Outdoor Feeding Without Antibiotics a Better Pattern for the Liver Health of Gushi Chicken
Lili JI Wei WANG Lin CHEN Bo HOU Jiamin ZHANG Ting BAI
Abstract A 180 d feeding trial was conducted to evaluate the effect of different feeding modes on liver of Gushi chicken. The chickens from 1-day-old were fed in the warm house for 30 d, and then divided into four groups by different feeding modes (O-noant, outdoor without antibiotics; O-ant, outdoor with antibiotics; D-noant, indoor without antibiotics; D-ant, indoor with antibiotics), respectively, to evaluate the effect on liver. The results showed that different feeding modes significantly influenced the growth, liver weight, subcutaneous fat thickness, liver fat and liver free amino acid contents. The outdoor feeding without antibiotic reflected remarkably (P﹤0.05) low liver weight and fat content with normal morphology. While, other groups showed different levels of fat metabolism disorder in the liver. Based on chicken liver healthy, the outdoor feeding without antibiotic was the optimal feeding mode for Gushi chicken.
Key words Feeding mode; Gushi chicken; Liver; physiology
Gushi chicken is a famous local high-quality product in China. It was famous both at home and abroad for its beautiful appearance, strong resistance, delicious meat, unique flavor, and rich nutrition[1-2]. Due to limitation in the raised number with traditional feeding mode, the indoor feeding mode occurred. Therefore, in order to prevent the diseases caused by increased breeding density, the antibiotics were used for preventing diseases and improving the growth performance of the chicken[3], which solves the problems of demand and survival, but bring a threat to the health of consumers[4-5]. Therefore, it is most important to find an optimal feeding mode for Gushi chickens to reduce the threat to human health.
Liver is the biggest organ of internal organs. It associated with the body lipid metabolism and protein synthesis, which plays an important role in synthesis, decomposition and metabolism[6-7]. In particular, ectopic fat accumulation occurs when the energy storage capacity of the adipose tissue was exceeded[8-9]. Moreover, in some special circumstances, such as drug stimulation, will affect the liver fat metabolism function[10]. Liver fat metabolism dysfunction can cause hepatocyte damage, then affect other functions of liver, and ultimately affect the health of body[11-14]. Thus, liver health was important to animals health.
Therefore, the objective of our research was to study the changes in chicken liver with different feeding modes and provide basic data for the appropriate feeding mode. Therefore, four feeding modes (O-noant, outdoor without antibiotics; O-ant, outdoor with antibiotics; D-noant, indoor without antibiotics; D-ant, indoor with antibiotics) were applied in Gushi chicken for 180 days and the liver indexes were evaluated in this research.
Materials and Methods
Gushi chickens, feed and the antibiotics
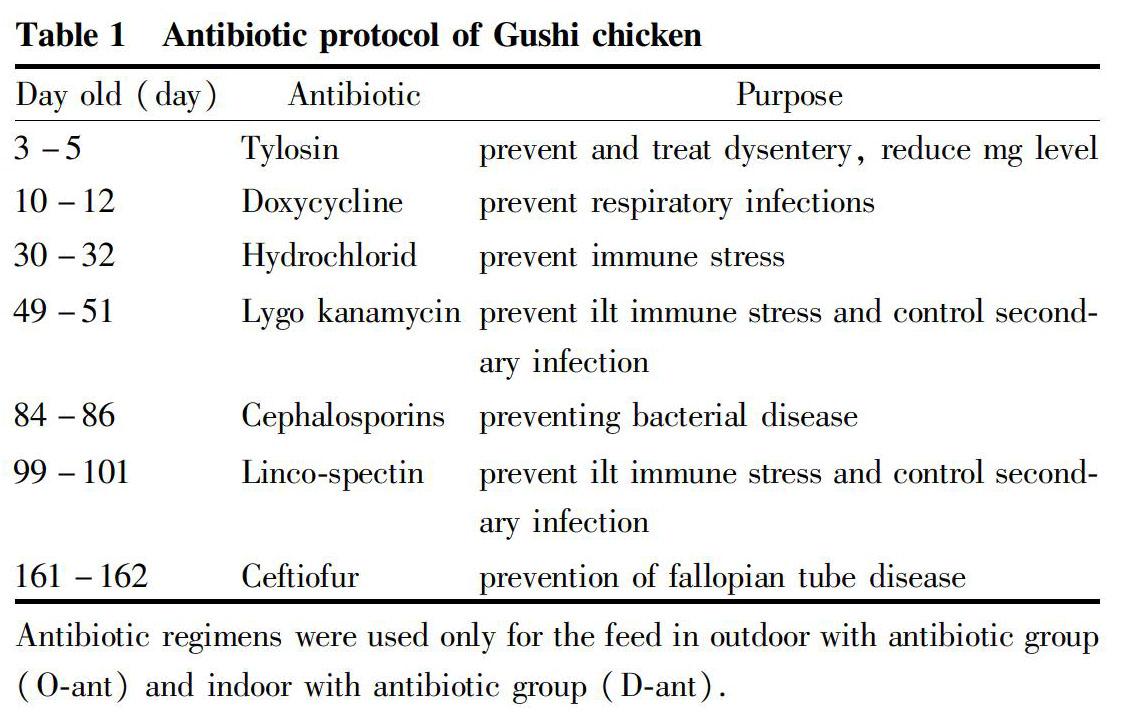
All experiments were in obedience to the guidelines of the Animal Care Committee from Chengdu University (permit No.CDU-20161012001). 1-day-old Gushi chickens were bought in Henan Sangao agriculture Co., Ltd. The 1 to 30-day-old chickens were fed in the warm house, the chickens were divided into 4 groups (O-noant, O-ant, D-noant, D-ant) after 30 d of feeding, then fed in the farm outdoor or indoor with or without antibiotics. Each group had 10 chickens and 6 repeats. The experiment was conducted from March to September. The outdoor groups were feeding in a farm about 200 m2 with trees and grass land, the temperature was 10-25 ℃. The area of indoor was 0.5 m×2.0 m×0.5 m, and the temperature was 15-25 ℃. The feed was all the same, which was made by corn, bean pulp, wheat bran and gunk. The antibiotics were feed as Table 1.
General indexes test
Ten chickens were randomly selected from each group, and their weight, liver weight, subcutaneous fat thickness and liver fat were measured. As for the measurement of content of liver fat, the crude fat content in liver was determined by Soxhlet extraction with 5 g of sample, and the rest was stored for the following test[15].
Gross pathology and Liver color evaluation
At the end of the growing period, three chickens from each group were randomly selected to be euthanized. The chickens were secured and the skin was cut from the abdomen down to the jaw along the midline of the abdomen, and then peeled off to the sides, so that the livers were fully exposed. In addition, the livers were sampled for color evaluation.
Objective liver color (CIE-L*a*b) measurement was carried out with Minolta colorimeter (Minolta CR-400, Konica Minolta, Japan), using illuminant D65 (Daylight at noon), 8 mm diameter aperture and a 10° standard observer. The same colorimeter was standardized prior color determination using the ceramic white tile as per the directions of the instrument manual. Color scans were collected under the CIE-LAB system. L* (lightness) is measured from black to white, a* (redness) has a negative value for green and a positive value for red and b* (yellowness) values have a negative value for blue and a positive value for yellow. Six measurements were taken per slice, and the averages were used in the statistical analysis[16-17].
Liver free amino acid analysis
The liver sample 6.00 g in 60 ml, 0.2 mol/L phosphate buffer (pH 6.5), was mechanical homogenized (6 000 rmp, 3 min), and centrifuged by 10 000 g, 4 ℃ for 20 min. 0.5 ml of the supernatant was adjusted to pH 2.0 with 3% salicylic acid solution, mixed with 0.25 ml of double distilled water, which then centrifuged (15 000 g, 4 ℃, 20 min) and determined with an automatic amino acid analyzer. Test condition: The eluent was citric acid buffer (pH 3.3-4.9), the color-substrate solution was ninhydrin: ethylene glycol methyl ether: sodium acetate buffer (2∶75∶25), and amino acids were tested at 570 nm, except hydroxyproline at 440 nm.
Liver HE staining
The liver sample was immersed in neutral formalin and fixed. After dehydration, transparent, and embedded in paraffin wax, the block was sliced to 5 μm thickness. Then, the sections were stained with H&E and observed under the microscope and photographed (Olympus, DP72, Olympus Imaging, Japan).
Liver oil red o staining
The fresh tissues were fixed in 4% par formaldehyde more than 24 h. After section and oil red O staining, the liver sections were observed under the microscope and photographed (Olympus, DP72, Olympus Imaging, Japan).
Statistical Analysis
Data were expressed as mean±SD of the number of animals used in each experiment. Statistical analysis was performed using two-way ANOVA and Tukey post hoc test. Values of P<0.05 were considered statistically significant.
Results and Analysis
General index test
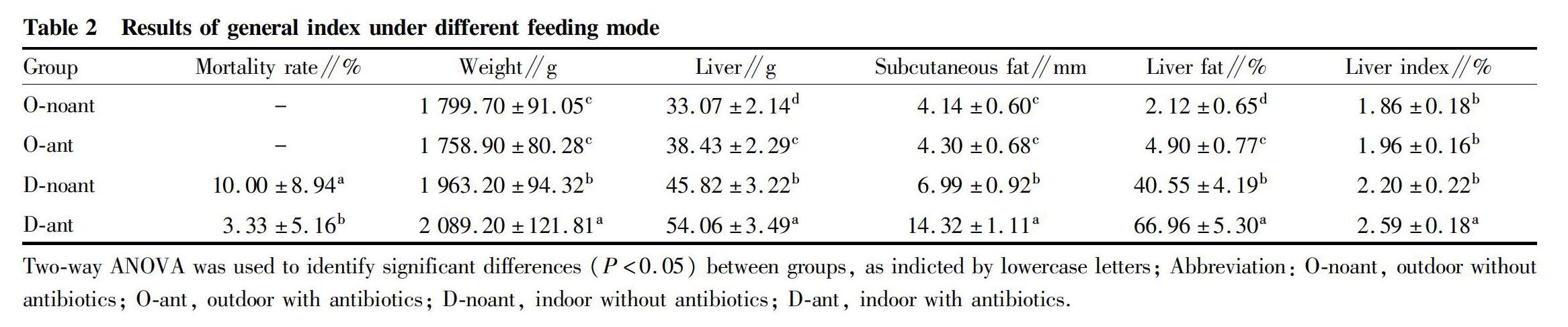
After 180 d of feeding, the results of the general index under different feeding mode were presented in Table 2. There were no deaths in groups O-noant and O-ant, while the mortality rates in groups D-noant and D-ant were (3.33± 5.16)% and (10.00±8.94)%, respectively. The chickens of group O-noant had the lowest liver weight, subcutaneous fat, liver fat, and liver index. However, there showed an opposite trend in D-ant group chickens. The weight, liver weight, subcutaneous fat thickness and liver fat content of the chickens in the outside groups (groups O-noant and O-ant) were significantly lower than those in the indoor feeding groups (groups D-noant and D-ant). Moreover, the antibiotic groups (groups O-ant and D-ant) were significantly higher than the no-antibiotic groups (groups O-noant and D-noant) in liver weight and liver fat content in the same feeding condition.
Gross pathology and Liver color evaluation
Photograph records of livers in different groups were showed in Fig. 1. The results showed that there were differences in the color of liver among chickens of different groups. The livers of group O-noant were ruddy and elastic. Large areas of livers in group O-ant were brownish yellow with blunt edges. The liver color of group D-noant was slightly yellow and their edges were blunt. The livers of group D-ant showed a completely abnormal color, which was yellow, greasy surface and crisp texture.
Our results about the color test results as light (L), red (a) and yellow (b) index showed that the L index was the same trend of change with the b index and opposite with the a. The O-noant group liver showed the lowest light (L, 40.54±1.75) and yellow (b, 11.50±2.06), and the highest red (a, 13.93±2.31). The chroma of yellow in B group was significance higher than O-noant group, while other chromas were in the same level. The color index L (66.45±5.93) and b (35.68±2.28) in D-ant group were the highest and index a (7.64±1.12) was the lowest.
Liver free amino acid (FAA) analysis
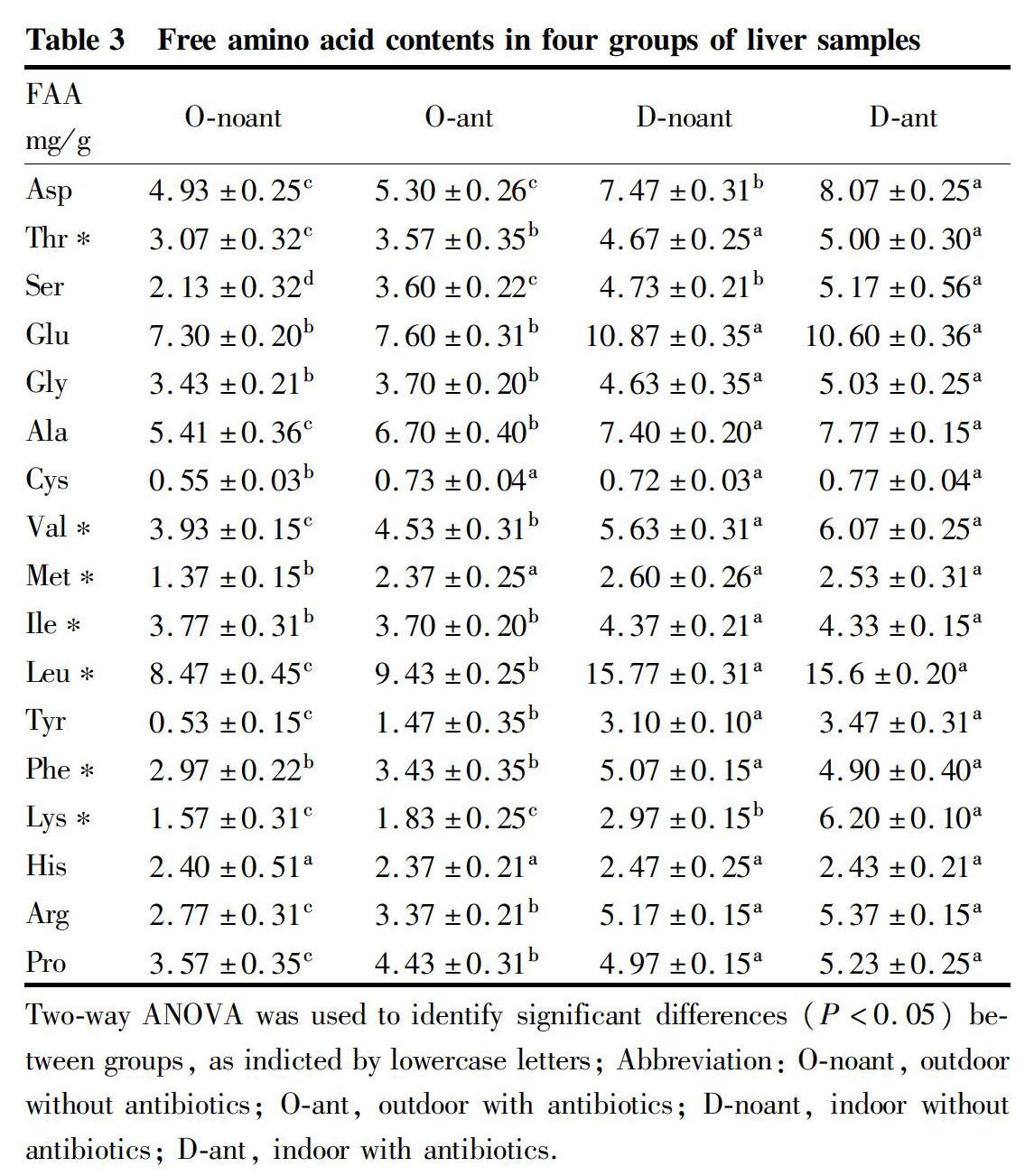
The contents of free amino acid in livers of different groups are presented in table 3. The total FAA contents varied between (58.08±0.69) and (98.54±0.87) mg/g, with the lowest in O-noant group and the highest in D-ant group. There were significant differences in total contents among the four groups. The indoor feeding groups were higher than the outdoor feeding groups. In the same feeding environment, the antibiotic groups were higher than the no-antibiotic groups. His was in the same level of the four groups. Thr, Ser, Gly, Glu, Ala, Val, Ile, Leu, Tyr, Phe, Lys, Arg, and Pro were in the different level of indoor and outside groups. The contents of water-soluble amino acids in groups O-noant and O-ant were (25.25±0.31) and (29.83±0.82) mg/g, respectively, which were in the same significant level.
Different types of amino acids contents in different group are shown in Fig.2. The water-soluble amino acid contents of indoor groups were significantly different from that in the outdoor groups. The contents of lipid-soluble amino acids were higher than the water-soluble in each group. The indoor groups lipid-soluble amino acid contents were significantly higher than the outdoor groups, and that in group O-ant was significantly higher than group O-noant.
Histopathological observation
To observe the changes of livers, pathological observation was carried out in the livers of different groups of Gushi chickens. The results of the tissue sections with HE staining are shown in Fig. 3. The histological examination revealed that the liver cells of group O-noant were morphologically normal, arranged densely, and the hepatic cords were arranged neatly (Fig. 3 A). There was more cytoplasm in visible liver cells in liver sections of group O-ant. Furthermore, a small amount of light staining material appeared in the cytoplasm, as well as a broadening in the intercellular space, swelling in the cells, and slight granule denaturation (Fig. 3 B). The liver of group D-noant had a large number of granules, as well as swelling in cells and obvious degeneration of granules (Fig. 3 C). The liver of group D-ant showed a large number of vacuoles, as well as swelling in cells and the hepatic cords were arranged irregularly (Fig. 3 D). All the results showed that indoor feeding (groups D-noant and D-ant) and feeding with antibiotic (O-ant and D-ant) could damage the liver of chickens.
In order to further confirm the components of liver vacuoles, the frozen sections of the liver were made with oil red O staining, and the results are shown in Fig. 4. The group O-noant showed scattered red fat droplets (Fig. 4 A). The red fat droplets increased in group O-ant (Fig. 4 B). The red fat droplets increased significantly in group O-ant (Fig. 4 C). The fat droplets in group D-ant showed an opposite trend compared with other groups, which were significantly big and wide, showing orange red (Fig. 4 D).
Agricultural Biotechnology2020
Discussion
It is now recognized that the food safety should control from the source of the food[18-19]. Ulteriorly, the meat safety had to retrospect to the animal feeding stage[20-21]. However, the popular intensive breeding led to limit the feeding space. In addition, antibiotic using to prevent disease will bring risks to meat safety[22-23]. The high-density intensive feeding was easy to spread the disease by air and excrement. Therefore, antibiotics are often used in the breeding process to prevent diseases and control mortality. However, in this study, we found that the death occurred only indoor feeding groups, which means that chickens raised in outdoors may be healthier. Moreover, our results showed that the Gushi chickens growth and fat accumulation in outdoor were slower than indoor. This could be because chickens raised in outdoor conditions have wider activity range, more feed consumption, large amount of energy for movement, and response to the temperature and the weather change. Furthermore, we found that the antibiotics were mainly affected the weight in the indoor feeding group. That might be the antibiotics keep the chickens a normal immune level in the grate density. The effect of antibiotics on the weight of chickens raised in outdoors was not significant, possibly because chickens raised in outdoors can accelerate antibiotics metabolism.
Liver is an important tissue for adipose metabolism[12,24]. According to the detection results, indoor feeding chicken had more adipose and greater demand in liver metabolism. Consequently, liver function compensation may occur, which leads to the increase in liver volume, weight, liver fat content and liver index. By comparison between the Anti and non-Anti, the liver weight, subcutaneous fat, liver coefficient and liver fat content of the non-Anti-chicken groups of the same feeding day were lower than those in the anti-feeding groups. Moreover, the weight of the non-anti-inside feeding group was significantly lower than the anti-inside feeding group. This may be due to the long-term intake of antibiotics on the liver damage[25-27]. Besides, the use of antibiotics mayhave effects on liver damage and increase the liver burden, which also influence the ability of liver fat metabolism, which result that the general index of antibiotics feeding group were higher than those in the no antibiotic feeding group, and indoor feeding groups more apparent. On the one hand, in the process of breeding, the liver needed to complete the fat metabolism. On the other hand, drug also needed to be metabolized. The liver was an important part of drug metabolism. Therefore, the feed containing antibiotic and conventional prevention medication will aggravate the burden on drug metabolism of the liver, which affected the fat metabolism of the liver and caused fat granule in the liver cells and the fat content in the liver increase, as well as the swelling in the cells and weight increase of the liver.
After the protein in the diet is digested, it is absorbed in the form of FAA through the intestine, and finally enters the liver through the portal vein of the liver, and most of the FAA will be transformed and metabolized in the liver[28-29]. Our results showed that the FAA content in the liver of the O-noant group was the lowest, while the FAA content in the D-ant group showed an opposite trend. In our experiment, the basic diet of all four groups of Gushi chickens was the same, but the FAA content in the liver of different group was significantly different, especially in the indoor groups, which were significantly higher than that in the outdoor groups. This might be because the O-noant group had healthier livers, which could convert the protein in the diet into FAA and be utilized by the body tissues. However, due to the addition of antibiotics in the indoor group, the metabolic pressure in the liver of Gushi chicken was increased, which damaged the basic functions of the liver. Therefore, the FAA could not be utilized and accumulated in the liver. Our results confirmed that outdoor feeding is beneficial to the liver health of Gushi chickens, and beneficial to the metabolism of FAA in the liver.
Vacuoles appear in cells due to the use of large number of organic solvents to dissolve fat during HE staining. In addition, when hydropic degeneration occurs in tissues, vacuoles also appear in cells. Therefore, it could not directly determine that the vacuoles occurred in the liver were hydropic or fatty degeneration[31-32]. Oily red O belonged to azo dye, and it was one of the very strong fat solvent and dye fat agent, which formed small fat drop when combined with triglyceride. By the HE and oil red O staining results, we can see the changes in the cell level directly. As is known to all, a small amount of fat exists in the liver during the normal fat metabolism of the liver cells. Chickens in the group O-noant had lower weight, lowest subcutaneous fat content, and liver fat content, which indicated that the energy and nutrition intake could provide organism against stress brought by a wide range of activities and environmental changes. This also suggests that chickens in the group O-noant consumed more energy than other three groups. Besides, the livers of chickens in group O-noant were within the normal range of anatomy and pathology. These results further confirm that outdoor non-antibiotics feeding is the best option for Gushi chicken compared to indoor feeding.
Conclusions
By the anatomy, pathological observation and general indexes test on the livers of Gushi chicken fed for 180 d in four feeding modes (O-noant, O-ant, D-noant, D-ant), it could be found that both long term feeding with antibiotics and limited range of feeding activities could affect the fat metabolism in chicken livers, which made an increase of the liver weight, liver index, subcutaneous fat thickness, and liver fat content. Besides, the fat metabolism disorder also occurred in livers. Consequently, no-antibiotic-outdoor feeding mode is recommended for raising Gushi chicken.
Availability of data and material
All data generated or analyzed are included in the article. All materials are available from the corresponding author, on reasonable request.
Acknowledgement
Ji Lili and Wang Wei were responsible for experimental design and article writing, Chen Lin was responsible for experimental animal breeding, and Hou Bo, Zhang Jiamin and Bai Ting were responsible for experimental data detection and analysis. cWe thank all authors for stimulating discussions and support.
References
[1] SUN GR, KANG XT, LI GX, et al. The Effect of different raising ways on the production performance of Gushi chicken[J]. Acta Agricultural Boreali-Sinica, 2006(21): 118-122
[2] ZHANG H, PEI SL, ZHAO SS, et al. Enhancement of SIgA secretion in duodenum of gushi chicken immunized with newcastle disease vaccine by IL-2[J]. Acta Agriculturae Jiangxi, 2010(22): 122-125
[3] MOORE P, EVENSON A. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick[J]. The Journal of biological chemistry, 1946(165): 437-441
[4] CHAISATIT C, TRIBUDDHARAT C, PULSRIKARN C, et al. Molecular characterization of antibiotic-resistant bacteria in contaminated chicken meat sold at supermarkets in Bangkok, Thailand[J]. Japanese Journal of Infectious Diseases, 2012(65): 527-534
[5] WANG W, FRIEDHELM J, CATHARIRA H, et al. Strengere Standards und intensive Kontrollen Fortschritt der Antibiotika-Vermeidung in der chinesischen Gefluegelerzeugung[J]. Fleischwirtschaft, 2016, 96(7): 24-27.
[6] BEDU E, CHAINIER F, SIBILLE B, et al. Increased lipogenesis in isolated hepatocytes from cold-acclimated ducklings[J]. American Journal of Physiology Regulatory Integrative & Comparative Physiology, 2002, 283(5): R1245-1253.
[7] BRADY L, ROMSOS DR, LEVEILLE GA. In vivo estimation of fatty acid synthesis in the chicken (Gallus domesticus) utilizing 3H2O[J]. Comparative Biochemistry & Physiology Part B Comparative Biochemistry, 1976, 54(3):403-407.
[8] BYRNE DC. Ectopic fat, insulin resistance and non-alcoholic fatty liver disease[J]. Proceedings of the Nutrition Society, 2013(72): 412-419.
[9] PERLA F, PRELATI M, LAVORATO M, et al. The role of lipid and lipoprotein metabolism in non-alcoholic fatty liver disease[J]. Children, 2017, 4(6): 46.
[10] SANTNGOSTINO A, LEONE MP, MAD R, et al. Effects of phenoxyacetic acid herbicides on chicken embryo liver drug metabolizing enzymes[J]. Basic & Clinical Pharmacology & Toxicology, 1991(68): 110-114
[11] AYDIN H, NIZAMLIO LU F. Quinolone antibiotic residues in raw milk and chicken liver in Konya Eurasian[J]. Journal of Veterinary Sciences, 2012(28): 154-158.
[12] FRAYN KN, ARNER P, YKI-J RVINEN H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease[J]. Essays in Biochemistry, 2006(42): 89-103
[13] LI F, YANG WY, LIANG R. Understanding the role of the liver in adjusting stress reactions[J]. Journal of Beijing University of Traditional Chinese Medicine, 1998(21): 20-23.
[14] SAMUEL V, PETERSEN K, SHULMAN G. Lipid-Induced insulin resistance: Unravelling the mechanism[J]. Lancet, 2010, 375(9733): 2267-2277.
[15] DASO AP, OKONKWO OJ. Conventional extraction techniques: Soxhlet and liquid–liquid extractions and evaporation[J]. American Cancer Society, 2015: 1437-1468.
[16] GAGAOUA M, PICARD B, MONTEILS V. Associations among animal, carcass, muscle characteristics, and fresh meat color traits in Charolais cattle[J]. Meat Science, 2018(140): 145.
[17] LI M, et al. Effects of protein phosphorylation on color stability of ground meat[J]. Food Chemistry, 2017(219): 304-310.
[18] MCMEEKIN TA, et al. Information systems in food safety management International[J]. Journal of Food Microbiology, 2006(112): 181-194.
[19] YU P, WU Y, HU Y, et al. Optimal policy for read rate in RFID food safety traceability system[J]. Transactions of the Chinese Society of Agricultural Engineering, 2008(24): 132-136.
[20] BAILONI L, CATTANI M. Impact of animal feeding on the nutritional value and safety of food of animal origin[M]. Springer Vienna, 2013.
[21] HUANG F, HAO P, WU H. Application of RFID middleware in agricultural product safety traceability system[J]. Transactions of the Chinese Society of Agricultural Engineering, 2008(24): 177-181.
[22] DOYLE MP, ERICKSON MC. Emerging microbiological food safety issues related to meat[J]. Meat Science, 2006(74): 98-112.
[23] LI K, LU D. Why the "factory" and intensive farming methods have been questioned (continued)——The harm and choice of "factory" farming for animal health and animal welfare[J]. Chinese animal health care, 2008(8): 15-21.
[24] WU Y, WANG Y, LI H. Research of fat metabolism factors in chicken liver[J]. Animal Husbandry & Veterinary Medicine, 2013(45): 91-95
[25] LI X. Commonly used antimicrobial agents and liver damage[J]. China Pharmacy, 1992(3): 35-36.
[26] SOBACK S, ZIV G, BOGIN E, et al. Pharmacokinetic changes of several antibiotics in chickens during induced fatty liver[J]. Research in Veterinary Science, 1987(43): 49
[27] WANG L. Antimicrobial agents and liver damage[J]. Central Plains Medical Journal, 1983(2): 040.
[28] GILBERT ER, WONG EA, WEBB KE. Peptide absorption and utilization: Implications for animal nutrition and health[J]. Journal of Animal Science, 2008(86): 2135-2155.
[30] NEWEY H, SMYTH DH. The intestinal absorption of some dipeptides[J]. J Physiol, 1959(145): 48-56
[31] TAN J, YANG G, CHEN R. Research on the liver fatty degeneration and function injury by high lipid feed and fat emulsion[J]. South China J Prev Med, 2011(37): 54-57.
[32] WANG M, CHENG CX, HE SH. The influence of chicken liver structure by aureomycin in diet[J]. Heilongjiang animal husbandry and veterinary, 2010: 146-147.
- 农业生物技术(英文版)的其它文章
- Investigation on Agronomic Characters of Dwarf Mutant 778 in Broomcorn Millet (Panicum miliaceum L.) and Analysis of Its Sensitivity to GA
- Construction of Technology System on Development and Repropagation in Vitro of Several Cultivars in Pear
- Construction of Camellia oleifera Cultivation Standardization System
- Nutrients Determination in Nuts from Different Torreya grandis Cultivars
- Photosynthetic Physiological Response to Drought Stress of Populus euphratica at Different Ages in Minqin
- Effects of UV-B Radiation on the Activity of Antioxidants in Flue-cured Tobacco Leaves

