Immunomodulatory and anticancer activity of Bombax ceiba Linn leaf extract
Neelima Sharma,Sneha Kispotta,Papiya Mitra Mazumder
Department of Pharmaceutical Sciences & Technology,Birla Institute of Technology,Mesra,Ranchi,India
ABSTRACT
Objective: To evaluate the immunomodulatory and anticancer activity of the methanolic extract of Bombax ceiba leaves in vitro and in vivo.
Methods: The antioxidant property of methanolic extract of Bombax ceiba leaves was determined by measuring hydrogen peroxide scavenging and DPPH scavenging activity.The effect on cellular immunity in vivo was determined by measuring neutrophil adhesion,carbon clearance,sheep red blood cell induced DTH response and cyclophosphamide-induced myelosuppression.In vitro anticancer activity was evaluated on human leukaemia cell line (HL-60) by MTT assay,caspase-3 activity,and cell cycle study.
Results: The methanolic extract of Bombax ceiba leaves showed antioxidant activity and significantly increased neutrophil adhesion,carbon clearance from blood,DTH response and cyclophosphamideinduced myelosuppression.The MTT assay showed a significant increase in the death of HL-60 cell line.A rise in caspase-3 activity and sub-G1 population in the presence of methanolic extract of Bombax ceiba leaves was observed.
Conclusions: The methanolic extract of leaves of Bombax ceiba L possesses anticancer activity,immunomodulatory activity,and antioxidant properties,proving its therapeutic usefulness in the treatment of immuno-compromised diseases and cancers.
KEYWORDS: Anticancer;Antioxidative;Bombax ceiba;Immunomodulatory
1.Introduction
The alternative system of medicine,which includes herbal extracts and formulations containing bioactive constituents,would be more valuable than combined therapy with less side effects and better patients' acquiescence.Herbal medicines have been used for a long time because of their good absorption,less toxicity,and easily availability.A large number of herbs have been used for the enhancement of the immune system and also as anticancer drugs in the form of Ayurvedic formulation either alone or in combination.Still,plants represent a large unexploited source of structurally novel compounds that might serve as a lead for the development of an innovative drug.
Bombax ceiba (B.ceiba) Linn belongs to the family Bombacaceae and is a vital medicinal plant cultivated throughout tropical and subtropical Asia.The plant is well known among the tribal people for the treatment of various diseases related to humans as well as animals.According to Ayurveda,it possesses anti-dysenteric,astringent,diuretic,stimulant,haemostatic,anti-diarrheal,cardiotonic,demulcent,and antipyretic effects[1].
Traditionally,different parts of B.ceiba Linn are being used for the treatment of various disorders.The roots of the plant are used for the treatment of wounds,diarrhea and dysentery while the gum is useful in burning sensation,pulmonary tuberculosis,enteritis and influenza.The flowers are good for skin problems and bark is demulcent and emetic.The fruits are useful in chronic inflammation and ulceration of kidney and bladder while the seeds are good in treating gonorrhoea.The literature reports reveal its antioxidative,anti-inflammatory,antihyperglycemic,antihyperlipidemic,immunomodulatory,and hepatoprotective activity[2-5].Different classes of compounds present in B.ceiba leaves are alkaloids,flavanoids,carbohydrates,quinones,cardiac glycoside,saponins,phenols,tannins and terpenoids[3,6].The polysaccharide fraction of B.ceiba flowers is reported to have immunomodulatory activity[7].Among the devastating and pandemic diseases,cancer is a major disease.Cancer is an uncontrolled proliferation of a normal cell that produces genetic instabilities and alterations within cells and tissues and transforms a normal cell into a malignant cell.As cancer weakens the immune system of the patient,the immunity is also an important concern for patients with cancer[8,9].Except for cancer,environmental pollutants and dietary habits also cause changes in the immune system.Although B.ceiba plant is of great biomedical importance,it is still lack of report regarding its anticancer activity.Therefore,the aim of our study was to determine the immunomodulatory activity (in vivo) and anticancer activity (in vitro)of the methanolic extract of B.ceiba leaves.
2.Materials and methods
2.1.Reagents and chemicals
HL-60 cell line (70%-80% confluent) was procured from National Centre for Cell Sciences,Pune.All chemicals used in this study were of highest grade purity available.1,1-Diphenyl-2-picrylhydrazyl(DPPH),3-(4,5-dimethyl-2-yl)-2,5-diphenyl tetrazolium bromide(MTT),and DEVD-AFC substrate were purchased from Sigma-Aldrich (St.Louis,MO).Fetal bovine serum (FBS),triton-×100,RPMI-1640 media,and antibiotic-antimycotic solution were purchased from Himedia Laboratories Ltd.(Mumbai,India).Methanol,sodium hydroxide,sulfuric acid,ascorbic acid,Tween 20 and hydrogen peroxide were purchased from Fine Chemicals.
2.2.Extract preparation
The leaves of B.ceiba L.were collected from the medicinal garden of Birla Institute of Technology,Mesra,Ranchi,Jharkhand during July-August 2017.The leaves of the plant were authenticated(Voucher number: PH/ BIT/367/2013) and shade dried for 40 d.The dried leaves were coarsely powdered and 250 g of dry powder of leaves was extracted with (500 mL) methanol (methanol:leave powder 10∶1) under reflux for 10 d.The extract was collected and filtered,and the filtrate was then concentrated by evaporating the solvent with a rotary evaporator under reduced pressure.
2.3.Preliminary phytochemical screening of methanolic extract of B.ceiba leaves (BCM)
The preliminary detection of phytochemical constituents in BCM was carried out using standard phytochemical screening tests.The qualitative tests for the identification of various plant constituents such as alkaloids (Mayer test),carbohydrates (Molisch test),glycosides (Keller kellian test),flavonoids (Shinoda test),phenols(Ferric chloride test),saponins (Foam test),sterols (Liebermann-Burchard test),tannins (Braymer test) and terpenoids (Salkowki test)were carried out with BCM[10-12].
2.4.Determination of antioxidative property
The antioxidant activity of the plant extract at 20,40,60,80,100 μg/mL and ascorbic acid (as a reference) was determined by hydrogen peroxide scavenging activity and DPPH scavenging activity[13,14].
2.4.1.Hydrogen peroxide scavenging activity
BCM and ascorbic acid at 20,40,60,80,100 μg/mL were added to hydrogen peroxide solution (0.6 mL,40 mM) which was prepared in phosphate buffer (pH 7.4).After 10 min,the absorbance was measured at 230 nm against the blank solution containing phosphate buffer.The H2O2scavenging activity of BCM and ascorbic acid was estimated by the following formula[13].
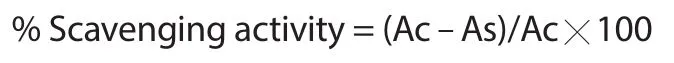
where Ac is the absorbance of the control and As is the absorbance of the sample.
2.4.2.DPPH radical scavenging assay
The sample solutions containing 1 mL of BCM at 20,40,60,80,100 μg/mL and 1 mL of DPPH solution (0.1 mL,in methanol) were prepared.Ascorbic acid solutions at different concentrations (same as extract) were used as the reference and the solution of 1 mL methanol and 1 mL DPPH was taken as control.The % inhibition was estimated by the following formula[14].
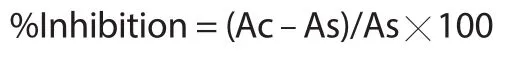
where Ac is the absorbance of the control and As is the absorbance of the sample.
2.5.Immunomodulatory activity of BCM
The immunomodulatory activity of BCM was determined in vivo.Healthy Swiss albino mice weighing 25-30 g were procured and housed in the clean at (23 ± 1) ℃ and relative humidity of 45%to 55% under 12 h light∶12 h dark cycle.The mice were fed with standard pellet diet and were allowed to acclimate to the laboratory conditions before the experiments.The drug solutions were prepared in distilled water and vehicle (Tween 80) for oral administration.The immunomodulatory activity was evaluated at the cellular level.Cellular immunity was determined by neutrophil adhesion test,carbon clearance assay,delayed-type hypersensitivity (DTH)reaction and cyclophosphamide-induced myelosuppression test.
2.5.1.Neutrophil adhesion test
For neutrophil adhesion test and carbon clearance assay,all mice were divided into 3 groups with 6 mice in each group.Mice in GroupⅠreceived only the vehicle and served as the control,GroupⅡ and Group Ⅲ were administered with BCM at 250 mg/kg and 500 mg/kg,respectively.The mice were treated with vehicle and BCM extract for 14 d in neutrophil adhesion test.After the treatment,blood samples of all mice were collected from the retro-orbital vein into heparinized vials and tested for estimation of total leukocyte count (TLC) and differential leukocyte count (DLC).Subsequently,the blood samples were incubated with nylon fibers (80 mg/mL)at 37 ℃ for 15 min.After incubation,the samples were examined again for estimation of TLC and DLC and the neutrophil index was determined.The percentage of neutrophil adhesion was calculated using the following equation[15].
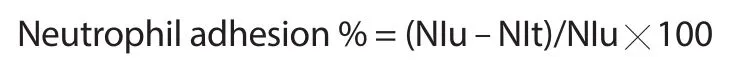
where NIu is the neutrophil index of the untreated blood sample and NIt is neutrophil index of the treated blood sample.
2.5.2.Carbon clearance test
For carbon clearance assay,the mice were treated with vehicle and BCM extract for 10 d.After treatment,all mice received an intravenous injection of colloidal carbon (30 mg in 0.3 mL Indian ink) via the tail vein.Following the ink injection,the blood samples were collected by retro-orbital plexus at the time gap of 0 and 15 min.Then the collected samples (0.1 mL) were mixed with sodium carbonate solution (4 mL).The absorbance was measured at 660 nm and phagocytic index K was calculated by applying the following formula[16].
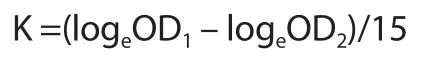
where OD1is the optical density at 0 min and OD2is the optical density at 15 min.
2.5.3.Sheep red blood cell (SRBC) induced DTH reaction(DTH response)
For DTH response and myelosuppression assays,all mice were divided into four groups with 6 animals in each group.Mice in Group Ⅰ were administered with only vehicle and served as control;Group Ⅱ received cyclophosphamide (50 mg/kg,i.p.)[17];Group Ⅲand Ⅳ were administered with low dose (250 mg/kg,oral) and high dose (500 mg/kg,oral) of BCM,respectively.
In DTH reaction,the mice were pretreated with vehicle and BCM for 14 d before the challenge.On the 15th day,after measurement of the footpad volume,all mice were challenged with SRBC (0.025×109cells) in the right paw and 0.025 mL of saline was administered in the left paw.After 24 h,the paw volume was measured again and the change was recorded.The rise in the paw volume was interpreted as an indication of cell-mediated immunity[18,19].
2.5.4.Cyclophosphamide-induced myelosuppression
For myelosuppression assay,the control group and cyclophosphamide group received Tween-80 (0.2%) solution as a vehicle,whereas animals in the treatment groups Ⅲ and Ⅳ received BCM (250 mg/kg and 500 mg/kg,oral,respectively) in Tween-80,daily for 14 d.On days 15,16,and 17,all the animals except the control group were injected with cyclophosphamide (50 mg/kg,i.p.) 1 h after the BCM administration.On day 18,the blood samples from all mice were collected and analysed for haematological parameters such as total erythrocyte count,total leucocyte count and haemoglobin content[20].
2.6.Anticancer activity of BCM
The anticancer activityof BCM extract was determined in vitro using HL-60 cell line.Cell density was adjusted to 1.5×106cells/mL and cells were treated with BCM at 1,10,25,50 and 100 μg/mL for different time periods.
2.6.1.Effect of BCM on cell viability
The effect of BCM on cell viability was assessed by MTT assay.The cells (1.5×104cells/well) were plated in a 96 well plate.Different concentrations of BCM were added and cells were incubated for 24 h at 37 ℃ with 5% CO2.MTT dye (10 μL,50 mg/mL) was added to each well 4 h before the completion of incubation time.The well plate was centrifuged,the supernatant was discarded and the resultant formazan crystals were dissolved by adding 100 μL DMSO in each well.The absorbance was read at 570 nm in a microtiter plate[21,22].According to the results of viability assay,BCM at 10,25,and 50 μg/mL were selected for further experiments.
2.6.2.Effect of BCM on caspase-3 activity
To determine the caspase-3 activity,the cells (3×106cells/well)were treated with different concentrations of BCM (10,25,and 50 μg/mL) for 1.5,3,and 6 h.After the completion of incubation time,the cells were resuspended in 50 μL lysis buffer for 10 min on ice.The cells were further centrifuged and the supernatant was collected.Reaction buffer (50 μL) and DEVD-AFC substrate (50 μm final concentration) were added to the supernatant and incubated for 2 h at 37 ℃.The resultant fluorescence was measured at 400 nm and 505 mm[23,24].
2.6.3.Effect of BCM on cell cycle study
The cell cycle study to measure sub-G1population was carried out using flow cytometry.Cells (1.5×106cells/mL) were incubated with BCM (10,25,and 50 μg/mL) for 18 h.The cells were collected,resuspended in 250 μL PBS and fixed with 500 μL ice-cold ethanol.The cell cycle study was performed after propidium iodide (PI)staining and the histogram was observed using FL-2 filter[23,24].
2.7.Statistical analysis
GraphPad Prism 7.03 was used.The measurement data were expressed as mean ± standard error of the mean (SEM) and analyzed using one-way analysis of variance (ANOVA).The significance level was set as α=0.05.
2.8.Ethical statement
The experiments were designed and conducted according to the guidelines of the committee for the purpose of the control and supervision of experiments on animals (CPSCEA) and the Institutional Animal Ethics Committee (Approval no.1972/PR/BIT/15/17/IAEC).
3.Results
3.1.Phytochemical screening of BCM
The preliminary phytochemical screening of BCM showed the presence of carbohydrates,alkaloids,glycosides,flavonoids,phenols,and tannins.Sterols were absent in the extract.
3.2.Antioxidative property of BCM
BCM at all concentrations showed the antioxidant property in a concentration-dependent manner as depicted by hydrogen peroxide assay (Figure 1A) and DPPH assay (Figure 1B).According to DPPH results,the half maximal inhibitory concentration (IC50) value of ascorbic acid was 74.56 μg/mL and that of BCM was 84.60 μg/mL.
3.3.Immunomodulatory activity of BCM
3.3.1.Effect of BCM on neutrophil adhesion
In the neutrophil adhesion test,the neutrophils adhered to the fibres during the incubation of blood with nylon fibres.Due to the adhesion of neutrophils,a decrease in the neutrophil count was observed.There was a significant increase (P<0.05) in the neutrophil adhesion after treatment of BCM at two doses as compared to the control(Table 1).
3.3.2.Effect of BCM on carbon clearance
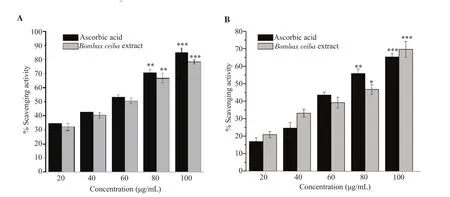
Figure 1.Effect of methanolic extract of Bombax ceiba leaves on hydrogen peroxide scavenging activity (A) and DPPH scavenging activity (B).All the values are expressed as mean ± SEM,n=3.*P<0.05,**P<0.01,***P<0.001 when compared to the control.
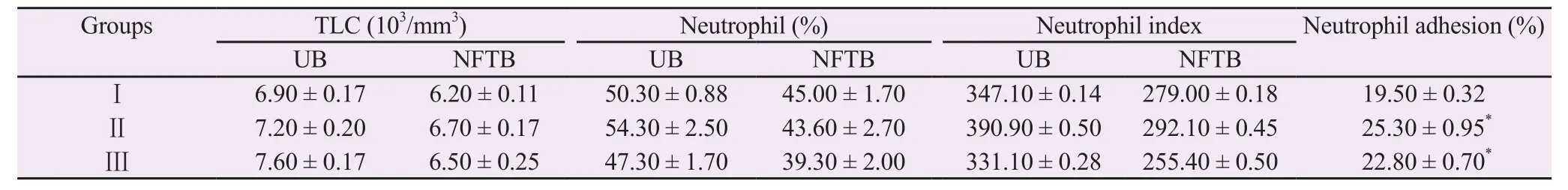
Table 1.Effect of methanolic extract of Bombax ceiba leaves on neutrophil adhesion.
The results of the carbon clearance test demonstrated that BCM significantly increased the phagocytic index.The clearance of colloidal carbon from the blood was increased after the administration of BCM.The clearance was more significant with the higher dose of the BCM (P<0.05).
3.3.3.Effect of BCM on DTH reaction
The effect of BCM on cell-mediated immune response was determined by DTH reaction,i.e.,by the footpad reaction to SRBC inoculation.The results revealed that BCM produced dose-dependent increases in DTH reaction [(1.46 ± 0.10) mm for 250 mg/kg and(1.49 ± 0.09) mm for 500 mg/kg]compared with GroupⅠ [(0.82 ±0.07) mm](P<0.05) and Group Ⅱ [(1.07 ± 0.05) mm].
3.3.4.Effect of BCM on cyclophosphamide-induced myelosuppression
The results of cyclophosphamide-induced myelosuppression assay indicated that treatment of cyclophosphamide at 50 mg/kg alone reduced the haemoglobin content and WBCs count significantly.Whereas the treatment of BCM (250 and 500 mg/kg) showed the restoration of haemoglobin content,RBCs and WBCs effectively(Table 2).
3.4.Anti-cancer property of BCM
3.4.1.Effect of BCM on cell viability
BCM at all concentrations decreased the cell viability of HL-60 cells in a concentration-dependent manner.Compared with the control group,the decrease in cell viability was not significant in the groups with BCM at 1 μg/mL [(98.90 ± 0.43)%]and 10 μg/mL[(96.02 ± 1.08)%](P>0.05);while,BCM produced significant cell death at 25 μg/mL [(75.14 ± 0.44)%](P<0.01),50 μg/mL [(68.91 ±0.21)%](P<0.001),100 μg/mL [(69.89 ± 0.09)%](P<0.001).
3.4.2.Effect of BCM on caspase-3 activity
As shown in Table 3,all treatment groups with BCM exhibited an increase in caspase-3 activity both in time- and concentrationdependent manner except for BCM at 10 μg/mL.The highest activity was observed at 6 h with 50 μg/mL of BCM.
3.4.3.Apoptotic DNA analysis
The cell population with hypodiploid DNA was determined by flow cytometry.It showed that the fraction of hypodiploid cell population in sub-G1phase was gradually increased from 4.8% (10 μg/mL BCM) to 28.6% (50 μg/mL BCM).The apoptotic cells in the normal population remained 3.4% over this duration (Figure 2).
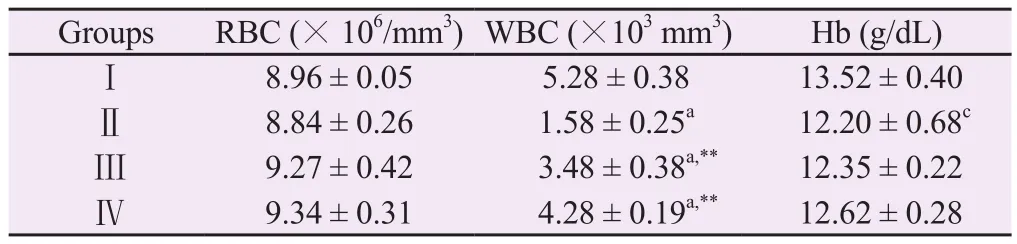
Table 2.Effect of methanolic extract of Bombax ceiba leaves on haematological parameters.
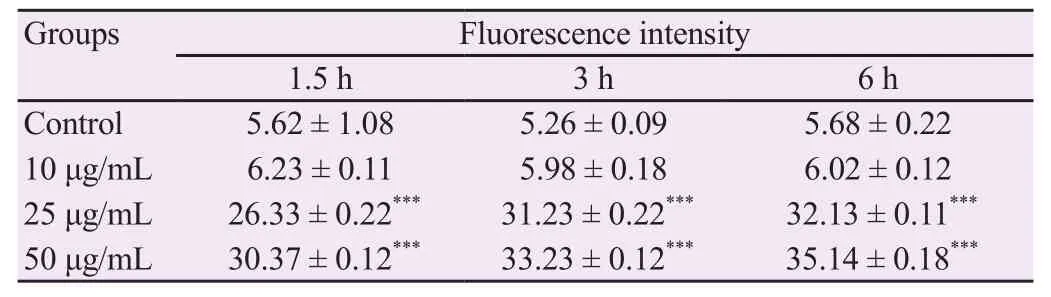
Table 3.Effect of methanolic extract of Bombax ceiba leaves on caspase-3 activity.
4.Discussion
In this study,the immunomodulatory and anti-cancer properties of BCM have been explored.The phytochemical screening showed the presence of alkaloids,flavonoids,carbohydrates,phenols etc.in BMC.Literature reports reveal that polyphenols and flavones are effective scavengers of free radicals and also help in the modulation of immune functions.The tannins,alkaloids and flavonoids of the plants may show beneficial effects in cancer by exerting antioxidant and immunomodulatory effects[25].
Neutrophils are related to the cell-mediated immunity and help in the clearance of foreign particles by recognition and movement toward the particle,phagocytosis,and abolishing the foreign particle.The neutrophil adhesion to nylon fibre produces a decrease in neutrophil counts.BCM at both doses showed a significant increase in the neutrophil adhesion.The effect of BCM on the reticuloendothelial system was analysed by the carbon clearance test.The phagocytic cells of the reticuloendothelial system play an important role in the removal of foreign particles from the blood[26].When colloidal carbon particles (ink) were injected directly into the blood,the macrophages cleared the particles from the systemic circulation.BCM showed a significant increase in the phagocytic index.Thus,it can enhance the activity of the reticuloendothelial system.
The activation of the T cells releases several lymphokines that activate and accumulate the macrophages,induce vasodilation and produce inflammation.It also enhances the phagocytosis and concentration of lytic enzymes to evade the foreign particles.These activities result in increased footpad thickness[19].In this study,BCM produced a significant dose-related increase in DTH response.It also restored cyclophosphamide-induced myelosuppression,i.e.depletion of T or B lymphocytes,deficiency of macrophages and a significant increase in WBC count.These results suggest that BMC could be effective in modulating the immune response and it may be used as a supportive treatment under immune-compromised conditions.The immunomodulatory property of BCM may be due to the presence of polyphenols and alkaloids.
Immunomodulatory drugs can be used to decrease myelosuppression and enhance immune response for cancer treatment.Regarding the anticancer activity,BCM induced a loss in the viability of HL-60 cells effectively in a concentration-dependent manner.The increase in cancer cell death indicates the anticancer property of BCM.The elevated levels of caspase-3 in the presence of BCM demonstrate the activation of the caspase-dependent pathway.Caspases are the proteins that cleave the main cellular components of the cells such as repair enzymes that are required for normal cellular functions.These caspases are characteristically activated during apoptosis and stimulate various lytic enzymes such as DNases that cleave the DNA in the nucleus[23].The increase in caspase-3 activity was observed in both time- and concentration-dependent manner.The highest activity was observed at 6 h with 50 μg/mL of the BCM.These results indicate that BCM may be cause caspase-dependent apoptotic cell death in HL-60 cells.
Apoptosis,the programmed cell death,generally occurs during the growth and aging processes to maintain the cell population in different tissues.During diseased conditions and in the presence of toxic agents,there are alterations in the normal apoptotic mechanisms.As the cell receives the signals to undergo apoptosis,a number of distinctive changes occur such as activation of caspases,pro- and anti-apoptotic proteins,etc[24].Apoptosis plays a vital role in the mechanism of anticancer drugs.The more effective anticancer agents can be developed by targeting the apoptotic pathways mainly in the tumors[27].The apoptotic cell death in HL-60 cell lines has been confirmed by cell cycle studies,the results imply that the fraction of hypodiploid cell population in sub-G1phase gradually increases from 4.8% (10 μg/mL) to 28.6% (50 μg/mL) as a consequence of the treatment with BCM.This confirms the activation of the apoptotic pathway by BCM.
Thus,it can be concluded that BCM possesses antioxidant,immunomodulatory as well as anti-cancer properties.Natural products are still significant sources of new drugs for the treatment of various diseases,such as cancer.A thorough investigation of B.ceiba L.is warranted regarding the mechanism as well as the active constituent(s),which will provide a better understanding of its activity against cancerous cells.
Conflict of interest statement
We declare that there is no conflict of interest.
 Asian Pacific Journal of Tropical Biomedicine2020年9期
Asian Pacific Journal of Tropical Biomedicine2020年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antidiabetic effect of Chrysophyllum albidum is mediated by enzyme inhibition and enhancement of glucose uptake via 3T3-L1 adipocytes and C2C12 myotubes
- Opuntia humifusa aqueous extract alleviates ethanol-induced gastric ulcer in a mouse model
- Allolobophora caliginosa coelomic fluid ameliorates gentamicin-induced hepatorenal toxicity in rats
- Resveratrol downregulates TGF-β1 and Smad3 expression and attenuates oxidative stress in CCl4-induced kidney damage in rats
