基于绵羊胚胎骨骼肌蛋白质组学的PI3K-AKT信号通路分析
王欣悦,石田培,赵志达,胡文萍,尚明玉,张莉
基于绵羊胚胎骨骼肌蛋白质组学的PI3K-AKT信号通路分析
王欣悦,石田培,赵志达,胡文萍,尚明玉,张莉
(中国农业科学院北京畜牧兽医研究所,北京 100193)
【】绵羊是重要的经济动物,其骨骼肌生长发育与产肉性能密切相关。胚胎期是绵羊骨骼肌生长发育的关键阶段,挖掘分析绵羊胚胎骨骼肌蛋白质组数据,为揭示绵羊肌肉发育重要时间节点、筛选绵羊胚胎骨骼肌生长发育调控蛋白质提供依据。本团队已对妊娠第85天、第105天和第135天的中国美利奴绵羊胚胎背最长肌进行串联质谱(tandem mass tag, TMT)蛋白质定量,鉴定到1316种差异丰度蛋白质。现利用GO、KEGG和R等方法对这些差异丰度蛋白质开展聚类、功能注释和通路分析等生物信息学分析。基于前期研究结果对差异丰度蛋白质进行R语言聚类,分析结果显示,cluster 5类蛋白在胚胎骨骼肌第105天具有较高丰度。对cluster 5 蛋白进行GO和KEGG富集分析发现,该类蛋白质参与胞内蛋白质代谢过程,显著富集于PI3K-AKT信号通路中,而在该信号通路中RAC-β丝氨酸/苏氨酸蛋白激酶X1(AKT2)具有较高表达丰度。蛋白质生物信息学结果表明,AKT2蛋白由481个氨基酸构成,AKT2蛋白理论分子量为55.58kD,由66个带正电荷的氨基酸残基和72个带负电荷的氨基酸残基组成,理论等电点为6.08,亲水性平均系数-0.454,属于亲水性蛋白。预测AKT2蛋白的481个氨基酸全部位于膜外,属于膜受体蛋白。AKT2蛋白有12个N-端糖基化位点,71个磷酸化位点,与蛋白酶K相似度为99%,属于蛋白酶催化亚基家族。绵羊胚胎骨骼肌蛋白质组数据发现,第105天是绵羊胚胎骨骼肌纤维由增殖分化到增大增粗的转折点,具有调控绵羊胚胎骨骼肌纤维生长发育作用的PI3K-AKT信号通路在该节点显著富集,AKT2是调控该信号通路的重要候选蛋白。综上,本研究结果对揭示胚胎骨骼肌生长发育及其调控分子机制具有重要理论指导意义。
绵羊(); 胚胎背最长肌; 蛋白质组学;生物信息学分析
0 引言
【研究意义】胚胎期是绵羊骨骼肌生长发育的重要时期。胚胎骨骼肌纤维在该时期发生增殖、分化、融合、增粗及成熟等生物过程,直接影响出生后骨骼肌的生长[1]。因此,分析绵羊胚胎骨骼肌蛋白质组学数据对阐明其生长发育机制、筛选重要调控蛋白具有重要意义。【前人研究进展】骨骼肌生长发育研究一直备受关注,早期研究较多的,又称,是一种肌肉生长抑制素,对家畜肌肉生长发育具有重要作用,其活性的丧失或降低会促进动物肌肉的发育。随后,发现、、和肌源性调节因子(myogenic regulatory factors,)调控肌源性祖细胞、成肌细胞和肌纤维的生长[2-4]。肌源性调节因子4(myogenic regulatory factor 4,)、肌源因子5(myogenic factor 5,)、肌源性分化因子(myogenic differentiation 1,)和肌细胞生成素()是决定肌纤维最终分化的调控因子,Six家族蛋白质是参与肌肉早期发育的转录因子,并在胚胎骨骼肌发育过程中发挥重要作用[5-6]。研究发现PI3K-AKT等信号通路与骨骼肌生长发育密切相关,可以诱导肌肉的生成、调控基因的表达和成肌分化[7-8]。绵羊骨骼肌结构特征研究表明,绵羊胚胎期第50天至第100天是肌纤维生长发育的关键阶段,此阶段以后肌纤维的种类、数量和状态不再发生变化[9-10]。【本研究切入点】蛋白组学研究技术为揭示家畜骨骼肌生长发育提供了有效的技术手段。目前,蛋白质组学技术广泛应用于猪、鸡、牛和羊等动物的骨骼肌生长发育研究。通过该技术,研究人员已挖掘出一批调控骨骼肌生长发育的关键蛋白[11-14]。但现阶段,对绵羊胚胎骨骼肌蛋白质组学的研究非常少。本团队前期利用TMT技术[15]对胚胎期第85天(D85N)、第105天(D105N)和第135天(D135N)的绵羊胚胎背最长肌进行蛋白质定量研究,并鉴定到1316种差异丰度蛋白。本研究在此基础上利用生物信息学技术对差异丰度蛋白质进行分析与筛选[16]。【拟解决的关键问题】通过进一步分析差异丰度蛋白,揭示绵羊胚胎骨骼肌重要发育时间节点、挖掘发育相关调控蛋白,分析预测候选调控蛋白功能与结构,为提高绵羊产肉性能、阐明绵羊胚胎骨骼肌生长发育蛋白质调控机制提供新思路。
1 材料与方法
试验于2018年7月在中国农业科学院北京畜牧兽医研究所完成。
1.1 前期绵羊胚胎骨骼肌蛋白质组学分析
选择体况良好、体重相近的中国美利奴绵羊成年母羊进行同期发情与人工输精。通过手术法采集妊娠D85N、D105N和D135N母羊的胚胎相同部位的背最长肌(每阶段3个生物学重复)为样品进行TMT蛋白质组学定量。通过对二级质谱数据进行Maxquant (v1.5.2.8)检索(数据库为NCBI Ovis aries Oar_v4.0 https://www.ncbi.nlm.nih.gov/genome/?term=Ovis+aries),设置D105N vs D85N、D135N vs D105N和D135N vs D85N 3个比较组进行分析,共鉴定到1316种差异丰度蛋白质。本试验将利用GO、KEGG和R等生物信息学数据分析软件和平台对这些差异丰度蛋白质进行分析和筛选。
1.2 差异丰度蛋白质聚类分析
为进一步分析差异丰度蛋白质功能,筛选调控绵羊胚胎骨骼肌生长发育候选蛋白,利用R中Mfuzz算法对前期定量到的1316种差异丰度蛋白进行表达模式聚类分析[17-18]。
1.3 cluster 5蛋白GO和KEGG分析
利用InterProScan v.5.14-53.0(http://www.ebi.ac. uk/interpro/)、KAAS v.2.0(http://www. genome.jp/ kaas-bin/kaas_main)、KEGG mapper V2.5(http://www. kegg.jp/kegg/mapper.html)和Perl module(v.1.31 https://metacpan.org/pod/Text::NSP::Measures::2D::Fisher)等软件对cluster 5蛋白进行功能注释及富集分析。
1.4 AKT2蛋白生物信息学分析
使用ExPASy网站的ProtParam(http://web.expasy. org/protparam/)预测和分析蛋白质的分子量、等电点等物理参数[19]; TMHMM软件(http://www.cbs.dtu.dk/ services/TMHMM-2.0/)对蛋白进行跨膜区域预测[20];使用Expasy(http://www.expasy.org/proteomics)软件分析蛋白质潜在的磷酸化和糖基化等位点[21-23];利用Protein Homology/analogY Recognition Engine V 2.0(Phyre2,http://www.sbg.bio.ic.ac.uk/phyre2/html/page. cgi?id=index)预测蛋白质的三级结构。
2 结果
2.1 差异丰度蛋白质表达模式聚类分析
R语言表达模式聚类分析表明,cluster 5蛋白在D105N时具有较高表达趋势(图1)。通过GO和KEGG分析发现,cluster 5蛋白质参与胞内蛋白质代谢过程,并显著富集于PI3K-AKT信号通路。同时,ATK2蛋白在PI3K-AKT信号通路中显著上调(图2-4)。
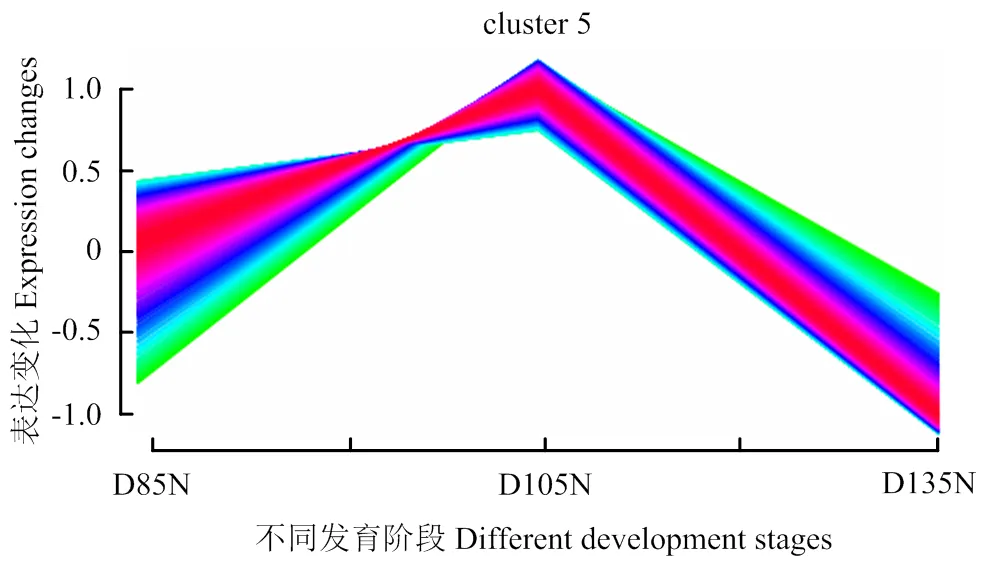
图1 差异丰度蛋白质表达模式聚类分析

Y轴:生物学过程Y: Enrichment index; X轴:富集指数X: Biology process

Y轴: 通路名称Y: Pathway name; X轴: Fisher精确测试P值X: Fisher’s exact test p-value

红色:显著富集的上调基因 Red:Significant enrichment up-regulation gene
2.2 AKT2蛋白生物信息学分析
2.2.1 AKT2蛋白的理化性质 AKT2蛋白由481个氨基酸构成。使用ProtParam在线软件分析AKT2蛋白的理化性质,推测其分子式为C2490H3865N673O724S24,分子量为55.58kD,理论等电点(pI)为6.08,半衰期均是30 h,不稳定系数32.36,属于稳定蛋白。脂肪系数为76.61,亲水性平均系数(GRAVY)是-0.454,属于亲水性蛋白。负电荷(Asp + Glu)氨基酸残基72个,正电荷(Arg + Lys)氨基酸残基66个。
2.2.2 AKT2蛋白跨膜结构分析及其潜在N-糖基化、磷酸化位点预测 TMHMM在线预测表明,AKT2蛋白的481个氨基酸没有位于细胞膜上和膜内,全部位于膜外,属膜受体蛋白(图5)。PSORT II Prediction分析结果表明AKT2蛋白主要在65.2% 胞质、4.3% 线粒体、17.4%细胞核、4.3%分泌包囊、4.3%细胞支架。使用NetNGlyc 1.0 Server和NetPhos 3.1 Server分别预测AKT2蛋白N-端糖基化和磷酸化情况,结果显示:AKT2蛋白有12个N-糖基化位点,71个磷酸化位点,其中26个丝氨酸(Ser)磷酸化位点、26个苏氨酸(Thr)磷酸化位点、19个酪氨酸(Tyr)磷酸化位点(图6)。
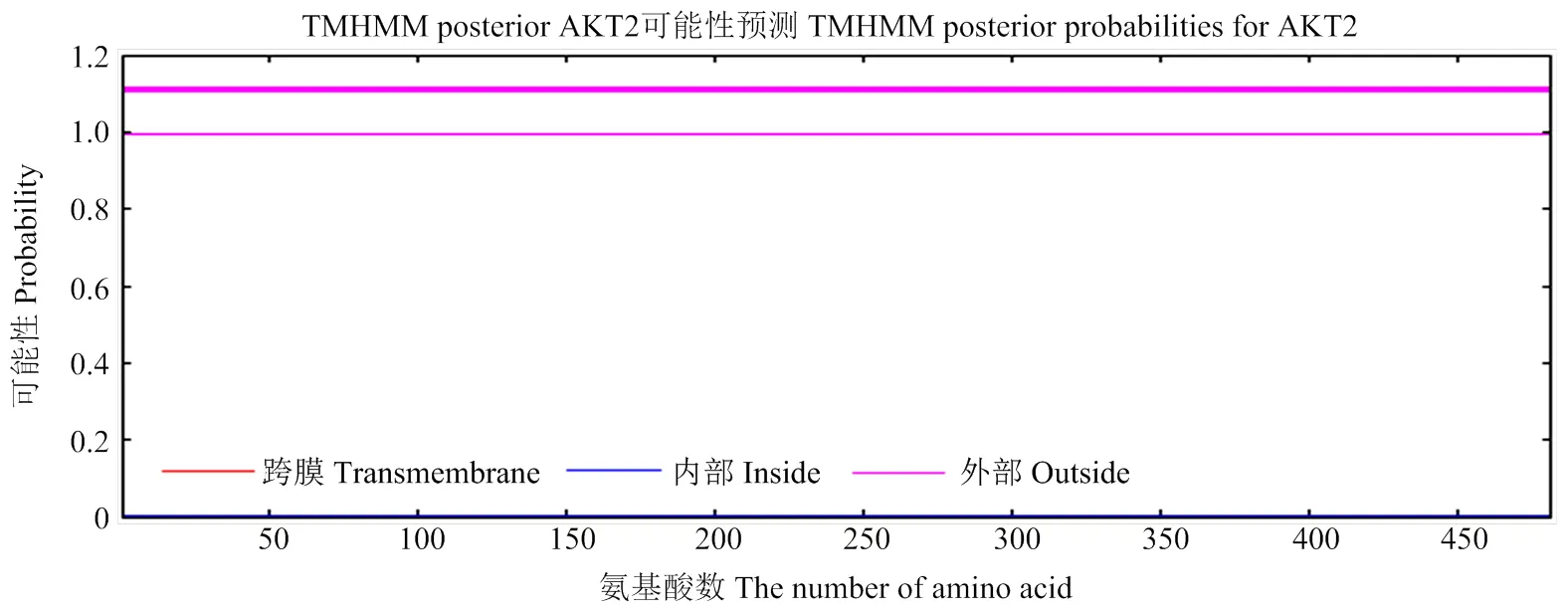
图5 AKT2蛋白跨膜结构分析

(a)AKT2 12个N-糖基化位点(a)12 N-glycosylation sites in AKT2;(b)AKT2 71个磷酸化位点(b)71 phosphorylation sites in AKT2
Fig .6 Prediction on glycosylation and phosphorylation sites of AKT2 protein
2.2.3 AKT2蛋白三级结构预测 PHYER2预测结果显示,AKT2蛋白具有α-螺旋及无规则卷曲等结构,三级结构整体呈晶体结构,与蛋白酶K相似度为99%,属于蛋白酶催化亚基(图7)。
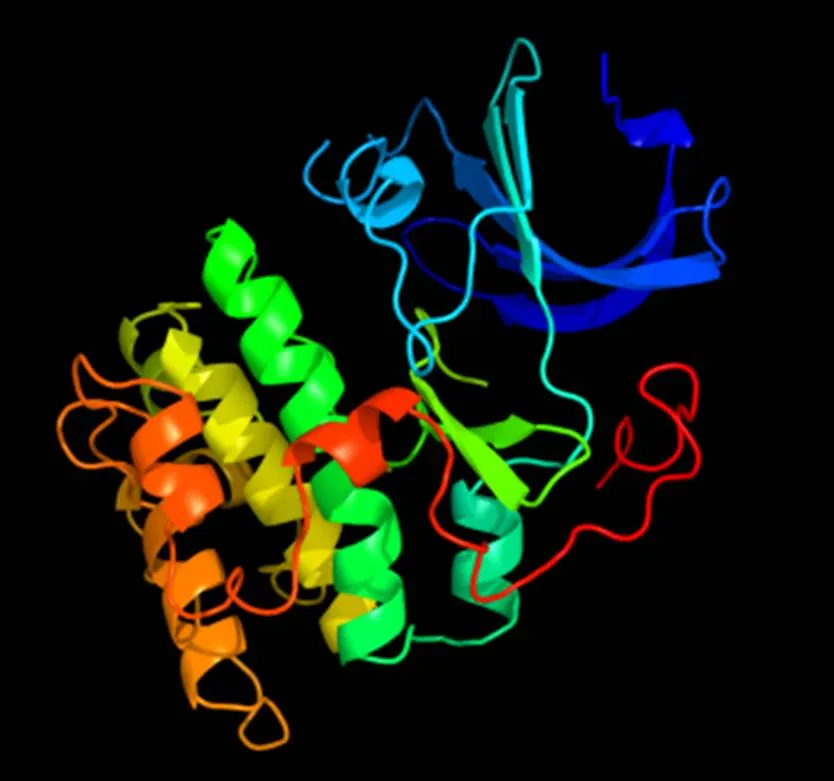
图7 AKT2蛋白3D结构预测
3 讨论
本文利用GO、KEGG和R语言等方法对差异丰度蛋白质进行聚类、功能注释和通路富集等生物信息学分析,分析结果对揭示绵羊胚胎骨骼肌生长发育关键窗口期、筛选调控蛋白具有重要意义。胚胎时期骨骼肌大部分由生肌节中的肌肉前体细胞发育而来,这些肌肉前体细胞会在初级生肌节中分化成单核肌肉细胞,初级生肌节最终生成脊椎动物早期的肌肉组织[24-25]。前期研究表明,绵羊胚胎骨骼肌纤维在胚胎期第85天至第105天增殖分化,在第105天至第135天增大增粗,而这些差异丰度蛋白质主要富集于能够调控肌纤维发生生长的代谢及氧化磷酸化等信号通路[8, 26-30]。本研究中,绵羊胚胎骨骼肌蛋白质组学数据R语言分析发现,cluster 5蛋白在胚胎发育第105天具有较高表达丰度(图1)。相关文献报道绵羊胚胎骨骼肌纤维在胚胎期第50天至第100天基本发育完成,并在第100天左右开始分化[31]。因此,初步判断D105N是调控绵羊胚胎骨骼肌发育转折点。
KEGG分析发现,cluster 5蛋白质在PI3K-AKT信号通路中显著富集。由此推断,PI3K-AKT信号通路可能对胚胎时期骨骼肌发育转折及调控具有重要的作用。PI3K-AKT信号通路参与骨骼肌生长发育,能够调控细胞周期、细胞凋亡和蛋白质合成等生物过程[32]。研究表明,PI3K-AKT信号通路能够调控肌浆蛋白形成,促进肌肉分化和肥大[33]。本研究发现PI3K-AKT信号通路在绵羊胚胎骨骼肌发育转折过程中具有重要作用,AKT作为第二信使在该通路中扮演重要的角色。PI3K-AKT信号通路下游的转导因子AKT/PKB在调节个体发育、生长和细胞存活过程中发挥着重要作用[34]。ATK是一种保守的丝/苏氨酸蛋白激酶,可以调控动物胚胎发育及幼体生长,而ATK2是ATK的不同亚基也具有相同作用[35]。在正常生理条件下,PI3K-AKT信号通路由受体酪氨酸激酶(RTK)激活,并通过活化PI3K诱导PIP3激活AKT,上调下游靶基因从而调节细胞周期及分化。本研究中,RAC-β丝/苏氨酸蛋白激酶X1(ATK2)显著富集于PI3K-AKT信号通路,成肌调控因子Myostatin作为AKT的活化因子之一,也可以通过激活PI3K-AKT信号通路来调控肌肉生长[36-38]。因此,PI3K-AKT信号通路可以通过调控和(肌酸激酶)骨骼肌发育分化标志分子表达来调控骨骼肌纤维发育及分化[39-40]。
AKT2蛋白在胞质比例较高,属于膜受体蛋白。由此可以推断,该蛋白可能在核膜上大量分布,并在蛋白质翻译时具有重要作用。而AKT2蛋白与蛋白酶催化亚基的三级结构具有较高的同源性,表明该蛋白可能是蛋白质翻译时重要的催化激活因子。同时,该蛋白的三级结构整体较为复杂,存在α-螺旋及无规则卷曲等结构,可能对配体或受体蛋白的识别和结合具有重要作用。该蛋白大量磷酸化修饰位点的发现表明,可逆磷酸化调控可能在实现AKT2蛋白质功能中起到重要作用。综上,AKT2蛋白不仅在PI3K-AKT信号通路中具有重要的信号传导及调控功能,还在绵羊胚胎骨骼肌发育分化时具有关键的调控作用,但AKT2蛋白调控肌纤维发育分化的分子机制还有待进一步验证和研究。
4 结论
通过GO二级注释、KEGG富集及R语言表达模式聚类等分析发现,蛋白质功能和富集通路均与个体发育和骨骼肌生长发育相关,第105天是绵羊胚胎骨骼肌纤维由增殖分化到增大增粗的转折点,PI3K-AKT信号通路对骨骼肌纤维生长发育转换具有调控作用。候选蛋白质生物信息学分析表明,ATK2具有重要催化调控功能,是调控PI3K-AKT信号通路信号传导的重要候选蛋白。
[1] BENTZINGER, C F, YU X W, RUDNICKI M A. Building Muscle: Molecular Regulation of Myogenesis., 2012, 4(2): 441-441.
[2] TAJBAKHSH S, BUCKINGHAM M. 6 The Birth of Muscle Progenitor Cells in the Mouse: Spatiotemporal Considerations., 1999, 48: 225-268.
[3] BUCKINGHAM, M. Skeletal muscle progenitor cells and the role of Pax genes., 2007, 330(6-7): 530-533.
[4] DONG Y, XIE M, JIANG Y, XIAO N, DU X, ZHANG W, TOSSER-KLOPP G, WANG J, YANG S, LIANG J, CHEN W, CHEN J, ZENG P, HOU Y, BIAN C, PAN S, LI Y, LIU X, WANG W, SERVIN B, SAYRE B, ZHU B, SWEENEY D, MOORE R, NIE W, SHEN Y, ZHAO R, ZHANG G, LI J, FARAUT T, WOMACK J, ZHANG Y, KIJAS J, COCKETT N, XU X, ZHAO S, WANG J, WANG W. Sequencing and automated whole-genome optical mapping of the genome of a domestic goat ()., 2013, 31(2): 135-141.
[5] MURPHY, M, KARDON G. Origin of vertebrate limb muscle: The role of progenitor and myoblast populations., 2011, 96: 1-32.
[6] KAWAKAMI, K, SATO S, OZAKI H, IKEDA K. Six family genes—structure and function as transcription factors and their roles in development., 2000, 22(7): 616-626.
[7] 史新娥, 吴国芳, 宋子仪, 路宏朝, 贾龙, 朱嘉宇, 杨公社. 阻断PI3K/AKT通路通过激活FoxO1抑制猪骨骼肌卫星细胞分化. 中国农业科学, 2014, 47(01): 154-160.
SHI X E, WU G F, SONG Z Y, LU H C, JIA L, ZHU J Y, YANG G S. Inhibition of PI3K/AKT pathway suppressing porcine skeletal muscle sattelite differentiation through activation of FoxO1 transcription factor., 2014, 47(1): 154-160. (in Chinese)
[8] LIU J, FU R, LIU R, ZHAO G, ZHENG M, CUI H, LI Q, SONG J, WANG J, WEN J. Protein profiles for muscle development and intramuscular fat accumulation at different post-hatching ages in chickens., 2016, 11(8): e0159722.
[9] ASHMORE, C R, ROBINSON D W, RATTRAY P, DOERR L. Biphasic development of muscle fibers in the fetal lamb., 1972, 37(2): 241-55.
[10] 李雪娇, 刘晨曦, 孙亚伟, 杨开伦, 刘明军. 德国美利奴羊胎儿期骨骼肌组织学结构发育特征研究. 西北农林科技大学学报(自然科学版), 2018, 332(5): 7-13.
LI X J, LIU C X, SUN Y W, YANG K L, LIU M J. Study on structure development characteristics of German Merion sheep fetal skeletal muscle tissue., 2018, 332(5): 7-13. (in Chinese)
[11] OUYANG H, WANG Z, CHEN X, YU J, LI Z, NIE Q. Proteomic analysis of chicken skeletal muscle during embryonic development., 2017, 8: 281.
[12] POLETI M D, REGITANO L C, SOUZA G H, CESAR A S, SIMAS R C, SILVA-VIGNATO B, OLIVEIRA G B, ANDRADE S C, CAMERON L C, COUTINHO L L. Longissimus dorsi muscle label- free quantitative proteomic reveals biological mechanisms associated with intramuscular fat deposition., 2018, 179: 30-41.
[13] ZHANG, X, CHEN Y, PAN J, LIU X, CHEN H, ZHOU X, YUAN Z, WANG X, MO D. iTRAQ-based quantitative proteomic analysis reveals the distinct early embryo myofiber type characteristics involved in landrace and miniature pig., 2016, 17(1): 137.
[14] HAMELIN, M, SAYD T, CHAMBON C, BOUIX J, LAVILLE E. Proteomic analysis of ovine muscle hypertrophy., 2007, 84(12): 3266-3276.
[15] THOMPSON A, SCHäFER J, KUHN K, KIENLE S, SCHWARZ J, SCHMIDT G, NEUMANN T, HAMON C. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS., 2003, 75(8): 1895-1904.
[16] 石田培, 王欣悦, 侯浩宾, 赵志达, 尚明玉, 张莉. 基于全转录组测序的绵羊胚胎不同发育阶段骨骼肌circRNA的分析与鉴定. 中国农业科学, 2020, 53(03): 642-657.
SHI T P, WANG X Y, HOU H B, ZHAO Z D, SHANG M Y, ZHANG L. Analysis and identification of circrnas of skeletal muscle at different stages of sheep embryos based on whole transcriptome sequencing., 2020, 53(3): 642-657. (in Chinese)
[17] 王素兰, 高华萍, 张菁, 叶翔. 基于稳定同位素标记和平行反应监测的蛋白质组学定量技术用于肝癌生物标志物的筛选和验证. 色谱, 2017, 35(9): 934-940.
WANG S L, GAO H P, ZHANG J, YE X. Stable isotope labeling and parallel reaction monitoring-based proteomic quantification for biomarker screening and validation of hepatocellular carcinoma., 2017, 35(9): 934-940. (in Chinese)
[18] KUMAR L, FUTSCHIK M E. Mfuzz: a software package for soft clustering of microarray data., 2007, 2(1): 5.
[19] GASTEIGER E, HOOGLAND C, GATTIKER A, WILKINS M R, APPEL R D, BAIROCH A. Protein identification and analysis tools on the ExPASy server., 2005: 571-607.
[20] SONNHAMMER E L, VON HEIJNE G, KROGH A. A hidden Markov model for predicting transmembrane helices in protein sequences., 1998, 6: 175-182.
[21] BLOM N, GAMMELTOFT S, BRUNAK S. Sequence and structure-
based prediction of eukaryotic protein phosphorylation sites., 1999, 294(5): 1351-1362.
[22] BLOM N, SICHERITZ-PONTéN T, GUPTA R, GAMMELTOFT S, BRUNAK S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence., 2004, 4(6): 1633-1649.
[23] STEENTOFT C, VAKHRUSHEV S Y, JOSHI H J, KONG Y, VESTER-CHRISTENSEN M B, KATRINE T, SCHJOLDAGER B, LAVRSEN K, DABELSTEEN S, PEDERSEN N B. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology., 2013, 32(10): 1478-1488.
[24] DENETCLAW W, CHRIST B, ORDAHL C P. Location and growth of epaxial myotome precursor cells., 1997, 124(8): 1601-1610.
[25] VENTERS S J, ORDAHL C P. Persistent myogenic capacity of the dermomyotome dorsomedial lip and restriction of myogenic competence., 2002, 129(16): 3873-3885.
[26] KAZANSKAYA O, GLINKA A, DEL BARCO BARRANTES I, STANNEK P, NIEHRS C, WU W. R-Spondin2 is a secreted activator of Wnt/β-catenin signaling and is required for Xenopus myogenesis., 2004, 7(4): 525-534.
[27] TAJBAKHSH S, BORELLO U, VIVARELLI E, KELLY R, PAPKOFF J, DUPREZ D, BUCKINGHAM M, COSSU G. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5., 1998, 125(21): 4155-4162.
[28] WANG Y X, ZHANG C L, RUTH T Y, CHO H K, NELSON M C, BAYUGA-OCAMPO C R, HAM J, KANG H, EVANS R M. Regulation of muscle fiber type and running endurance by PPARδ., 2004, 2(10): e294.
[29] ZIZOLA C, KENNEL P J, AKASHI H, JI R, CASTILLERO E, GEORGE I, HOMMA S, SCHULZE P C. Activation of PPARδ signaling improves skeletal muscle oxidative metabolism and endurance function in an animal model of ischemic left ventricular dysfunction., 2015, 308(9): 1078-1085.
[30] WANG X Y, SHI T P, ZHAO Z D, HOU H B, ZHANG LProteomic analyses of sheep () embryonic skeletal muscle., 1750 (2020) 10:1750.
[31] 李雪娇, 刘晨曦, 杨开伦, 刘明军. 德美羊与中美羊胎儿期骨骼肌组织学结构发育特征差异性研究. 草食家畜, 2017 (04):1-6.
LI X J, LIU C X, YANG K L, LIU M J. Study on differentiation of fetal skeletal muscle development characteristics between German and Chinese merino sheep., 2017 (04):1-6. (in Chinese)
[32] BAI L, LIANG R, YANG Y, HOU X, WANG Z, ZHU S, WANG C, TANG Z, LI K. Microrna-21 regulates pi3k/akt/mtor signaling by targeting tgfβi during skeletal muscle development in pigs., 2015, 10(5): e0119396.
[33] ROMMEL C, BODINE S C, CLARKE B A, ROSSMAN R, NUNEZ L, STITT T N, YANCOPOULOS G D, GLASS D J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI (3) K/Akt/mTOR and PI (3) K/Akt/GSK3 pathways., 2001, 3(11): 1009.
[34] NICHOLSON K M, ANDERSON N G. The protein kinase B/Akt signalling pathway in human malignancy., 2002, 14(5): 381-395.
[35] AMIROUCHE A, DURIEUX A-C, BANZET S, KOULMANN N, BONNEFOY R, MOURET C, BIGARD X, PEINNEQUIN A, FREYSSENET D. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle., 2008, 150(1): 286-294.
[36] JI M, ZHANG Q, YE J, WANG X, YANG W, ZHU D. Myostatin induces p300 degradation to silence cyclin D1 expression through the PI3K/PTEN/Akt pathway., 2008, 20(8): 1452-1458.
[37] TRENDELENBURG A U, MEYER A, ROHNER D, BOYLE J, HATAKEYAMA S, GLASS D J. Myostatin reduces Akt/TORC1/ p70S6K signaling, inhibiting myoblast differentiation and myotube size., 2009, 296(6): C1258-C1270.
[38] 孙伟, 王鹏, 丁家桐, 马月辉, 关伟军, 储明星, 李碧春, 吴文忠陈玲. 湖羊Myostain和Myogenin基因表达的发育性变化及与屠宰性状的关联分析. 中国农业科学, 2010, 43(24): 5129-5136.
SUN W, WANG P, DING J T, MA Y H, GUAN W J , CHU M X, LI B C, WU W Z, CHEN L. Developmental changes of gene expression of myostain and myogenin genes and their association analysis with carcass traits in Hu Sheep.2010, 43(24): 5129-5136. (in Chinese)
[39] 李晶, 张云生, 李宁, 胡晓湘, 石国庆, 刘守仁, 柳楠. PI3K/AKT信号通路调控 Myogenin和MCK基因的表达. 遗传, 2013, 35(5): 637-642.
LI J, ZHANG Y S, LI N, HU X X, SHI G Q, LIU S R, LIU N. Expression of Myogenin and MCK genes regulated by PI3K/AKT pathway,2013, 35(5): 637-642. (in Chinese)
[40] FIGUEROA A, CUADRADO A, FAN J, ATASOY U, MUSCAT G E, MUNOZ-CANOVES P, GOROSPE M, MUNOZ A. Role of HuR in skeletal myogenesis through coordinate regulation of muscle differentiation genes., 2003, 23(14): 4991-5004.
The Analysis of PI3K-AKT Signal Pathway Based on the Proteomic Results of Sheep Embryonic Skeletal Muscle
WANG XinYue, Shi TianPei, ZHAO ZhiDa, HU WenPing, Shang MingYu, Zhang Li
(Institute of Animal Sciences, Chinese Academy of Agriculture Sciences, Beijing 100193)
【】Sheep is an important economic livestock and its skeletal muscle growth and development have a deep bond with meat production traits. The sheep embryonic period is an essential stage for skeletal muscle growth, analyzing and mining the proteome data of sheep embryonic skeletal muscle in this period has a great significance to reveal the muscle development process and screen their key regulation proteins.【】The longissimus dorsi of Chinese merino sheep at embryonic age of 85 days, 105days and 135days were selected for protein qualification by using tandem mass tag (TMT) and 1316 differential abundance proteins were obtained finally. GO, KEGG and R bioinformatic methods were used to cluster, annotate and analyze the differential abundance proteins. And the candidate proteins were testified by using bioinformatic methods.【】Based on the previous results, the cluster analysis on differential abundance proteins illustrated that the cluster 5 proteins were significantly expressed on embryonic age of 105 dayswith high abundance.GO and KEGG analysis on cluster 5 proteins showed these proteins were significantly involved in protein metabolism biology process and notably enriched in PI3K-AKT signal pathway in which RAC-beta serine/threonine-protein kinase isoform X1(ATK2) has a high abundance. Meanwhile, the results of bioinformatics showed that the AKT2 was composed of 481 amino acids and the theoretical molecular weight was 55.58kD. It consists of 66 positively charged amino acid residues and 72 negatively charged amino acid residues, the theoretical isoelectric point was 6.08, the hydrophilic average coefficient was -0.454, 12 N-terminal glycosylation sites and 71 phosphorylation sites were found in AKT2. The homology of AKT2 and protein kinase-like (PK-like) was 99% and it belongs to the family of protein kinases catalytic subunit.【】The proteome data analysis of sheep embryonic skeletal muscle showed that embryonic age of 105 daysis a key point of sheep embryonic skeletal fiber cell from proliferation and differentiation to hypertrophy. The PI3K-AKT signaling pathway which has function of regulating growth and development of embryonic skeletal muscle fibers was significantly enriched, and ATK2 is a keycandidateregulation protein in this pathway. To summarize, the study has a theoretical guiding significance to reveal the growth and development and its molecular regulation mechanism of embryonic skeletal muscle.
sheep (); embryonic longissimus dorsi; proteomic; bioinformatics analysis

10.3864/j.issn.0578-1752.2020.14.018
2019-08-29;
2020-03-30
国家自然基金联合基金重点支持项目(U1503285)、中国农业科学院基本科研业务费重大项目储备计划 (Y2017XM02)
王欣悦,E-mail:wxyanimalgenetic@163.com。通信作者张莉,E-mail:zhangli07@caas.cn
(责任编辑 林鉴非)

