The Effect of Magnesium on Accumulations of Primary Metabolites and Yield of Blumea balsamifera
Dan WANG Huiping LAN Yingbo ZHANG Xuan HU Xiaolu CHEN Yuxin PANG Fulai YU
Abstract [Objectives] This study was conducted to investigate the effects of magnesium on the yield of Blumea balsamifera (L.) DC. and the accumulation of primary metabolites that affect yield of the medicinal material.
[Methods] The annual seedlings of B. balsamifera were selected as experimental materials. The treatment concentrations of magnesium (Mg) were set as 0, 1.5, 15 and 150 mg/ml supplied by MgSO4·7H2O. The yield of the medicinal material was measured dynamically. And the content of total sugar was determined by 3, 5-dinitrosalicylic acid colorimetry; the content of crude protein was determined by the Kjeldahl method; the ash content was determined by the high-temperature burning method; the crude fat content was determined with a crude fat instrument; and the crude fiber content was determined by the acid-base washing and weighing method.
[Results] Mg significantly increased the yield of B. balsamifera medicinal material, especially 15 mg/ml Mg. It was found that in September, October and November, 1.5 mg/ml and 15 mg/ml Mg significantly increased the contents of primary metabolites including total sugar, ash, crude protein, crude fat and crude fiber, and 150 mg/ml of Mg increased the accumulation of total sugar, ash, crude protein and crude fiber to different degrees, but had certain inhibitory effect on the accumulation of crude fat. In December, the application of Mg inhibited the accumulation of total sugar, ash and crude protein to different degrees, but significantly promoted the accumulation of crude fat and fiber.
[Conclusions] This study provides a theoretical basis for clarifying the effects of different concentrations of magnesium on plant growth.
Key words Magnesium (Mg); Blumea balsamifera; Yield; Primary metabolites; Total sugar; Ash; Crude protein
Received: March 23, 2020 Accepted: May 8, 2020
Supported by National Natural Science Foundation of China (81403035); Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (1630032019004); Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (1630032020002).
Dan WANG (1982-), female, P. R. China, associate researcher, PhD, devoted to research about standardized production and quality control mechanism of Chinese medicinal materials (South China medicinal plants).
*Corresponding author. E-mail: fulai.yu@163.com.
The Ainaxiang medicinal material refers to leaves and shoots of plant Blumea balsamifera (L.) DC. in Asteraceae. It is widely used in Hainan, Guizhou, Yunnan and other provinces, and is also a medicine commonly used by the Li and Miao nationalities. B. balsamifera has the effects of removing wind and dampness, warming middle energizer to stop diarrhea, promoting blood circulation and detoxifying[1]. In addition to the traditional clinical application of B. balsamifera, it is a raw material for extracting natural borneol[2-3], which is widely used in medicine, cosmetics, food and other fields. At present, B. balsamifera has been planted in large areas in Guizhou and Hainan. Scholars have found that fertilization can significantly increase the yield of medicinal material B. balsamifera. He et al.[4-5] confirmed that 3 kinds of farm fertilizers (barnyard manure, oil cake and firewood ash), 3 kinds of monochemical fertilizers (urea, superphosphate and potassium chloride) and compound fertilizers can significantly improve the economic output and biological yield of B. balsamifera. Moreover, studies have found that nitrogen fertilizer can increase the yield and effective ingredient content of B. balsamifera, but excessive nitrogen fertilizer will lead to a decline in yield and quality in practice. The studies of Wang et al.[6-8] showed that in the slow growth period of B. balsamifera in winter, the application of calcium, magnesium (Mg), manganese and naphthaleneacetic acid, gibberellin and plant growth regulator DA-6 can significantly promote the growth of B. balsamifera, and increase the biomass of leaves, stems and roots. Lan et al.[9] found that the combined application of N, P and K can significantly increase the yield of B. balsamifera and the accumulation of relative content of l-borneol, and the effects on yield ranked as N>K>P. However, there is no report about the effect of Mg on the accumulation of primary metabolites and the yield of B. balsamifera medicinal material during the growth period. The yield of medicinal materials is mainly composed of substances produced by the primary metabolism of plants such as total sugar, ash, crude protein, crude fat, and crude fiber. Therefore, studying the effect of Mg on the accumulation of primary metabolites in B. balsamifera medicinal material can help understand better its effect on the yield of the medicinal materials.
Mg is an essential element for plants and plays a vital role in plant growth and development. Mg has photosynthetic physiology and enzyme activation functions, and affects active oxygen metabolism, carbon and nitrogen metabolism, and gene expression[10-11]. Studies have shown that Mg can promote the accumulation of yield and active ingredients of medicinal plants[12]. In this study, on the basis of the previous research, the effects of different concentrations of Mg on the accumulation of primary metabolites and yield of the medicinal material in B. balsamifera in the growth period were more accurately investigated by the pot cultivation method using perlite as the substrate and Hoagland nutrient solution for providing basic nutrition under the spraying of Mg on the leaves.
Materials and Methods
Materials
Plant materials and pretreatment
Uniformly growing B. balsamifera seedlings were selected as the experimental materials, and were identified as B. balsamifera (L.) DC. by associate researcher Yu of Tropical Crops Genetic Resources Institute, Chinese Academy of Tropical Agricultural Sciences. The seedlings were cultured by the hydroponic method using perlite as the substrate. Nutrition was provided for B. balsamifera with Hoagland nutrient solution. The concentration of various components in the Hoagland nutrient solution were as follows: KH2PO4 136 mg/L, H3BO3 2.86 mg/L, CuSO4·5H2O 0.08 mg/L, ZnSO4·7H2O 0.22 mg/L, Na2MoO4·2H2O 0.52 mg/L, MnSO4·H2O 1.81 mg/L, Fe-EDTA 2.5 mg/L, KNO3 510 mg/L and Ca(NO3)2 945 mg/L. Water was supplemented once a day, and the nutrient solution was supplemented every 7 d. Foliar application of Mg was performed in August and mid-September, respectively, using MgSO4·7H2O. A total of 4 concentrations of 0, 1.5, 15 and 150 mg/ml were set, respectively. Sampling was performed at the end of September, October, November and December. The yield of B. balsamifera and the total metabolites including sugar, total ash, total protein, crude fat and crude fiber were determined.
Methods
Determination of yield of the medicinal material
The shoots and leaves of B. balsamifera were collected, and measured for yield of the medicinal material with an electronic scale after dried in the shade.
Determination of total sugar content
The 3, 5-dinitrosalicylic acid colorimetric method (DNS colorimetric method) was applied to determine the total sugar content[13]. A certain amount of B. balsamifera (100.00 mg) was accurately weighed and added with 6 mol/L HCl (2 ml) and distilled water (3 ml), followed by heating to hydrolyze for 30 min. After the hydrolysate was cooled, one drop of phenolphthalein indicator was added, and the liquid was neutralized with 6 mol/L NaOH to reddish and then filtered, while repeatedly flushing the test tube and filter paper with distilled water. The filtrate was collected into a 50 ml volumetric flask, and diluted to constant volume. A certain amount of the solution (2 ml) was transferred to a 10 ml volumetric flask, and diluted to constant volume, giving the total sugar extract. A certain amount of the total sugar extract (1 ml) was transferred to a 20 ml test tube, added with DNS developing agent (1 ml), and heated in a boiling water bath for 6 min to allow color development. The solution was immediately cooled to room temperature, and added with distilled water (3 ml), followed by mixing well. Color comparison was performed at a wavelength of 520 nm, and the total sugar content was calculated according to formula.
Determination of ash content
Referring to "Chinese Pharmacopoeia" (2015 edition)[1], the high-temperature burning method was used to determine the ash content of B. balsamifera medicinal material.
Determination of crude protein content
The Kjeldahl method was applied to determine the crude protein content[14]. The medicinal material B. balsamifera accurately weighed (1.000 g) was added into a digestion tube, and then added with concentrated sulfuric acid (10 ml) and a mixture of copper sulfate and potassium sulfate with a ratio of 1∶3 (6 g) to digest the sample. The crude protein content was finally determined using a Kjeldahl nitrogen analyzer.
Determination of crude fat content
The crude fat content was determined by the Soxhlet extraction method using ether[14]. The medicinal powder (2.0 g) was weighed and dried at 105 ℃ for 30 min, and then put in a filter paper tube (a layer of absorbent cotton was plugged at the bottom of the tube and dried at 105 ℃ for 30 min), and the upper part was plugged with absorbent cotton. The sample was pressed and added into an extraction cylinder, and added with petroleum ether (50 ml). The crude fat content was determined with a crude fat meter.
Determination of crude fiber content
The crude fiber content was determined by the acid-base washing and weighing method using a crude fiber tester[14]. A certain amount of the B. balsamifera powder (2.0 g) was weighed and directly added to the glass sand core crucible. The weight of the glass sand core crucible and the medicinal material was determined using a crude fiber tester. The powder was first soaked with H2SO4 and then with KOH. The remaining component was crude cellulose. After acid-base washing, the glass sand core crucible burned at 500 ℃ and weighed, and the crude fiber content was calculated.
Data analysis
Excel was used for data entry and chart drawing. SPSS 16.0 software was used for statistical analysis and One-way ANOVA analysis of variance, and multiple comparisons were performed by Duncan test.
Results and Analysis
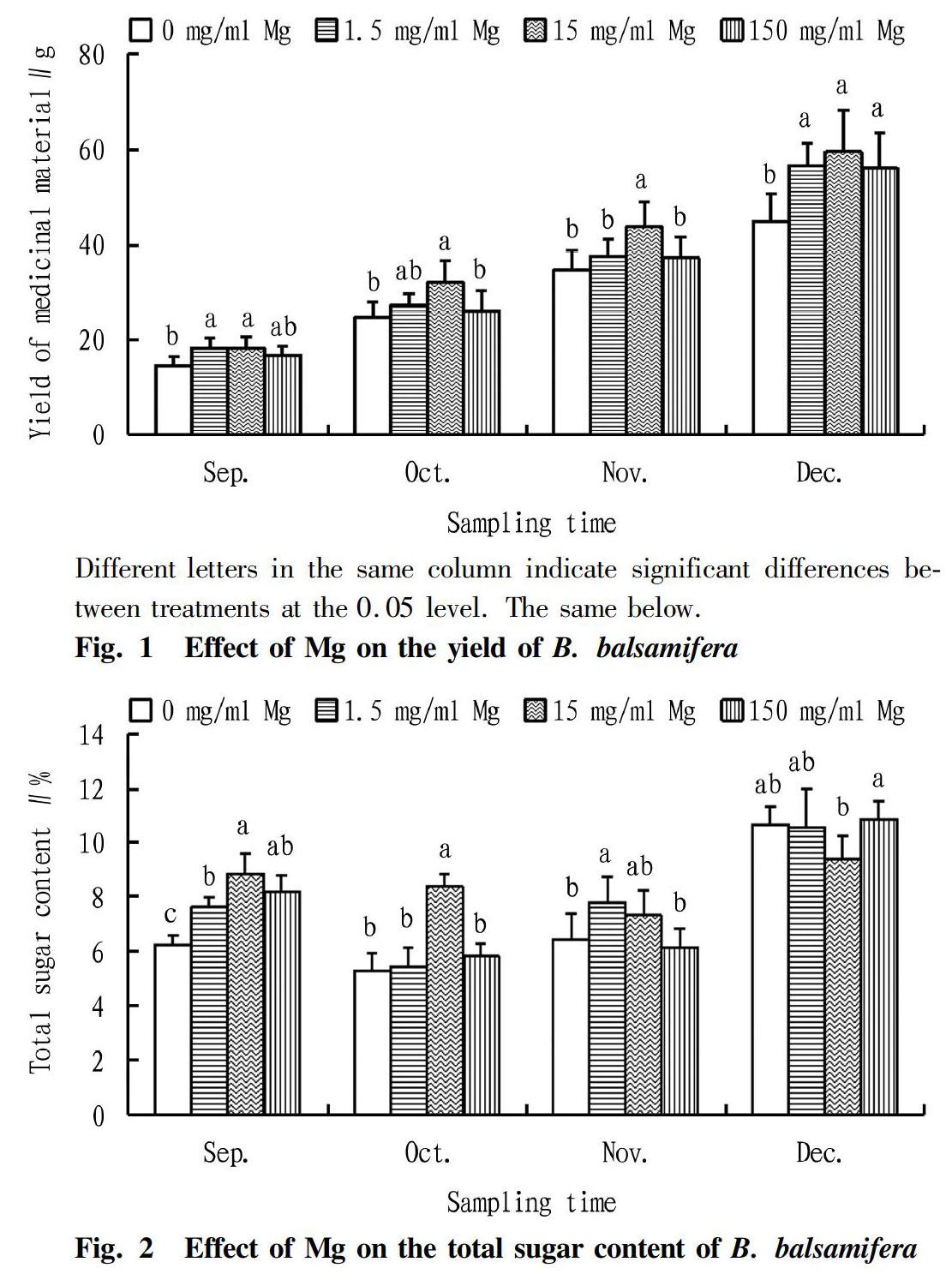
Effect of Mg on the yield of B. balsamifera
It could be seen from Fig. 1 that with the extension of the treatment time, the yield of B. balsamifera medicinal material showed a continuously increasing trend under different concentrations of Mg treatment. In September, there was a significant difference in the yield of the medicinal material between treatments (P<0.05). The yield of the medicinal material increased significantly by 25.49% and 25.08% in the 1.5 and 15 mg/ml Mg treatments, respectively, compared with the 0 mg/ml Mg treatment. In October and November, the differences in the yield of the medicinal material between treatments were extremely significant (P<0.01). Compared with other three treatments, 15 mg/ml Mg significantly increased the yield of the medicinal material. In December, the differences between the treatments were not significant (P>0.05).
Effect of Mg on the total sugar content of B. balsamifera
It could be seen from Fig. 2 that with the extension of the treatment time, the total sugar content of B. balsamifera showed a trend of first decreasing and then increasing under different concentrations of Mg treatment. The results of analysis of variance showed that in September, October and November, the total sugar content of B. balsamifera leaves was significantly different between the treatments (P<0.01). Especially in September and October, the total sugar content under the treatment of 15 mg/ml Mg was significantly higher than those under other treatments, and compared with the treatments of 0, 1.5 and 150 mg/ml Mg, it increased by 40.99%, 15.86% and 7.94% in September, respectively, increased by 57.93%, 54.24% and 43.45% in October, respectively. In November, the total sugar content under the 1.5 mg/ml Mg treatment was the highest, but there was no significant difference from the total sugar content under the 15 mg/ml Mg treatment.
Agricultural Biotechnology2020
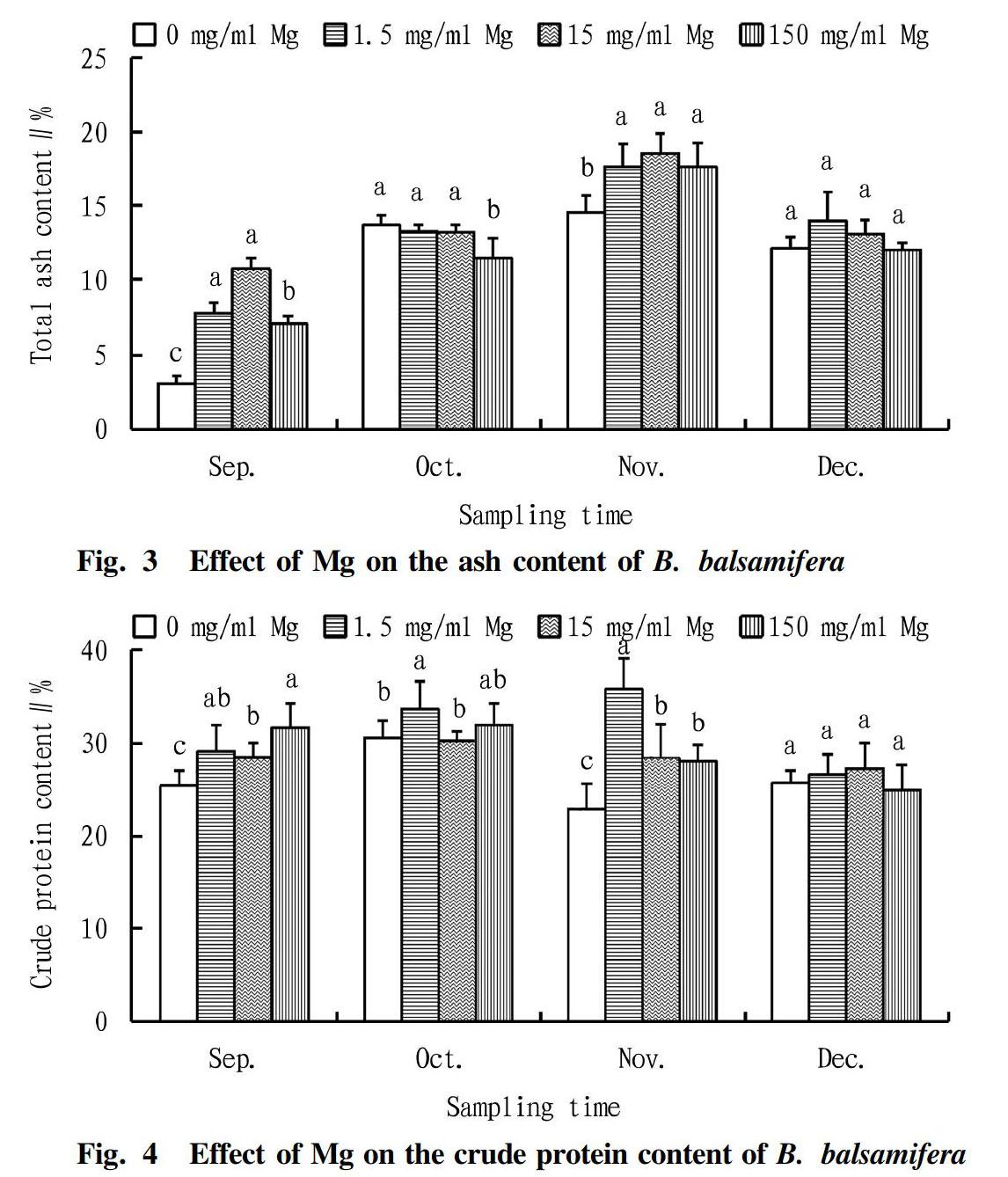
Effect of Mg on the ash content of B. balsamifera
It could be seen from Fig. 3 that with the extension of the treatment time, the ash content of B. balsamifera under different concentrations of Mg showed a trend of first increasing and then decreasing. The analysis of variance showed that the differences in ash content between the treatments in September were extremely significant (P<0.01), and the differences in October and November were significant (P<0.05). In September, the ash contents of the 1.5 and 15 mg/ml Mg treatments were higher than those of the 0 and 150 mg/ml Mg treatments. The 150 mg/ml Mg treatment was higher than the 0 mg/ml Mg treatment. In November, the values of the three Mg treatments were significantly higher than that of the 0 mg/ml Mg treatment, increasing by 21.55%, 27.66% and 21.62%, respectively. However, in October, the 150 mg/ml Mg treatment had the lowest ash content, which was significantly lower than other treatment groups.
Effect of Mg on the crude protein content of B. balsamifera
It could be seen from Fig. 4 that with the extension of the treatment time, the crude protein content of B. balsamifera showed a trend of increasing first and then decreasing under different concentrations of Mg treatment. In September and November, the differences between the treatments were significant (P<0.05). Compared with the 0 mg/ml Mg treatment, the crude protein contents of the 1.5, 15 and 150 mg/ml Mg treatments increased significantly by 14.72%, 12.04% and 24.80% in September, respectively, and increased by 56.39%, 24.00% and 22.56% in November, respectively.
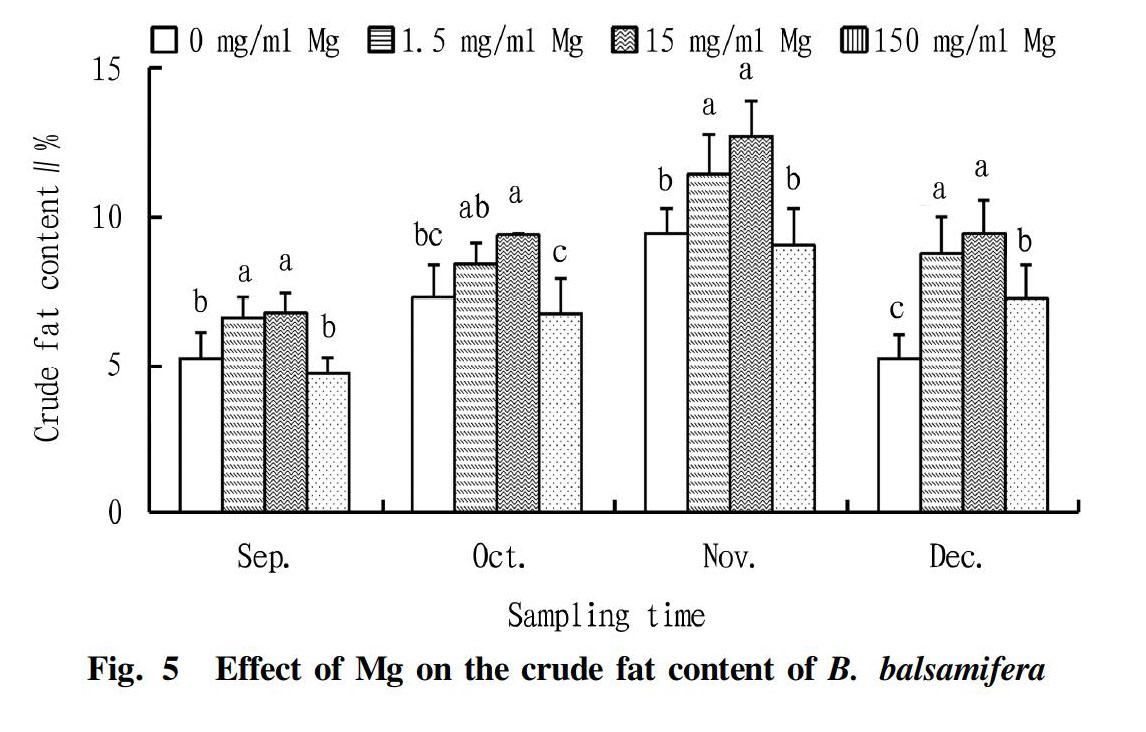
Effect of Mg on the crude fat content of B. balsamifera
It could be seen from Fig. 5 that with the extension of the treatment time, the crude fat content of B. balsamifera showed a trend of increasing first and then decreasing under different concentrations of Mg treatment. The analysis of variance showed that the differences in crude fat content between the treatments were extremely significant (P<0.01). The crude fat contents of the 1.5 and 15 mg/ml Mg treatments were significantly higher than those of other treatments. In November, compared with the 0 and 150 mg/ml Mg treatments, the crude fat contents of the 1.5 and 15 mg/ml Mg treatments increased significantly by 21.01%, 26.35%, and 34.74%, 40.68%, respectively.
Effect of Mg on the crude fiber content of B. balsamifera
It can be seen from Fig. 6 that with the extension of the treatment time, the crude fiber content of B. balsamifera under different concentrations of Mg treatment showed a trend of first decreasing and then increasing. According to the analysis of variance, in September, the differences between the treatments were extremely significant (P<0.01), and in November, the differences were significant (P<0.05). In September, the crude fiber contents of the 1.5 and 15 mg/ml Mg treatments were significantly higher than those of the 0 and 150 mg/ml Mg treatments, increasing by 46.27%, 19.01%, and 53.41%, 24.82%, respectively. However, in November, the crude fiber contents of the 15 and 150 mg/ml Mg treatments were higher than other treatments.
Conclusions and Discussion
Mg is one of the essential nutrients for plant growth and development. Mg plays a very important role in many physiological metabolic processes such as plant photosynthesis and carbon and nitrogen metabolism[15]. The application of Mg fertilizer is beneficial to the formation of plant chlorophyll, promoting photosynthesis, improving net photosynthetic rate, and can delay leaf senescence and improve its anti-aging ability. Meanwhile, it is conducive to plant growth and development, and provides a good material basis for plant growth and development[16]. The results of this study showed that the application of Mg in the growth period of B. balsamifera significantly increased the yield of medicinal material, which is consistent with the result of Wang[8] applying Mg in the growth retardation period of B. balsamifera in Hainan in winter. It indicates that Mg is needed in both the growth period and the growth retardation period of B. balsamifera to promote the growth and development of B. balsamifera and the accumulation of the medicinal material. It is mainly because that Mg is an important component of chlorophyll, which can promote the formation of chlorophyll and participate in the photosynthesis of plants and the synthesis of carbohydrates, fats, lipids, proteins and nucleic acids[17-18], thus it promoted the accumulation of the yield of B. balsamifera medicinal material in the growth period. Many studies have confirmed that Mg can promote the growth and yield of Pseudostellaria heterophylla, betel nut, corn, and potatoes[16,19-21].
Plant primary metabolites are closely related to the absorption and transport of carbon and nitrogen substances. In this study, the substances such as total sugar, ash, crude protein, crude fat and crude fiber that affect the yield of B. balsamifera were investigated. It was found that in September, October and November, 1.5 and 15 mg/ml Mg significantly increased the contents of total sugar, ash, crude protein, crude fat and crude fiber in B. balsamifera, which might be due to the fact that Mg is an activator of various enzymes in plants, which has a very important role in plant carbon metabolism and affects plant fixation of carbon, solar energy conversion efficiency, photosynthesis, etc.[22]. Some studies have pointed out that Mg can regulate the activity of photosynthetic metabolism RuBP carboxylase and PEP carboxylase, strengthen the fixation of CO2, and promote carbon assimilation of photosynthesis and transport of photosynthetic products[23], and Mg deficiency will lead to carbon metabolism disorders and affect the accumulation of plants primary metabolites including total sugar, ash and crude fiber. Moreover, Mg ions are also involved in the activation process of amino acids in protein synthesis, which in turn affects protein synthesis in plants[21]. Mg ions can also increase the activity level of nitrogen metabolism rate-limiting enzymes (nitrate reductase, NR) in plants, thereby regulating NO-3 reduction and directly affecting the level of nitrogen metabolism. Mg can activate glutamine synthetase, DNA and RNA polymerase, and is an essential element for RNA synthesis in the nucleus. Once lack of Mg, the net synthesis of RNA in plant stops immediately, resulting in a rapid slowdown of protein synthesis. Mg is directly related to plant carbon and nitrogen metabolism processes[24]. Therefore, the proper concentration of Mg now promotes the accumulation of total sugar, ash, crude protein, crude fat, and crude fiber in B. balsamifera. Wang[21] found that Mg can increase corn leaf area, increase chlorophyll content, photosynthetic capacity and soluble sugar and starch content, and promote carbon metabolism in corn. Mg can increase the contents of soluble sugar, starch and soluble protein in wheat, enhance wheat carbon metabolism, and promote its growth and development. Mo et al.[16] found that Mg deficiency will lead to a decrease in the yield of P. heterophylla, and a decrease in ash and extract contents. Li[25] found that Mg can improve carbon metabolism and nitrogen metabolism of chrysanthemum leaves. Wang et al.[26] found that Mg can increase the biomass, antioxidant enzymes and per unit area active ingredient yield of two-year-old B. balsamifera.
References
[1] Chinese Pharmacopoeia Commission. Chinese pharmacopoeia[M]. Beijing: China Medical Science Press, 2015. (In Chinese)
[2] WANG D, LAN HP, ZHANG YB, et al. Effects of magnesium on biomass, antioxidant enzyme activity and accumulation of active ingredients in two-year-old Blumea balsamifera[J]. Chinese Journal of Tropical Agriculture, 2018, 38(7): 50-56. (in Chinese)
[3] State Administration of Traditional Chinese Medicine. Chinese materia medica[M]. Shanghai: Shanghai Scientific and Technical Publishers. 1999. (in Chinese)
[4] HE YN, DING Y, XIAN FR, et al. Effects of different fertilizers on biological yield and active ingredient content of Blumea balsamifera[J]. Guizhou Agricultural Sciences, 2005, 33(5): 53-57. (in Chinese)
[5] HE YN, DING Y, XIAN FR, et al. Effect of N nutrition on yield and active ingredient in Blumea balsamifera[J]. Guizhou Agricultural Sciences, 2006, 34(2): 28-30. (in Chinese)
[6] WANG D, LAN HP, ZHANG YB, et al. The effect of magnesium on biomass and accumulation of nutrient elements in Blumea balsamifera[J]. Chinese Journal of Tropical Crops, 2018, 39(11): 2126-2131. (in Chinese)
[7] WANG D, MA QS, FAN ZW, et al. The effect of calcium element on biomass and contents of effective constituents in Blumea balsamifera in slow growth period of winter[J]. Agricultural Science & Technology, 2016(2): 358-361, 437.
[8] WANG D, FAN ZW, PANG YX, et al. Effect of exogenous magnesium on the biomass and active ingredient content of Blumea balsamifera in the slow growth period in winter[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2015(4): 75-79. (in Chinese)
[9] LAN HP, WANG D, YANG Q, et al. Effects of combined application of N, P and K on the yield and quality of Blumea balsamifera[J]. Guizhou Agricultural Sciences, 2017, 45(1): 107-111. (in Chinese)
[10] DAYANE MEIRELES DA SILVA, KAMILA REZENDE DZIO DE SOUZA, LISSA VASCONCELLOS VILAS BOAS, YE, et al. The effect of magnesium nutrition on the antioxidant response of coffee seedlings under heat stress[J]. Scientia Horticulturae, 2017(224): 115-125.
[11] CANIZELLA, MOREIRA A, MORAES LAC, et al. Efficiency of magnesium use by common bean varieties regarding yield, physiological components, and nutritional status of plants B. T[J]. Communications in Soil Science and Plant Analysis, 2015(46):1376–1390.
[12] LIN MX, ZHANG XD, QIU MH, et al. Magnesium physiological function in plants and diagnosis and application of magnesium nutrition[J]. Chinese Journal of Tropical Agriculture, 2016, 36(3): 39-43. (in Chinese)
[13] CHEN YQ. Biochemical experiment methods and techniques[M]. Beijing: Science Press, 2002. (in Chinese)
[14] WANG D, WAN CX, WANG WQ, et al. Effect of molybdenum on accumulation of glycyrrhizic acid based on material ingredients distribution[J]. Chinese Traditional and Herbal Drugs, 2013, 44(8): 1037-1042. (in Chinese)
[15] DING YC, JIAO XY, NIE D, et al. Effects of fertilization with different nitrogen sources and magnesium on cabbage yield, quality and nutrient absorption[J]. Chinese Journal of Eco-Agriculture, 2012, 20(8): 996-1002. (in Chinese)
[16] MO XC, LI JL, ZHAO Z, et al. Effects on the yield and quality of Pseudostellaria heterophylla by treatments of calcium and magnesium deficiency[J]. Modern Chinese Medicine, 2014, 16(7): 547-550, 555. (in Chinese)
[17] JIN XL, MA CL, CHEN LS. Advances in research on magnesium deficiency in plants[J]. Subtropical Agriculture Research, 2012, 8(2): 118-122. (in Chinese)
[18] LYU SB, WANG G, BAI YX, et al. Effects of different magnesium application rate on growth, yield and quality of flue-cured tobacco K326[J]. Journal of Anhui Agricultural Sciences, 2017, 45(26): 41-43. (in Chinese)
[19] LI J, CAO XM, LIU LY, et al. Effects of magnesium on photosynthetic characteristics and chloroplast ultrastructure of areca seedlings[J]. Journal of Plant Nutrition and Fertilizer, 2019, 25(11): 1949-1956. (in Chinese)
[20] YUAN JL, YANG LL, WANG YP, et al. Effects of low magnesium stress on carbohydrate accumulation and distribution in test-tube potato seedlings[J]. Agricultural Research in the Arid Areas, 2018, 36(4): 200-206. (in Chinese)
[21] WANG DD. Effects of foliar application of iron and magnesium fertilizers on physiological metabolism and growth of corn and wheat seedlings[D]. Yangling: Northwest A&F University, 2016. (in Chinese)
[22] GRZEBISZ W. Crop response to magnesium fertilization as affected by nitrogen supply[J].. Plant and Soil, 2013, 368(1-2): 23-39.
[23] ZHANG S. Physiological mechanism of magnesium relieving inhibition of high temperature on wheat grain filling[D]. Nanjing: Nanjing Agricultural University, 2017. (in Chinese)
[24] LIU XQ, SHI XJ, ZHAN F, et al. On the nutrition element magnesium[J]. Hunan Agricultural Sciences, 2005(6): 41-43. (in Chinese)
[25] LI L. Effects of nitrogen, molybdenum and magnesium nutrition and organic acids on the growth and quality of medicinal chrysanthemum[D]. Nanjing: Nanjing Agricultural University, 2016. (in Chinese)
[26] WANG D, LAN HP, ZHANG YB, et al. Effects of magnesium on biomass, antioxidant enzyme activity and accumulation of active ingredients in two-year-old Blumea balsamifera[J]. Chinese Journal of Tropical Agriculture, 2018, 38(7): 50-56. (in Chinese)
- 农业生物技术(英文版)的其它文章
- Investigation on Agronomic Characters of Dwarf Mutant 778 in Broomcorn Millet (Panicum miliaceum L.) and Analysis of Its Sensitivity to GA
- Construction of Technology System on Development and Repropagation in Vitro of Several Cultivars in Pear
- Construction of Camellia oleifera Cultivation Standardization System
- Nutrients Determination in Nuts from Different Torreya grandis Cultivars
- Photosynthetic Physiological Response to Drought Stress of Populus euphratica at Different Ages in Minqin
- Effects of UV-B Radiation on the Activity of Antioxidants in Flue-cured Tobacco Leaves

