coli, Staphylococcus aureus and Bacillus subtilis were analyzed.
[Results] The extract had a low-susceptibility inhibitory effect to the three kinds of bacteria, and the minimal inhibitory concentrations (MICs) corresponding to S. aureus, E. coli and B. subtilis were 200, 175 and 150 mg/ml, respectively. The minimal bactericidal concentrations (MBCs) corresponding to E. coli and S. aureus were both 500 mg/ml.
[Conclusions] This study provides theoretical support for the wide application of H. hedyotidea.
Key words Hedyotis hedyotidea (DC) Merr; Antibacterial effect; Extract; Efficacy
Hedyotis hedyotidea (DC) Merr is a plant of Hedyotis in Rubiaceae, which is distributed in Guangdong, Guangxi and Yunnan, and commonly used in Chaoshan area. H. hedyotidea is rich in a variety of biological components[1-2]. It is used as a medicine to treat diseases such as wind-heat type common cold, cough with lung heat, heat stroke and high fever, enteritis and carbuncles, but related research literatures are very limited. Staphylococcus aureus and Escherichia coli are the most common pathogenic bacteria, and Bacillus subtilis has broad-spectrum drug resistance. In this study, with above three kinds of bacteria as objects, the in-vitro bacteriostatic effect of H. hedyotidea ethanol extract was analyzed, aiming to provide research support for the application of this herb.
Materials and Methods
Experimental materials
H. hedyotidea was collected in Jiexi County, Guangdong. E. coli, S. aureus and B. subtilis were provided by the Microbiology Laboratory of Hanshan Normal University.
Main reagents: Beef extract; peptone; agar; sodium chloride, sodium hydroxide and absolute ethanol, all analytically pure.
Main instruments: Multifunctional pulverizer; constant-temperature water bath; rotary evaporator; vacuum filter; LRH biochemical incubator; steam sterilizer.
Experimental methods
Preparation of H. hedyotidea ethanol extract
The fresh H. hedyotidea was subjected to impurity removal, cleaning and drying. The material was pulverized into powder, which was sieved with a No. 4 sieve and stored in dark place for later use.
A certain amount of H. hedyotidea powder (50 g) was accurately weighed into a flask and added with 75% ethanol (300 ml) at room temperature for 1 h. The mixture was reflux-extracted in a 70 ℃ water bath for 2 h and vacuum filtered, obtaining a filtrate, which was preserved. The residue was reflux-extracted twice with 75% ethanol (200 ml) in a water bath. The three filtrates were mixed and concentrated to 50 ml (1 g/ml). The concentrate was stored at 4 ℃ in a sealed place in dark. It should be sterilized with a sterile microporous membrane (0.22 μm) before use.
Preparation of bacterial suspensions
An appropriate amount of activated E. coli or S. aureus strain was scratched and inoculated into liquid medium (4 ml), and cultured on a shaker for 24 h (37 ℃, 130 r/min) to make a bacterial suspension.
The activated B. subtilis was inoculated on a slant solid medium and cultured at 37 ℃ for 7 d. The spores was washed off with sterile saline solution and heated at 65 ℃ for 30 min to make a 4.5 ml of spore suspension[3].
Plate colony counting
The corresponding bacterial suspension (0.2 ml) was pipetted into a solid medium at 46 ℃ and mixed. After solidification, it was cultured at 37 ℃ for 24 h. Plates with a number of colonies between 30 and 300 CFU (colony-forming unit) without colonies growing in a sprawl pattern were selected to count the total number of colonies. Below 300 CFU, the number of colonies was recorded, and above 300 CFU, it was not countable.
Antimicrobial susceptibility test (oxford cup method)
The corresponding bacterial suspension (1×106 CFU/ml, 0.2 ml) was accurately coated evenly on a beef extract peptone plate. Later, three Oxford cups (inner diameter 6 mm, outer diameter 8 mm, height 10 mm) were put on the surface of the bacterial culture medium in a triangular pattern with the cup edge not less than 10 mm away from the inner edge of the culture dish and the cup spacing not less than 20 mm. In the test group, the H. hedyotidea ethanol extract (1 g/ml, 0.2 ml) was added to each cup; the cups of the negative control was added with 75% ethanol, respectively; and the blank control was added with normal saline. Each treatment was set with three replicates. Culture was performed at 37 ℃ for 24 h. The inhibition zones which were completely transparent and uniformly shaped were selected for measurement, and a diameter greater than 8.0 mm was judged to have an antibacterial effect.
Determination of minimal inhibitory concentration (MIC)
A two-fold dilution method was used to prepare the antimicrobial concentration gradients of the H. hedyotidea extract (500, 250, 125, 62.5, 31.3, 15.6, 7.8, 3.9, 0 mg/ml), and the corresponding bacterial suspension (106 CFU/ml, 0.2 ml) was added to each, followed by mixing and cultivation at 37 ℃ for 24 h. The turbidity of the culture solution was observed, and the range of minimal inhibitory concentration (MIC) was preliminarily determined. A saline control was set up simultaneously. The concentration gradients of the extract were further determined accurately within the range of the minimal inhibitory concentration preliminarily determined, so as to determine the more accurate minimal inhibitory concentration.
Determination of minimal bactericidal concentration (MBC)
According to the results of the antimicrobial susceptibility test, a certain amount of the culture solution (0.2 ml) in the test tube observed to have no bacterial growth by naked eyes was drawn up and evenly coated on a solid culture dish. After the culture solution was dried, it was cultured upside down at 37 ℃ for 24 h. The blank control group was also coated and cultured. The drug concentration corresponding to the culture solution with an average colony count of less than 5 in the plate was the MBC of the bacterial strain.
Results and Analysis
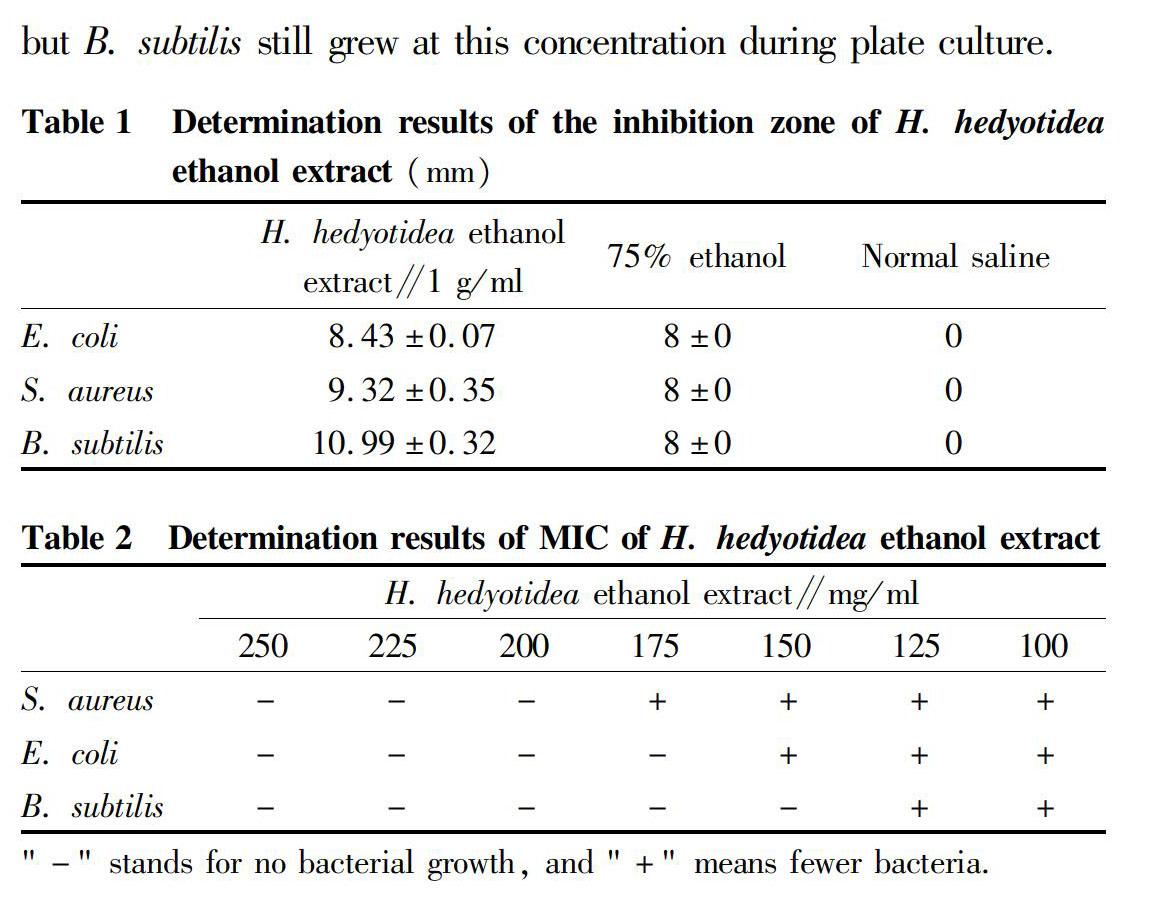
Determination results of the inhibition zone of H. hedyotidea ethanol extract
The H. hedyotidea ethanol extract had a low-susceptibility antibacterial effect against E. coli, S. aureus and B. subtilis[4]. The inhibitory effect of this extract on B. subtilis was relatively obvious, as shown in Table 1.
Determination results of minimal inhibitory concentration (MIC) of H. hedyotidea ethanol extract
The MIC ranges of the three bacterial strains were determined to be 125-250 mg/ml by preliminary test. Seven concentration gradients (250, 225, 200, 175, 150, 125, 100 mg/ml) within this range were set for the secondary test. The results are shown in Table 2.
Determination results of MBC of H. hedyotidea ethanol extract
From the test results, it could be seen that the H. hedyotidea ethanol extract had an MBC of 500 mg/ml for E. coli and S. aureus,but B. subtilis still grew at this concentration during plate culture.
Conclusions and Discussion
The H. hedyotidea ethanol extrac had a low-susceptibility inhibitory effect on S. aureus, E. coli and B. subtilis, and the inhibitory effects on B. subtilis and S. aureus were relatively stronger. The minimal bactericidal concentrations against E. coli and S. aureus in vitro were both 500 mg/ml. The research results suggest that the herb H. hedyotidea has a definite therapeutic effect on bacterial diseases such as bacterial enteritis and carbuncles.
References
[1] CHEN YF, TAO SH, YU JZ, et al. Screening of anti-inflammatory and analgesic effective parts of Hedyotis hedyotidea[J]. Traditional Chinese Drug Research and Clinical Pharmacology, 2012, 23(1): 17-19. (in Chinese)
[2] YUAN ZH, LIU Y, SHANG LX, et al. Isolation and content determination of hydrangenol in leaves of Hedyotis Hedyotidea (DC) Merr by HPLC[J]. Chinese Journal of Information on Traditional Chinese Medicine, 2018, 25(03): 94-97. (in Chinese)
[3] HUANG J, WEI T. Comparison of survival time of bacterial suspensions prepared by three kinds of dilutions[J]. Chinese Journal of Health Laboratory Technology, 2015, 25(12): 1948-1951. (in Chinese)
[4] PAN LW, LUO ZP, CHEN JY. Study on the chemical constituents in leaves of Clerodendrum bungei and antibacterial effect of its extract[J]. Popular Science & Technology, 2018, 20(10): 18-20. (in Chinese)
- 农业生物技术(英文版)的其它文章
- Investigation on Agronomic Characters of Dwarf Mutant 778 in Broomcorn Millet (Panicum miliaceum L.) and Analysis of Its Sensitivity to GA
- Construction of Technology System on Development and Repropagation in Vitro of Several Cultivars in Pear
- Construction of Camellia oleifera Cultivation Standardization System
- Nutrients Determination in Nuts from Different Torreya grandis Cultivars
- Photosynthetic Physiological Response to Drought Stress of Populus euphratica at Different Ages in Minqin
- Effects of UV-B Radiation on the Activity of Antioxidants in Flue-cured Tobacco Leaves

