Enhanced Production of Natural Carotenoids from Genetically Engineered Rhodobacter sphaeroides Overexpressing CrtA
Zhiping ZHAO Jiahui DU Hongfan CHEN Lili JI Jiamin ZHANG Wei WANG
Abstract Carotenoids act as precursors of vitamin A, antioxidants, enhancers of immunity, and are thus widely used in food and pharmaceutical industry. Microbial fermentation is one of the most important solutions for production of natural carotenoids. Rhodobacter sphaeroides is one of most promising bacteria employed for large scale production of carotenoids. In the present study, crtA located in the carotenoids biosynthesis pathway in R. sphaeroides was amplified by PCR. The overexpression vector pRKcrtA was constructed and subsequently transferred into R. sphaeroides, producing the genetically engineered strain R. sphaeroides 2.4.1/pRKcrtA overexpressing crtA. The carotenoid production from the genetically engineered strain was significantly increased. Fermentation procedure was optimized for further enhanced carotenoids production.
Key words Carotenoids; crtA; Rhodobacter sphaeroids; Fermentation; Overexpression; Optimization
Carotenoids are valuable molecules and widely used in food, pharmaceutical, poultry and cosmetics industries[1]. It has been well demonstrated that carotenoids can act as vitamin A precursors[2-3]. Carotenoids have coloring properties, which exhibit various colors including yellow, orange, red and purple colors[4]. On the other hand, carotenoids exhibit powerful antioxidant capacity[5], and thus have attracted much attention of the industries and researchers all over the world. Due to the antioxidant potential, carotenoid-rich plant foods have been suggested as promising treatment for inflammatory bowel diseases[6]. Carotenoids have shown multiple functions such as scavenging free radicals, inhibition of angiogenesis and prevention of cell propagation, which are closely correlated with the antioxidant properties[7-8].
Carotenoids are vital for human being because the human body is not capable to synthesize carotenoids in vivo, which thus need to be supplied by the intake of food or supplements[9]. Currently, production of carotenoids is mainly obtained through chemical synthesis, extraction from plants and microbial fermentation[1]. However, extraction from plants is relatively limited by low yields and high production costs. Furthermore, problems including seasonal and geographic variability still exist which are out of control. Production of carotenoids by chemical synthesis will generate hazardous wastes that can affect the environment. All these effects lead to microbial production of carotenoids becoming more and more popular.
Carotenoids are the most widespread pigments in nature and are present in photosynthetic bacteria, some species of fungi, algae and plants[4]. Photosynthetic bacteria and yeast are two main resources for microbial fermentation to produce natural carotenoids[10-12]. Among them, R. sphaeroides is one of the most excellent bacteria for production of carotenoids. The cell cultures of this bacterium are in dark red, which is resulted from the biosynthesis of carotenoids. Normally, this bacterium will form photosynthetic apparatus including light-harvesting 1, light-harvesting 2 and reaction center under low oxygen tension and light intensity[13]. Carotenoids are very important photopigment for the formation of photosynthetic apparatus, which will protect the light-harvesting complexes against damage caused by the photogeneration of singlet oxygen.
Genomic sequence of R. sphaeroides has been completely performed and a crt operon for biosynthesis of carotenoids is located in the chromosome I of R. sphaeroides[14]. The crt operon is comprised of crtA, crtI, crtB, crtC, crtD, crtE and crtF, which is regulated by many transcriptional regulators including AppA, PpsR, TspO and RegA/RegB[15]. crtA encodes spherene oxygenase, catalyzing the transform of hydroxyspheroidene into hydroxyspheroidenone. In the present study, the genetically engineered R. sphaeroides overexpressing crtA gene was constructed to increase total carotenoids production.
Materials and Methods
Bacterial strains and growth conditions
R. sphaeroides strains were grown at 30 ℃ in malate minimal medium[16]. Growth under micro-aerobic conditions was performed as described in our previous study[17]. E. coli strains were grown at 37 ℃ in flask filling Luria-Bertani medium. Antibiotics were added to the growth medium at the following concentrations when necessary: 200 μg/ml ampicillin, 20 μg/ml tetracycline for E. coli, and 1.5 μg/ml tetracycline for R. sphaeroides.
Construction of DNA plasmids
The crtA was amplified from R. sphaeroides genomic DNA by PrimeSTAR HS DNA polymerase (TAKARA) with the primers of crtA-F: GGGGTACCATGCAGACTGTCACGCTC, crtA-R: CGAG CTCTCAGGCGTTCTCTTTGC. After verification by electrophoresis, the crtA fragment was ligated into pMD18-T and sequenced. Then, the crtA fragment was released from pMD18-T-crtA by Kpn I and Sac I and subsequently ligated into pRKpuf[18] digested by the same enzymes, producing the crtA overexpression vector pRKcrtA.
Construction of genetically engineered R. sphaeorides
The constructed plasmid DNA was transferred into R. sphaeroides 2.4.1 by using the E. coli S17-1 as the donor as described in the previous study[17]. The genetically engineered R. sphaeroides termed 2.4.1/pRKcrtA.
Production of total carotenoids from the genetically engineered strain
Colonies of the conjugant were selected and cultured under micro-aerobic conditions in dark at 30 ℃ until OD660 reached approximately 0.6. Pre-cultures were respectively inoculated into 100-ml flasks filled with malate minimal medium with 1.5 μg/ml tetracycline at the ratio of 1% and grown under micro-aerobic conditions in dark at 30 ℃ for 48 h. Carotenoids were extracted and quantified as described in our previous study[19].
Effects of inoculate amount on the yield of carotenoids from genetically engineered R. sphaeroides strain
A single colony was inoculated in a 50 ml-flask filled with 40 ml of malate minimal medium and grown under micro-aerobic growth conditions in dark at 30 ℃ until the OD660 was about 0.6. Pre-cultures were inoculated into five 100-ml flasks filled with 80 ml of malate minimal media at 1%, 2%, 4%, 6% and 8%, then grown in dark at 30 ℃ for 48 h. Total carotenoids were extracted from different cell cultures and quantified, respectively. The experiment was repeated three times.
Effects of oxygen tension on the yield of carotenoids from genetically engineered R. sphaeroides strain
Oxygen tension was controlled by changing the ratio of liquid volume to total flask volume. A single colony was inoculated in a 50 ml-flask filled with 40 ml of malate minimal media and grown under micro-aerobic conditions in dark at 30 ℃ until the OD660 was about 0.6. Pre-cultures were respectively inoculated into five 100-ml flasks filled with 50, 60, 70, 80 and 90 ml of malate minimal media at 1:50 and grown in dark at 30 ℃ for 48 h.
Effects of fermentation time on the yield of carotenoids from genetically engineered R. sphaeroides strain
A single colony was inoculated in a 50 ml-flask filled with 40 ml of malate minimal medium and grown under micro-aerobic growth conditions in dark at 30 ℃ until the OD660 was about 0.6. Pre-cultures were respectively inoculated into five 100-ml flasks filled with 80 ml of malate minimal medium at 1% and grown in dark at 30 ℃ for 24, 36, 48, 60 and 72 h. Total carotenoids were extracted from the cell cultures and quantified, respectively. The experiment was repeated three times.
Effects of fermentation temperature on the yield of carotenoids from genetically engineered R. sphaeroides strain
A single colony was inoculated in a 50 ml-flask filled with 40 ml of malate minimal medium and grown under micro-aerobic conditions in dark at 30 ℃ until the OD660 was about 0.6. Pre-cultures were inoculated into five 100-ml flasks filled with 80 ml of malate minimal medium at 1:100 and grown in dark at 23, 27, 30, 33 and 37 ℃ for 48 h. Total carotenoids were extracted from the cell cultures and quantified, respectively. The experiment was repeated three times.
Data analysis
All experiments were repeated for 3 times. Turkey test and GraphPad Prism software were used to analyze the data trend.
Results and Discussion
Construction of the overexpression vector pRKcrtA
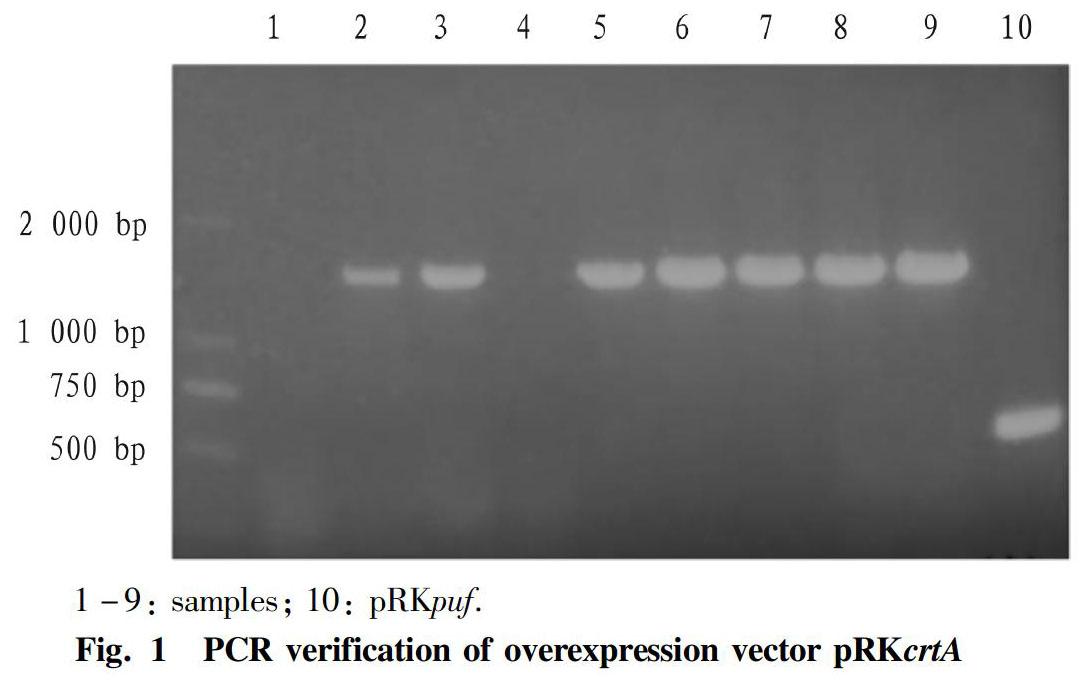
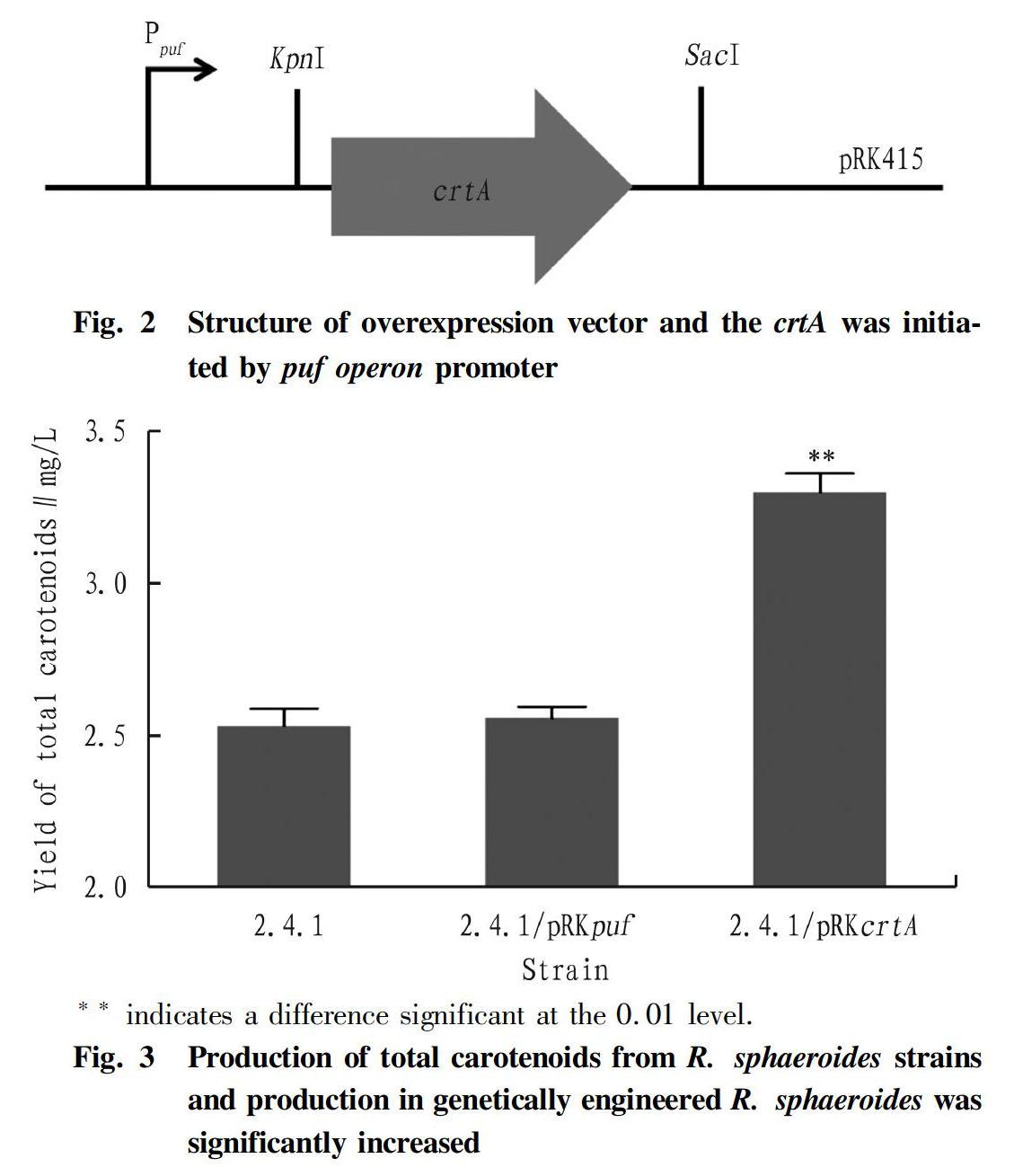
The crtA gene fragment was amplified from R. sphaeroides genomic DNA and ligated into pMD18-T cloning vector and sequenced. After that, the crtA fragment was cut and purified by gel extraction, which subsequently ligated into pRKpuf, producing the overexpression vector pRKcrtA verified by PCR with M13 primers, as seen in Fig. 1. The size of fragment amplified from the empty vector pRKpuf was about 500 bp. So, the size of the PCR fragment for overexpression vector with the crtA should be about 1 500 bp. Clearly, samples except sample 1 and sample 4 were correct. The structure of overexpression vector was described as Fig. 2. The crtA gene was initiated by the powerful puf operon promoter, which was used and worked well as described in our previous study[20].
Increased production of carotenoids from genetically engineered R. sphaeroides overexpressing crtA
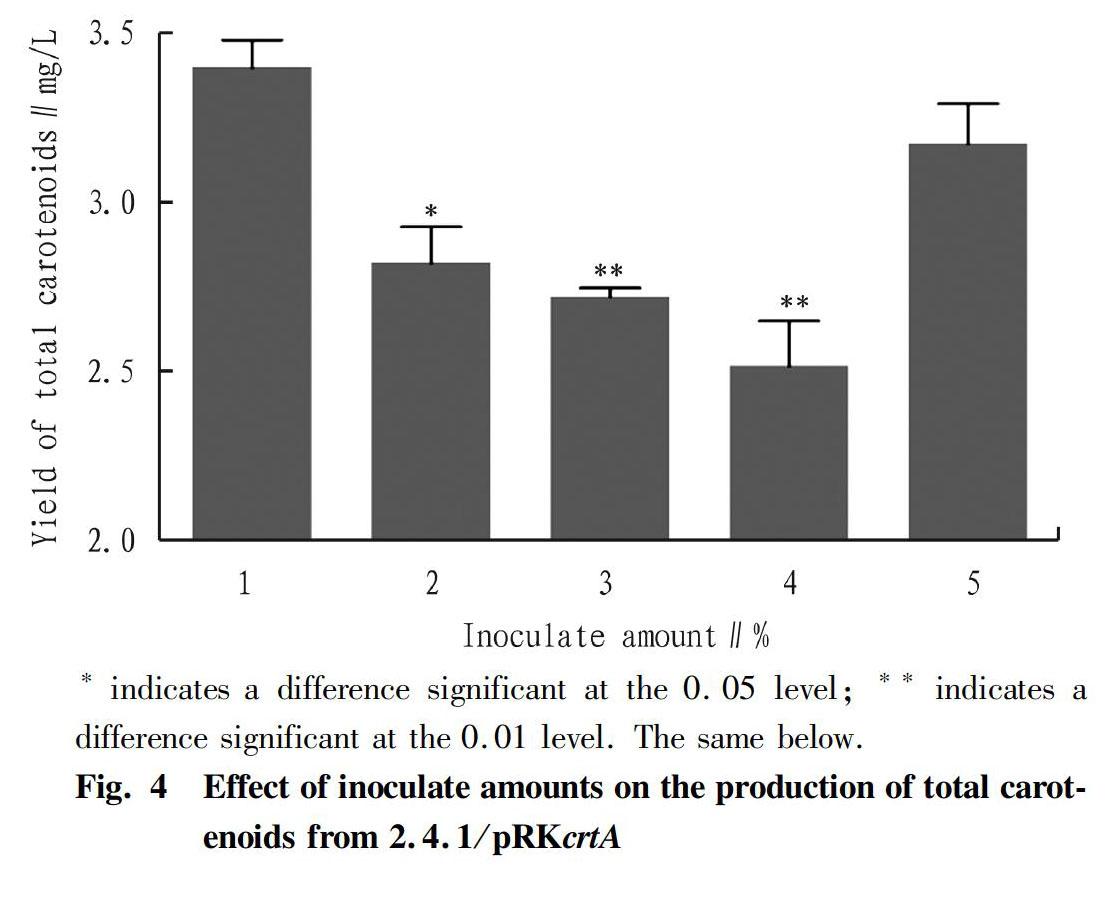
Total carotenoids were extracted from the wild type R. sphaeroides 2.4.1, R. sphaeroides 2.4.1 harboring the empty vector pRKpuf (2.4.1/pRKpuf) and genetically engineered R. sphaeroides 2.4.1 overexpressing pRKcrtA (2.4.1/pRKcrtA), as shown in Fig. 3. The production of total carotenoids from wild type 2.4.1, 2.4.1/pRKpuf and 2.4.1/pRKcrtA was 2.53, 2.56 and 3.30 mg/L, respectively. The production of total carotenoids from the genetically engineered R. sphaeroides 2.4.1/pRKcrtA was significantly increased by 30.26% and 29.21% compared to the 2.4.1 and 2.4.1/pRKpuf, respectively. Although, the production of total carotenoids from all the strains were low, the expression vector pRKpuf was broad-host-range vector[21]. The expression vector pRKcrtA could be transferred into high carotenoids yield R. sphaeroids reported previously[22].
Effects of inoculate amount on the yield of carotenoids from genetically engineered R. sphaeroides strain
The effect inoculate amount on the production of total carotenoids were shown in Fig. 4. The production of total carotenoids from the genetically engineered 2.4.1/pRKcrtA with the inoculate amounts of 1%, 2%, 4%, 6% and 8% was 3.36, 3.13, 3.09, 3.01 and 3.27, respectively. It can be concluded that the inoculate amount strongly affected production of total carotenoids. Compared to the carotenoids production from cell cultures inoculating at 1%, the production of total carotenoids with the inoculate amounts of 4%, 6%, 8% and 10% was decreased by 6.85%, 8.04%, 10.71% and 2.68%, respectively. 1% was the optimized inoculate amount for production of carotenoids from genetically engineered strain, which agreed well with our previous study[19].
Effects of oxygen tension on the yield of carotenoids from genetically engineered R. sphaeroides strain
When oxygen tension is higher, the puf operon promoter activity will be repressed by PpsR regulator because the PpsR will bind puf operon promoter[23-24]. The effect of oxygen tension on the production of carotenoids was performed, as observed in Fig. 5. The oxygen tension in the shaken flask is dependent on the volume of medium to total volume of flask shaking at steady rotation. When the medium volume to flask volume was 50%, the oxygen tension was relatively higher, resulting in lower production of carotenoids. The carotenoids production was increased with the increase of medium from 50% to 80%. However, when the medium volume to flask volume was over 90%, the production of carotenoids was decreased. One of the reasons was probably that lower oxygen tension would influence the growth of cell cultures. On the other hand, the carotenoid production from cell cultures when the medium volume to flask volume was 80%, was much higher than other ratio of medium volume to flask volume. Thus, 80% was the optimized ratio of medium volume to flask volume for production of carotenoids from genetically engineered R. sphaeroides.
Zhiping ZHAO et al. Enhanced Production of Natural Carotenoids from Genetically Engineered Rhodobacter sphaeroides Overexpressing CrtA
Effects of fermentation temperature on the yield of carotenoids from genetically engineered R. sphaeroides strain
Fermentation temperature affected the production of carotenoids strongly, as seen in Fig. 6. From 23 to 30 ℃, the production of carotenoids was increased with the increase of incubation temperature. However, when the incubation temperature was over 30 ℃, the production of carotenoids was decreased. The production of carotenoids from cell cultures incubated at 23, 27, 30, 33 and 37 ℃ was 0.57, 1.59, 3.36, 2.97 and 2.28 mg/L, respectively. On the other hand, the production of carotenoids isolated from cell cultures incubated at 30 ℃ was significantly higher than that isolated from cell cultures incubated at 23, 27, 33 and 37 ℃. 30 ℃ was the optimized temperature for production of carotenoids from genetically engineered R. sphaeroides.
Effects of fermentation time on the yield of carotenoids from genetically engineered R. sphaeroides strain
Effect of fermentation time on the production of carotenoids was analyzed, as shown in Fig. 7. Production of carotenoids from cell cultures incubated for 48 h was significantly higher than that from cell cultures incubated for 24 and 36 h. No significantly difference between the carotenoids production from cell cultures incubated for 48 h and cell cultures incubated for 60 and 72 h was observed. 48 h was the optimized fermentation time for the production of carotenoids from genetically engineered R. sphaeroides, which agreed well with our previous study[19].
Conclusions
In the present study, the genetically engineered R. sphaeroides overexpressing crtA was constructed. As expected, the carotenoids from the genetically engineered strain were significantly increased compared to the control. The fermentation procedure for enhanced production of carotenoids was optimized and optimized procedure were: inoculate amount 1%, volume of medium to flask volume 80%, fermentation temperature 30 ℃ and fermentation time 48 h.
References
[1] MATA-GOMEZ LC, MONTANEZ JC, MENDEZ-ZAVALA A, et al. Biotechnological production of carotenoids by yeasts: an overview[J]. Microb Cell Fact, 2014(13): 12.
[2] TOTH G. Determination of vitamin A, vitamin A precursors and cytoprotective carotenoids in animal and human blood[J]. Acta Physiol Hung, 1984, 64(3-4): 319-324.
[3] SIMPSON KL. Relative value of carotenoids as precursors of vitamin A[J]. Proc Nutr Soc, 1983, 42(1): 7-17.
[4] MAOKA T. Carotenoids as natural functional pigments[J]. J Nat Med, 2019.
[5] GAREWAL H, MEYSKENS F, FRIEDMAN S, et al. Oral cancer prevention: the case for carotenoids and anti-oxidant nutrients[J]. Prev Med, 1993, 22(5): 701-711.
[6] KAULMANN A, PLANCHON S, RENAUT J, et al. Proteomic response of inflammatory stimulated intestinal epithelial cells to in vitro digested plums and cabbages rich in carotenoids and polyphenols[J]. Food Funct, 2016, 7(10): 4388-4399.
[7] BOLHASSANI A. Cancer chemoprevention by natural carotenoids as an efficient strategy[J]. Anticancer Agents Med Chem, 2015, 15(8): 1026-1031.
[8] BOHN T. Carotenoids, chronic disease prevention and dietary recommendations[J]. Int J Vitam Nutr Res, 2017, 87(3-4): 121-130.
[9] DAMS S, HOLASEK S, TSIOUNTSIOURA M, et al. An encapsulated fruit, vegetable and berry juice powder concentrate increases plasma values of specific carotenoids and vitamins[J]. Int J Vitam Nutr Res, 2019: 1-10.
[10] HENKE NA, WIEBE D, PEREZ-GARCIA F, et al. Coproduction of cell-bound and secreted value-added compounds: Simultaneous production of carotenoids and amino acids by Corynebacterium glutamicum[J]. Bioresour Technol, 2018(247): 744-752.
[11] LEE JJ, CHEN L, CAO B, et al. Engineering Rhodosporidium toruloides with a membrane transporter facilitates production and separation of carotenoids and lipids in a bi-phasic culture[J]. Appl Microbiol Biotechnol, 2016, 100(2): 869-877.
[12] BONA-LOVASZ J, BONA A, EDERER M, et al. A rapid method for the extraction and analysis of carotenoids and other hydrophobic substances suitable for systems biology studies with photosynthetic bacteria[J]. Metabolites, 2013, 3(4): 912-930.
[13] DHAENE SE, CROUCH LI, JONES MR, et al. Organization in photosynthetic membranes of purple bacteria in vivo: the role of carotenoids[J]. Biochim Biophys Acta, 2014, 1837(10): 1665-1673.
[14] CHOUDHARY M, FU YX, MACKENZIE C, et al. DNA sequence duplication in Rhodobacter sphaeroides 2.4.1: evidence of an ancient partnership between chromosomes I and II[J]. J Bacteriol, 2004, 186(7): 2019-2027.
[15] ZEILSTRA-RYALLS J, GOMELSKY M, ERASO JM, et al. Control of photosystem formation in Rhodobacter sphaeroides[J]. J Bacteriol, 1998, 180(11): 2801-2809.
[16] REMES B, BERGHOFF BA, FORSTNER KU, et al. Role of oxygen and the OxyR protein in the response to iron limitation in Rhodobacter sphaeroides[J]. BMC Genomics, 2014(15): 794.
[17] ZHAO Z, HU Z, LIANG Y, et al. One-step purification of functional light-harvesting 2 complex from Rhodobacter sphaeroides[J]. Protein Pept Lett, 2010, 17(4): 444-448.
[18] HENDRISCHK AK, FRUHWIRTH SW, MOLDT J, et al. A cryptochrome-like protein is involved in the regulation of photosynthesis genes in Rhodobacter sphaeroides[J]. Molecular Microbiology, 2009, 74(4): 990-1003.
[19] TANG Y, NIE X, YU Y, et al. Optimization of fermentation process for the production of functional carotenoids from Rhodobacter sphaeroides treated by nitrosoguanidine[J]. Journal of Chemical and Pharmaceutical Research, 2016, 8(6): 367-371.
[20] TANG K, WANG J, WANG W, et al. Production of functional coenzyme Q10 from genetically engineered Rhodobacter sphaeroides[J]. Advance Journal of Food Science and Technology, 2019, 17(3): 48-53.
[21] BILLENKAMP F, PENG T, BERGHOFF BA, et al. A cluster of four homologous small RNAs modulates C1 metabolism and the pyruvate dehydrogenase complex in Rhodobacter sphaeroides under various stress conditions[J]. J Bacteriol, 2015, 197(10): 1839-1852.
[22] LIU SL, ZHANG GM, LI XK, et al. Enhancement of Rhodobacter sphaeroides growth and carotenoid production through biostimulation[J]. Journal of Environmental Sciences, 2015(33): 21-28.
[23] FEDOTOVA Y, ZEILSTRA-RYALLS J. Analysis of the role of PrrA, PpsR, and FnrL in intracytoplasmic membrane differentiation of Rhodobacter sphaeroides 2.4.1 using transmission electron microscopy[J]. Photosynth Res, 2014, 119(3): 283-290.
[24] JAGER A, BRAATSCH S, HABERZETTL K, et al. The AppA and PpsR proteins from Rhodobacter sphaeroides can establish a redox-dependent signal chain but fail to transmit blue-light signals in other bacteria[J]. J Bacteriol, 2007, 189(6): 2274-2282.
- 农业生物技术(英文版)的其它文章
- Testis of Male Tilapia Under Methomyl Stress: Transcriptome Changes and Signal Pathway Analysis
- Functional Analysis of Dunaliella salina Calmodulin Kinase Gene
- First Report of Phomopsis Leaf Spot on Patchouli [Pogostemon cablin (Blanco) Benth.] Caused by Diaporthe arecae in China
- Effects of Plant Growth Regulators on Tillering Ability of Ophiopogon japonicus cv
- Effect of Irrigation and Fertilization on Population Structure and Yield of Wheat
- Production and Cultivation Technology of Selenium-enriched Pueraria thomsonii Benth

