Opuntia humifusa aqueous extract alleviates ethanol-induced gastric ulcer in a mouse model
Chi-Yeol Yoo,Hyeong-U Son,,Alshammari Fanar,Hee-Jung Choi,Md Badrul Alam,Sang-Han Lee,✉
1Department of Food Science and Biotechnology,Kyungpook National University,Daegu 41566,Korea
2Food and Bio-Industry Research Institute,Kyungpook National University,Daegu 41566,Korea
ABSTRACT
Objective: To investigate the effect of Opuntia humifusa aqueous extract on gastric ulcers.
Methods: An ethanol-induced model was used to examine the protective effect of Opuntia humifusa against gastric ulcers.The gastric ulcer index was evaluated via clinical observation and image analysis.Various inflammatory indicators were determined by RTPCR and Western blotting assays.
Results: The gastric ulcer index was reduced to 8% in the group treated with Opuntia humifusa aqueous extract compared with that in the control group.RT-PCR analysis revealed that MUC5AC expression was reduced to 39% in the control group compared with the non-treated group,whereas the omeprazole and Opuntia humifusa aqueous extract-treated groups increased the expression to 95% and 79%,respectively.Moreover,the expressions of various cytokines including TNF-α,IL-1β,and IL-6 were increased in the control group,while decreasing in Opuntia humifusa aqueous extract-treated group.Opuntia humifusa aqueous extract also suppressed the expressions of iNOS,COX-2,and its transcription factor NF-κB and increased mucus content considerably as compared to the control group.
Conclusions: These results suggest that Opuntia humifusa aqueous extract is suitable as an alternative remedy for gastric ulcer treatment.
KEYWORDS: Opuntia humifusa;Gastric ulcer;Gastric lesions;Image analysis;Mucin
1.Introduction
Gastric ulcers have a profound impact on human health worldwide.Gastric ulcers combine to duodenal ulcers to form peptic ulcer disease,which is the most common gastrointestinal disorder[1].Major factors that impact gastric ulcers are stress,alcohol,tobacco,steroids,food irritants,nutritional deficiencies,and Helicobacter pylori infection[2,3].However,deterioration of antioxidant capacity owing to an increase in reactive oxygen species (ROS) and prostaglandin production is associated with ulcerative lesions.Acute gastric ulcers are reportedly induced by a single dose of indomethacin,ethanol,aspirin,and water immersion-restraint stress[4];such models have been used to monitor a variety of factors that affect gastric injury treatment[5],thereby demonstrating that different factors/agents can cause different types of ulcers[6].Moreover,gastric ulcers are treated by signal mediators,including prostaglandins,cytokines,and matrix metalloproteinases,which are needed to maintain gastrointestinal homeostasis for regulating gastrointestinal lesion development or healing mechanism[7].
Additionally,mucin is a major polymeric glycoprotein component of the mucosal surface (10-μm sized granules with a fiber-like membrane),which plays an important role in preserving mucosal expression integrity.It is present in various systemic secretions from the gastrointestinal mucosa,respiratory mucosa,female genital mucosa,middle ear,and conjunctiva.Moreover,mucin is a polypeptide in which oligosaccharides are linked by O-linked glycosylation,and the sugar structure of mucins has different composition ratios depending on the tissue and disease.Moreover,mucus represses cell adhesion of cancer cell growth,immune recognition,and gastric epithelial cells.In addition,the risk of a malignant transformation increases and adversely affects intrusion and metastatic ability.In these cases,steroids and non-steroidal antiinflammatory drugs are generally used for treating mild symptoms.However,systemic long-term administration may cause side effects such as immunosuppression and gastric ulcer.From this viewpoint,it is important to search for natural anti-inflammatory products whose safety has been established[8].Additionally,in Western medicine,omeprazole is a widely used drug for gastric ulcer treatment;human and animal experiments have revealed that omeprazole can be substituted by benzimidazole,which is used to treat gastric ulcer and gastric hyperplasia because of the latter's strong ability to inhibit gastric acid secretion.
Opuntia humifusa (O.humifusa),a member of the Cactaceae family,is a perennial plant and widely distributed across semiarid regions,such as Central and South America.O.humifusa has also been cultivated in large quantities in Asan and Uiseong,Republic of Korea,and is known as “Cheon-nyun-cho” in Korean[9].It has biological benefits such as radical scavenging and antiinflammatory activities[10],exerts streptozotocin-mediated antidiabetes effect[11]and anti-asthmatic effect by regulating the expression levels of IL-4 and IL-13[12]as well as ameliorates acute pancreatitis[13].In particular,the plant contains mucilage,which consists of carbohydrates in the form of pectin,arabinose,galactose,galacturonic acid,and rhamnose[14,15]and can ameliorate gastric ulceration.Therefore,our research focused upon the effect of O.humifusa aqueous extract on gastric ulcers.
2.Materials and methods
2.1.Reagents
The following reagents were purchased: ethyl alcohol (99.9%,Merck),TRIZOL reagent (Invitrogen,Carlsbad,CA),omeprazole(O104,Sigma-Aldrich,St.Louis,MO),caffeic acid (C0625,Sigma-Aldrich),ferulic acid (PHR1791,Sigma-Aldrich),gallic acid(G7384,Sigma-Aldrich),chlorogenic acid (C3878,Sigma-Aldrich),quercetin (Q4951,Sigma-Aldrich),and rutin (R5143,Sigma-Aldrich).
2.2.Sample preparation
O.humifusa (1 kg) was obtained from Uiseong Cluster Association,Republic of Korea;The Association Group supervised cultivation to manage standard O.humifusa.The voucher specimen (2017-OH) was deposited in the Food Enzyme Biotechnology Laboratory,Kyungpook National University,Daegu,Korea.O.humifusa was dried and homogenized in a commercial mixer (JSHR-270D,JSR,Gongju,Korea).The partial remaining powder (100 g) was mixed with distilled water at a 1:10 w/v ratio and extracted using a shaking incubator (Korea) at 45 ℃ for 24 h.After extraction,the mixture was filtered using WHATMAN filter paper No.1 and lyophilized(Lyophilizer,Ilshin BioBase,Dongducheon,Korea),after which it was dissolved in distilled water for further investigation.
2.3.Animal care
Five-week-old specific pathogen-free male BALB/c mice (average body weight,20-23 g) were purchased from Samtaco Korea (Osan,Korea).The experimental animals were subjected to laboratory purification for 1 week in the animal breeding room of Kyungpook National University Experimental Animal Research Center.The mice were freely fed a pellet-type solid feed Samtaco Korea (Osan,Korea) and allowed to drink water freely.
2.4.Ethical statement
This experimental procedure was performed strictly according to in-house guidelines and the current protocol conformed with the Guidelines Research and Ethical Issues of the International Society of Pain Medicine.Kyungpook National University Industry Foundation (KNUIF-2017-0069).
2.5.Ethanol-induced gastric ulceration model
Ethanol-induced gastric ulceration model was performed as described previously[16,17]with minor modifications.The mice were randomly divided into four groups (n=5),namely,normal control,ethanol control (ethanol induction and saline treatment),omeprazole group (ethanol induction and omeprazole treatment,3 mg/kg) and O.humifusa aqueous extract-treated group (ethanol induction and O.humifusa aqueous extract,100 mg/kg via p.o.injection with 200 μL saline).The concentration of O.humifusa aqueous extract was fixed since 100 mg/kg treatment was sufficiently effective against gastric ulcer in our previous study (data not shown).Omeprazole was used as the positive control.The animals were fasted and allowed to consume only purified water for 18 h.Before ethanol induction,mice were subjected to the oral administration of each sample and maintained without water ad libitum for 30 min.All mice groups,except normal control,were orally administered with 8 mg/kg ethanol.After 30 min,the mice were intoxicated and laid down because of ethanol induction,but no death occurred.The mice were then euthanized by CO2gas,and subsequently,gastric tissues were isolated.
2.6.Image analysis of gastric ulcers
Using image analysis,we examined striped erythema or pointlike lesions and various damaged gastric tissues for measuring ulcer index scores.Photographed image was divided into ulcer lesion or none because of its distinct color.Ulcer area was calculated as selected pixel by Image J program.To maintain the consistency of the measured images,we controlled the light source and the digital light raw file.We took pictures in a dark room using a controlled box in a restricted space to avoid environmental disturbances caused by sunlight (Hyundai Fomax E400,Seoul,Korea).A Nikon digital camera was used (D5100;Nikon Co.,Tokyo,Japan).The Nikon digital camera D5100 was equipped with AF-S Micro NIKKOR 40 mm f/2.8 G lens (Nikon Co.,Tokyo,Japan) and a Nikon MLL3 wireless remote-control system (Nikon Co.,Tokyo,Japan).In the first experiment,the subject was placed in a small studio with a diffuser and a background fabric cover,followed by a color image scale (QP card 203,QPcard AB,Helsingborg,Sweden).Color images are innovative tools that control color profiles and enable color reproduction[18].All photograph data were conducted on same condition for calculating gastric lesions area.Images of gastric mucosal organs were saved as original files and colors were adjusted using a QP Calibration v1.21 software (QPcard AB,Helsingborg,Sweden).
2.7.Measurement of gene expression (MUC5AC,TNF-α,IL-6,IL-1β,COX-2,GAPDH) by RT-PCR
Total cellular RNA was prepared using TRIZOL®reagent according to the manufacturer's instruction.After mice were euthanized,gastric tissues were isolated and the total RNA was extracted as described.One mL of TRIZOL®reagent was mixed with frozen stomach tissues(approximately 100 mg).The total RNA content was analyzed by projecting a multi-label counter (VICTOR-3 spectrophotometer,Perkin Elmer,Turku,Finland) at 260 nm.After the course of the reaction,each product was analyzed using 1% agarose gel electrophoresis by performing EtBr staining.The gene product was identified by comparing with the 1-kb DNA ladder (Solgent in Korea).For semi-quantitative analysis,the relative intensities of each set of gene products were quantified using a Molecular Imager(ChemiDoc XRS+,Bio-Rad,Philadelphia,PA,USA).PCR reaction was carried out in a programmable thermal cycler (Perkin Elmer,model 9700).
2.8.Immunohistochemical analysis
Immunohistochemical analysis was performed as described in the previous experiment[19].Briefly,the stomach tissue was cut into larger curvatures and embedded in paraffin.Thereafter,the section was cut to a size of 5 mm and placed on a slide warmer for 16 h.Xylene was used for deparaffinization,after which each section was serially diluted with 70%-100% alcohol for 60 min.After washing,the sections were incubated with 10% goat serum at RT for 1 h to avoid non-specific binding.The sections were subsequently stained with antibody (iNOS and NF-κB).
2.9.HPLC analysis
Experiments were performed on a Shimadzu Prominence HPLC system (Shimadzu,Kyoto,Japan).The extract samples were eluted at a rate of 10 mg/mL and filtered through a syringe filter (0.2 μm,Pall Life Sciences,Ann Arbor,MI).A C18 column (4.6 × 250 mm,5 μm) was used,with a flow rate and column temperature of 1 mL/min and 30 ℃,respectively;the detection wavelengths were set at 254 and 324 nm.The mobile phase was 1% formic acid: MeOH at a ratio of 80:20.
2.10.Statistical analysis
Data are expressed as mean ± standard deviation (SD).Statistical values were calculated by ANOVA with Tukey post-hoc test program using SPSS 21.0 software[20].P<0.05 was considered statistically significant.
3.Results
3.1.Effect of O.humifusa aqueous extract on gastric ulcer
Severe lesions in the stomach were phenotypically con firmed.As shown in Figure 1A,gastric ulcer lesions resulted in a light peach to red coloration in the inner tissues lining the stomach.Red-colored areas were clearly seen in the images,demonstrating tissue damage in the ethanol control group,and red-colored spots/lesions were measured in black color using Image J for alternative comparison.For easily comparing the area values,white and black colors through binary were shown (Figure 1).Image analysis revealed that the omeprazole-treated group (positive control) showed a significant decrease in the positive area,whereas the O.humifusa aqueous extract-treated group showed the index was reduced to 8% in the positive area compared with the ethanol group (Figure 1B).These results indicated that the activity of O.humifusa aqueous extract was similar to omeprazole.
3.2.Relevant gene expression by RT-PCR
Expression of MUC5AC was decreased to 39% in the ethanol control group compared with the normal control group.Treatment with O.humifusa aqueous extract as well as omeprazole increased the expression to 79% and 95%,respectively (Figure 2B).Additionally,the ethanol control group increased TNF-α,IL-6,IL-1βand COX-2 expressions,while omeprazole and O.humifusa aqueous extract decreased these expressions (Figure 2C-F).The effect of O.humifusa aqueous extract was comparable to that of omeprazole.
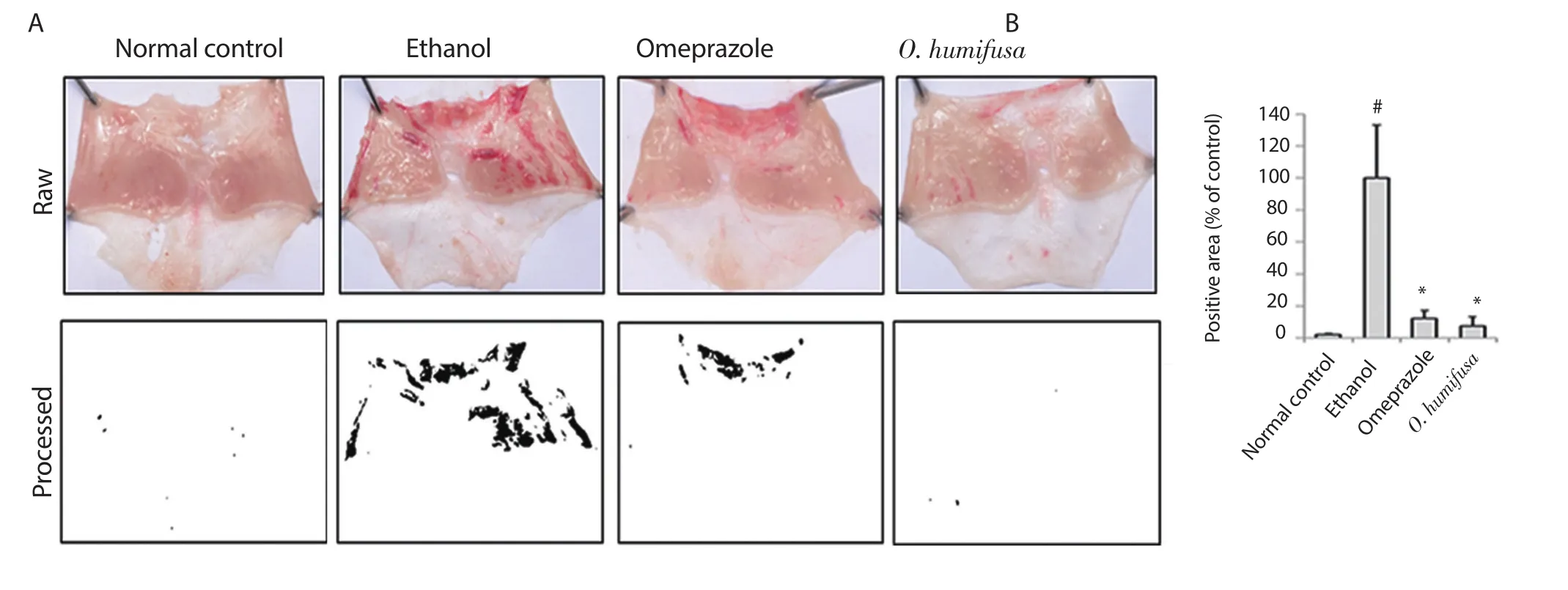
Figure 1.Effects of Opuntia humifusa (O.humifusa) aqueous extract on mucosal tissues in an ethanol-induced gastric ulcer model.(A) Images of gastric ulcers in the experimental groups.(B) Positive area was measured according to gastric lesion index as shown in Figure 2A and expressed as the intensity relative to control.Data are expressed as mean ± standard deviation (SD,n=5 animals/group).#P<0.05 and *P<0.01 compared with the normal control group and the ethanol-administered control group,respectively.
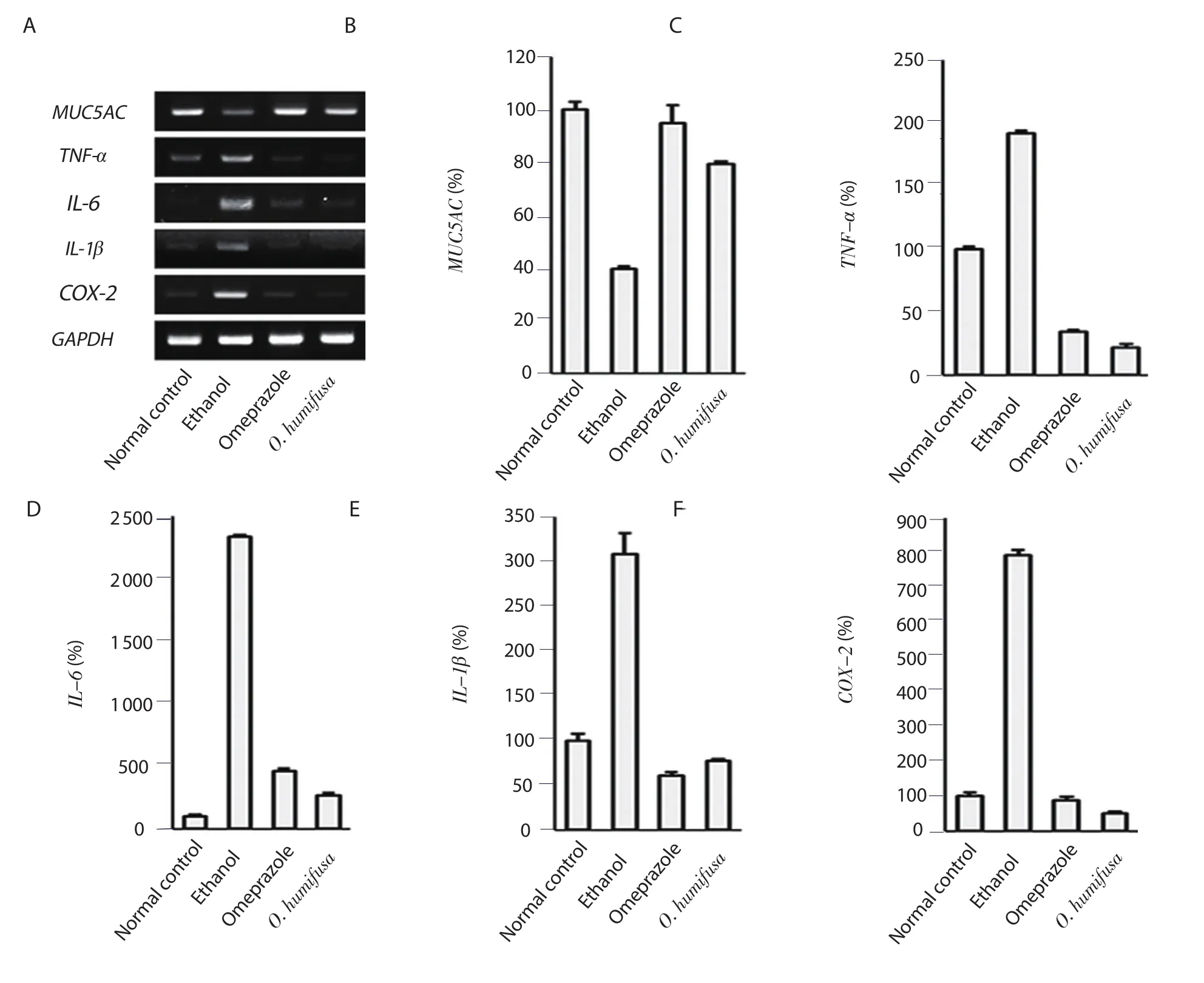
Figure 2.Analyses of RT-PCR for measuring the inhibition of mRNA expression by O.humifusa aqueous extract.Comparative analysis of mRNA expression in ethanol-induced ulcer lesions after treatment with O.humifusa aqueous extract was performed using RT-PCR.(A) the detected PCR band.(B-F) the gene expressions of MUC5AC,TNF-α,IL-6,IL-1β,and COX-2 mRNAs,respectively.
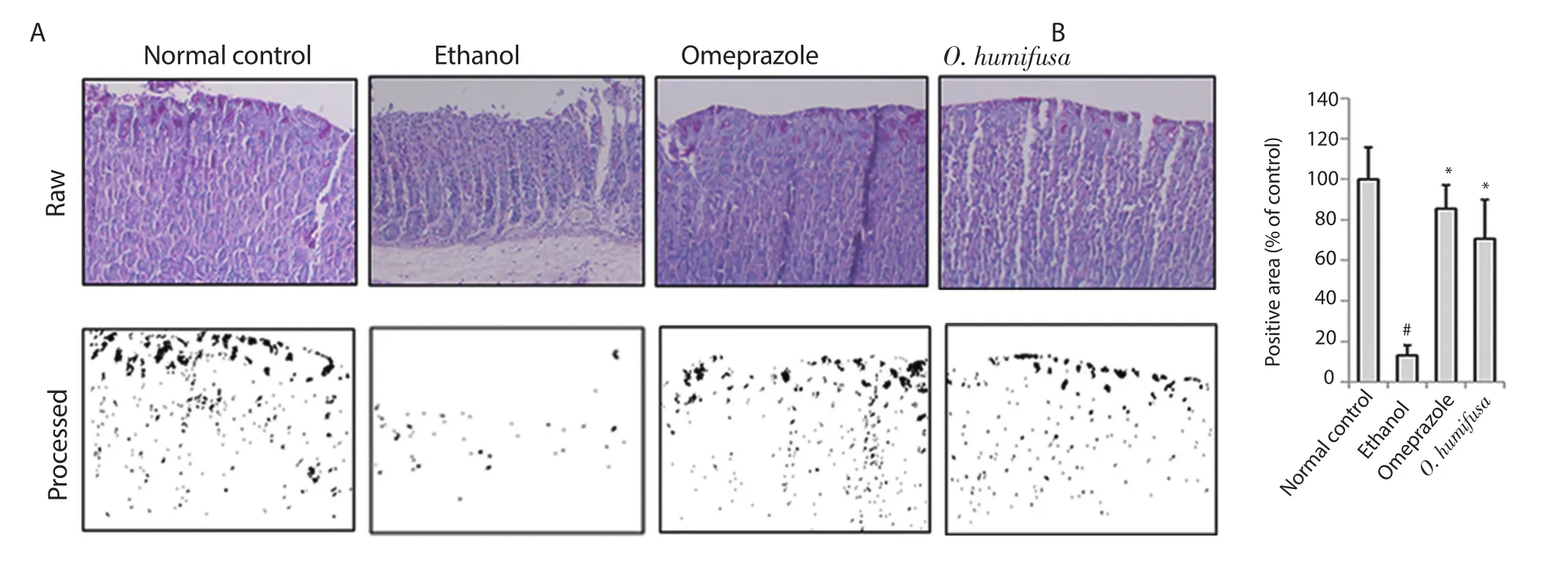
Figure 3.Immunohistochemical analyses by PAS staining.Immunohistochemistry was applied to compare the protective effects of O.humifusa aqueous extract via clinical observation or image analysis.(A) Dark violet blots indicate PAS-responsive cells.(B) Relative band intensities are shown in bar graph.Significant differences (P<0.05,ANOVA with Tukey post-hoc) when compared with the normal control (#) or the ethanol control (*) group.PAS: periodic acid Schiff.
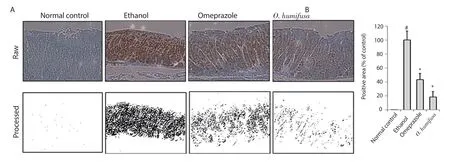
Figure 4.Expression of inducible nitric oxide synthase (iNOS) by immunohistochemical analysis.(A) Dark violet blots indicate iNOS-responsive cells.(B)Relative band intensities are shown in bar graph.Significant differences (P<0.05,ANOVA with Tukey post-hoc) when compared with the normal control (#) or the ethanol control (*) group.
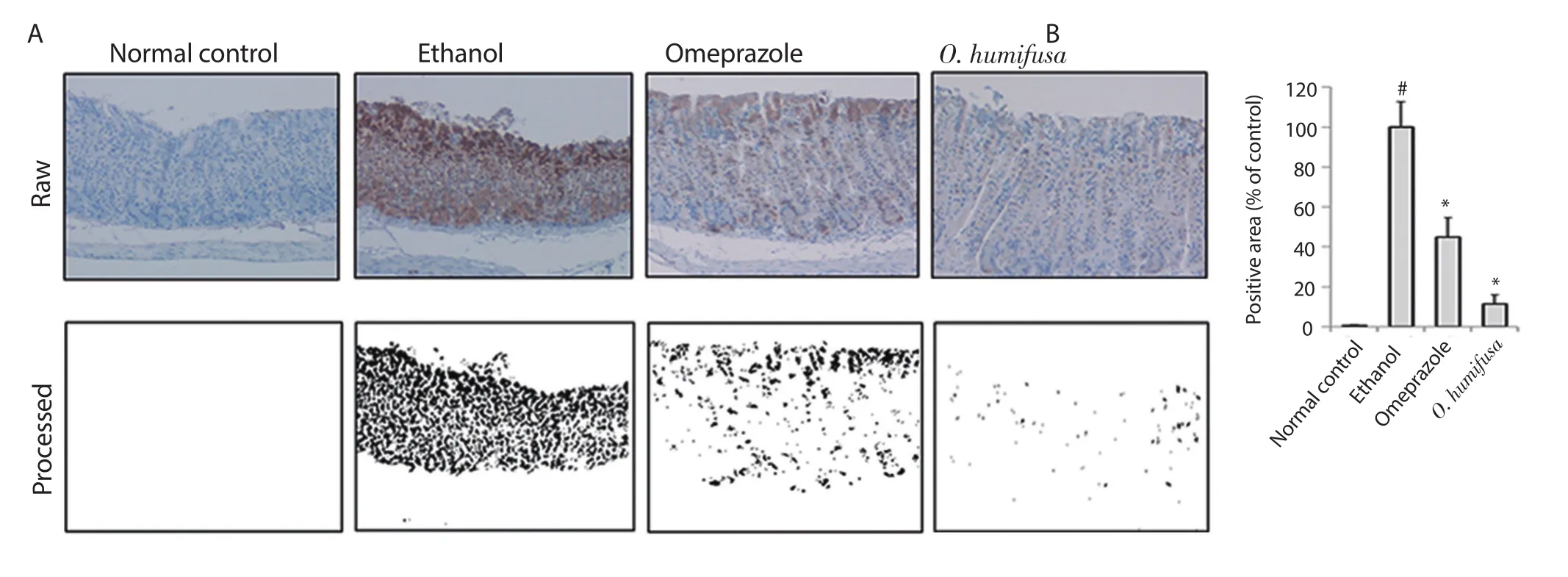
Figure 5.Expression of NF-κB by immunohistochemical analysis.(A) Dark violet blots indicate NF-κB-responsive cells.(B) Relative band intensities are shown in bar graph.Significant differences (P<0.05,ANOVA with Tukey post-hoc) when compared with the normal control (#) or the ethanol control (*)group.
3.3.Immunohistochemical results
The distribution of responsive molecules in stomach tissues was measured by periodic acid Schiff (PAS) staining.In the normal control group,dark pink coloration was observed on the surface of the stomach tissue sections owing to PAS staining.The control group showed a reduced number of lesions in the barrier spot (Figure 3).For more accurate determination of the symptoms,image analysis was performed with a similar protocol as followed for measuring gastric ulcer index.A viscous molecule,such as a polysaccharide,was depicted as a black area,and the other parts were depicted as a white area (Figure 3).Quantification of these black areas revealed a 87% reduction in the ethanol group compared with the normal control group,whereas 14% and 29% reduction were observed in the omeprazole and O.humifusa aqueous extract-treated groups,respectively,indicating a protective effect against gastric ulcer(Figure 3).Moreover,inducible nitric oxide synthase (iNOS)expression in stomach tissues was observed after ethanol induction,although it was not observed in the normal control group.The results of the antibody-bound area were shown in Figure 4.Omeprazole treatment showed a 57% reduction in antibody-bound areas compared with an assumed value of 100% for the ethanol group.In contrast,O.humifusa aqueous extract reduced iNOS expression more significantly (Figure 4).Moreover,O.humifusa aqueous extract restored NF-κB expression towards normal (Figure 5).Image analysis further confirmed the results of quantitative analysis (Figure 5).
3.4.HPLC analysis of O.humifusa aqueous extract
O.humifusa aqueous extract was analyzed for identifying the compounds by HPLC analysis (Figure 6).The active components present in the O.humifusa aqueous extract included 384.1 μg/g gallic acid,258.8 μg/g rutin,147.3 μg/g chlorogenic acid,and 503.5 μg/g ferulic acid.However,quercetin and caffeic acid were not detected.
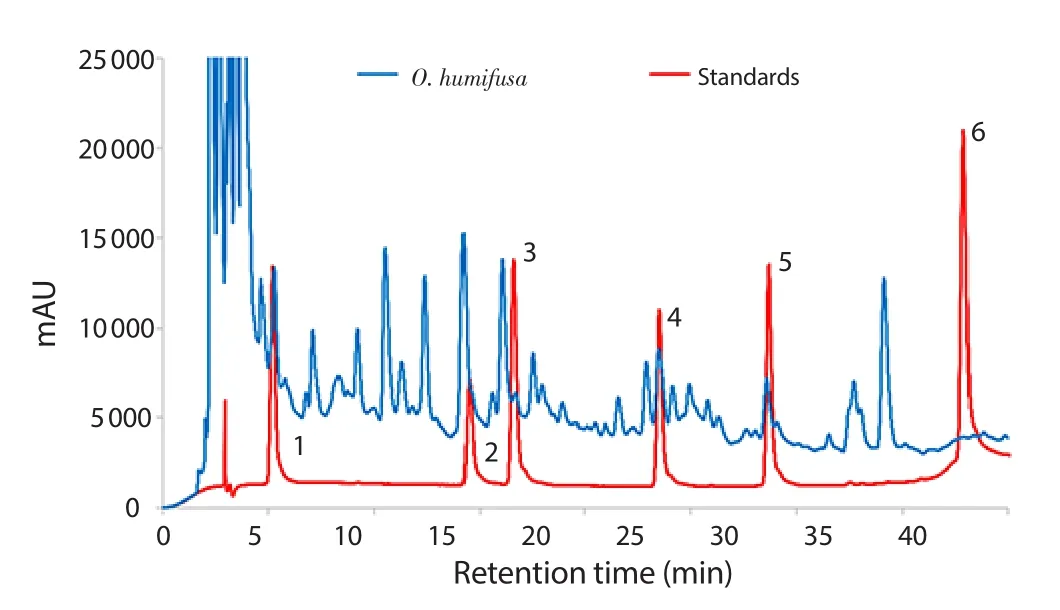
Figure 6.HPLC analysis of O.humifusa aqueous extract.Standards (red line)were clearly separated and the sample (blue line) was loaded and compared with standards.(1) gallic acid,(2) chlorogenic acid,(3) caffeic acid,(4)ferulic acid,(5) rutin,and (6) quercetin.
4.Discussion
We evaluated the gastroprotective effects of O.humifusa aqueous extract on an ethanol-induced animal model.The plant is well-known to contain higher concentrations of polyphenolics,flavonoids,and dietary fibers than any other subtropical plants.The extracts of O.humifusa exhibit anti-inflammatory[21,22],anti-diabetic,anti-aging,skin whitening,anticancer,and antioxidative effects[23].However,the anti-ulcer effects of O.humifusa remain unclear.Therefore,we investigated the gastroprotective ability of this plant extract using an ulcer model.
Contrastingly,it has been well-documented that MUC5AC encodes mucin in human beings.A part of the conformational structure in MUC1 and MUC6 consists of small recurring fragments of amino acids;consequently,the core region of mucin undergoes structural modification,which in turn substantially modifies its function[24].Out of the nine investigated genes,so far,chromosome 11 was found as the locus of MUC5AC,MUC5B,and MUC6.Moreover,TNF-α,a very well-known cytokine that causes systematic inflammation,is the functional modulator of gastric mucosal lesions.Acute inflammation reaction caused gastric ulceration,which was followed by neutrophil infiltration into the gastric mucosa.It has recently been revealed that gastric mucosal lesions can cause activation of the caspase-3-induced apoptotic pathway[25,26].Preliminary experimental observations reveal that gastroprotective effects of O.humifusa are considerably predominant with the inhibition of TNF-α,IL-1β,and IL-6,whereas these proinflammatory cytokines played a significant role in ethanol-instigated gastric ulcers[27].TNF-α expression levels were drastically decreased with O.humifusa aqueous extract treatment.In the inactivated state,NF-κB is retained in the cytoplasm IκB protein[28].When the tissues are exposed to various inflammatory stimulators,IκB kinase induces IκB protein phosphorylation and causes decomposition of IκB complexes.This event of NF-κB/IκB balance transferred to the nucleus and caused transcriptional activation of other proinflammatory cytokines including TNF-α and IL-6[29,30].The present results suggested that the downregulation of the NF-κB signaling pathway was mediated by ethanol-induced COXs,NOSs,and other inflammatory cytokines during gastric ulcer induction.Therefore,we investigated mRNA expression by examining COX-2,IL-6,IL-1βand TNF-αlevels.The data unveiled that the downregulation of the NF-κB signaling pathway was mediated by ethanol-induced gastric COX isoenzymes and prostaglandins (data not shown).Moreover,COX-2 is predominant in inflamed areas.In a parallel experiment,it was concluded that regulation of gene expression between COX-1 and COX-2 as well as cytokines is closely associated with inflammation homeostasis[31-33].Screening of several food extracts confirmed the existence and expression of ulcer inflammatory biomarkers.COX-2 is an anti-inflammatory drug target.Moreover,IL-6 is a cytokine and is known to be a major regulator of acute and chronic inflammation as well as a stimulant of T and B cells.Cytokine stimulates neutrophils,macrophages,and lymphocytes in inflammatory sites to generate toxic products,ROS,and lysosomal enzymes that cause damage of various tissues during the development of gastric ulcer[34].Previous studies have demonstrated that mRNA levels of IL-6 are significantly higher in inflamed tissues of gastric ulcers[35].The overproduction of IL-6,IL-1β,and/or TNF-α is responsible for inflammatory disease progression,and antibody-based therapy has been shown to significantly reduce inflammation by blocking inflammatory signals[36].
Image analysis program is a fascinating tool for measuring phenotypic symptoms using photographic data,including immunohistochemical images,generated from computer programs,such as ImageJ,Adobe Photoshop,and Metamorph software[37].Because the process eliminates unnecessary information,which made it easy to confirm any differences,we can measure the individual areas of damaged tissues by concurrently estimating binary viewing of lesion index and image analysis.Moreover,image analysis has been applied to various biochemical data and is one of methods for analyzing amplified PCR bands or protein spots detected by antibodies after electrophoresis and quantitatively calculating the band intensities[38];it also allows easy counting of cells (or nuclei) with the mammalian cell culture feature[39,40].Furthermore,because it is difficult to quantify immunohistochemical data by observation through naked eyes,image analysis is useful to quantitatively calculate the proportion of stained cells as well as stained areas[41].The advantages of the image analysis method were described in a previous study in which the results of image analysis were compared by employing the spectrophotometric method[42].When it was plotted as a graph based on a linear equation,the coefficient of determination (R-square) value indicated data validation.
In conclusion,the current experimental study provided evidence for the protective effects of O.humifusa aqueous extract in a mouse model with ethanol-induced gastric ulcers,which were mediated via suppression of gastric inflammation and oxidative stress.Additionally,this study also reported a high antioxidant activity of this plant extract.HPLC analysis showed a large number of peaks which validated the presence of active compounds.Although chemical drugs for gastric ulcers are commercially available at present,the use of natural materials is preferred.The abovementioned finding highlights the use of O.humifusa aqueous extract as an effective and secure strategy for regulating and managing gastric ulcers.Further information is warranted by exploring the probable potency of O.humifusa aqueous extract as an adjunct for managing gastric ulcers.Moreover,if certain obstacles pertaining to image analysis are overcome,it can be a long-lasting and useful tool for establishing phenotypic properties even for other target diseases.
Conflict of interest statement
The authors declare no conflicts of interest.
 Asian Pacific Journal of Tropical Biomedicine2020年9期
Asian Pacific Journal of Tropical Biomedicine2020年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antidiabetic effect of Chrysophyllum albidum is mediated by enzyme inhibition and enhancement of glucose uptake via 3T3-L1 adipocytes and C2C12 myotubes
- Allolobophora caliginosa coelomic fluid ameliorates gentamicin-induced hepatorenal toxicity in rats
- Immunomodulatory and anticancer activity of Bombax ceiba Linn leaf extract
- Resveratrol downregulates TGF-β1 and Smad3 expression and attenuates oxidative stress in CCl4-induced kidney damage in rats
