Antidiabetic effect of Chrysophyllum albidum is mediated by enzyme inhibition and enhancement of glucose uptake via 3T3-L1 adipocytes and C2C12 myotubes
Benjamin Kingsley Harley,Rita Akosua Dickson,Isaac Kingsley Amponsah,Robert A Ngala,Dorice Berkoh,4,Theophilus Christian Fleischer
1Department of Pharmacognosy and Herbal Medicine,School of Pharmacy,University of Health and Allied Sciences,Ho,Ghana
2Department of Pharmacognosy,Faculty of Pharmacy and Pharmaceutical Sciences,College of Health Sciences,Kwame Nkrumah University of Science and Technology,Kumasi,Ghana
3Department of Molecular Medicine,School of Medical Sciences,College of Health Sciences,Kwame Nkrumah University of Science and Technology,Kumasi,Ghana
4Department of Basic and Applied Biology,School of Sciences,University of Energy and Natural Resources,Sunyani,Ghana
ABSTRACT
Objective: To investigate the in vivo and in vitro antidiabetic potential of Chrysophyllum albidum.
Methods: The effects of oral treatment with hydro-ethanolic extract(125,250 and 500 mg/kg) of the stem bark of Chrysophyllum albidum and glibenclamide for 21 d on glucose level,serum enzyme markers for liver function,lipid profile,total protein,serum urea,serum creatinine,and body weight were evaluated in experimental diabetic rats administered with 45 mg/kg of streptozotocin.In vitro assays including glucose uptake in C2C12 cells and 3T3-L1 adipose tissues,α-glucosidase and α-amylase inhibition were employed to evaluate the possible mechanism of hypoglycemic action of the extract.DPPH and nitric oxide radical antioxidant activity of the extract was also measured.
Results: The increased levels of blood glucose,triglycerides,lowdensity lipoprotein,total cholesterol,serum aspartate,and alanine transaminases,creatinine,and urea in the diabetic animals were reduced significantly (P<0.01) after treatment with Chrysophyllum albidum extract.The decreased total protein and high-density lipoprotein concentrations were normalized after treatment.In addition,the extract significantly (P<0.01) increased the transport of glucose in 3T3-L1 cells and C2C12 myotubes and exhibited considerable potential to inhibit α-amylase and α-glucosidase.It also demonstrated potent antioxidant action by scavenging considerably DPPH and nitric oxide radicals.
Conclusions: Chrysophyllum albidum stem bark extract exhibits considerable antidiabetic effect by stimulating glucose uptake and utilization in C2C12 myotubes and 3T3-L1 adipocytes as well as inhibiting the activities of α-amylase and α-glucosidase.
KEYWORDS: Antidiabetic;C2C12 myotubes;α-glucosidase inhibition;α-amylase inhibition;Glucose uptake;Chrysophyllum albidum
1.Introduction
Diabetes mellitus describes a cluster of metabolic disorders defined by elevated glucose levels.The diabetic population worldwide is predicted to rise to 693 million worldwide from its current figure of 451 million in the next 25 years with the majority of this increase coming from Sub-Saharan Africa[1].Type 2 diabetes dominates the disease burden in Africa,with less than 10% of diabetic cases presenting as type 1.This has been attributed to rapid urbanization and economic development with its attendant behavioural changes such as sedentary lifestyles and unhealthy eating habits[2].The costly nature of available synthetic hypoglycemic drugs in addition to their adverse effects limits their use in low- and middle-income countries like Ghana.
Ghana has a pluralistic healthcare system that combines both traditional and biomedical systems.This is an attempt by the government in ensuring quality and equitable health care,especially for the rural poor and marginalized in society who are not able to afford conventional healthcare[3].In some rural communities,herbal medicine is the only form of health care that is available,affordable and accessible[4].For chronic diseases such as diabetes,the majority of the populace resort to the use of medicinal plant products as they believe these have demonstrated efficacy for such conditions.There is,therefore,the need to identify affordable anti-diabetic agents that can improve blood glucose levels without the side effects of current therapy.This has brought a renewed interest in natural products as they offer new approaches for managing chronic diseases,including diabetes[5].
In most developing countries,with a high poverty line,the majority of the populace go through stressful conditions to feed their families.Work-related stress leads to oxidative stress which has been implicated in diabetic micro- and macro-vascular complications[6].Decreasing oxidative stress with antioxidant compounds has been shown to prevent the development of diabetes and its complications[7].Thus,medicinal plants with hypoglycemic actions,particularly those with high levels of strong antioxidant compounds,can be useful adjuncts in the treatment of the disease[8].
Chrysophyllum albidum (C.albidum) G.Don (Sapotaceae)called “Adasema” by the Akan communities in Ghana is a tree well distributed in the coastal regions of West Africa.The antiplasmodial,antihistaminic,anti-inflammatory and antimicrobial properties of the plant have been reported[9].The ethanol extract of the seed cotyledons and root bark demonstrated moderate antihyperglycemic and hypolipidemic effects in alloxan-induced diabetic rats[10,11].Phenolic compounds namely epicatechin,epigallocatechin and procyanidin B5 were isolated from the stem bark whereas the alkaloids skatole,eleagnine and tetrahydro-2-methylharman have been reported from the seed testa[12,13].Traditionally,the stem bark is used in treating sleeping sickness,yellow fever,diabetes and malaria[14].However,its antidiabetic properties have not been fully explored.
The present study aimed to investigate the hypoglycemic and antihyperlipidemic effects of the hydro-ethanolic extract from the stem bark of C.albidum on streptozotocin (STZ)-induced diabetic rats and elucidate its mechanism of action.
2.Materials and methods
2.1.Reagents and chemicals
The 3T3-L1 adipocytes and C2C12 myoblasts were bought from American Type Culture Collection whiles newborn calf serum,penicillin/streptomycin,L-glutamine,Dulbecco's modified Eagle's Medium (DMEM),horse serum and fetal calf serum (FCS) for their growth and differentiation media were sourced from Gibco (Berlin,Germany).Griess reaction reagent,acarbose,2-deoxy-D-glucose,STZ,pancreatic α-amylase,glibenclamide,dinitrosalicylic acid,rat intestinal acetone powder,2,2-diphenyl-1-picrylhydrazyl (DPPH),3-isobutyl-1-methylxanthine,p-nitrophenyl-α-D-glucopyranoside and accutase were purchased from Sigma (St.Louis,MO,USA).Solvents and chemicals used were of analytical grade.
2.2.Plant sample collection,extraction,and fractionation
The stem bark of C.albidum was harvested from the medicinal plant garden of Kwame Nkrumah University of Science and Technology (KNUST) and authenticated at the Herbarium of the Botany Department of the University of Ghana (UG),Legon.Herbarium specimen (KNUST/HM1/2013/S004) was deposited at the Department of Herbal Medicine,KNUST,Kumasi.The stem barks were dried at room temperature and coarsely powdered.The powder (1.2 kg) was extracted for 48 h with 70% ethanol using a 2 L Soxhlet apparatus.The liquid extract obtained was concentrated under reduced pressure at 40 ℃ with a Buchi R-100 rotavap to obtain a brown residue (CAB) (Yield: 9.7% w/w).About 80 g of the extract was successively solvent fractionated by partitioning the recosntituted extract in distilled water with chloroform (CHCl3),ethyl acetate (EtOAc) and n-butanol (ButOH) to obtain four fractions:CHCl3,EtOAc,ButOH and aqueous (AQ) parts,respectively.
2.3.Experimental animals
Male Sprague Dawley rats that weighed between 230-250 g were bought from Noguchi Memorial Institute for Medical Research(NMIMR),UG,Legon and housed in stainless steel cages lined with saw dust at the animal house of the Department of Pharmacology,KNUST,fed on a standard pellet diet and allowed to drink water at will.The rats were kept at a temperature of 25 ℃ with overhead incandescent illumination on a 12 h light-dark cycle and allowed to acclimate to the laboratory's conditions and circumstances for 7 d.
2.4.Acute toxicity studies
Acute oral toxicity of CAB was performed on male Sprague Dawley rats according to the Organization of Economic Cooperation and Development (OECD) Guidelines number 425.Brie fly,Sprague Dawley rats placed in groups of five,fasted overnight and allowed to drink water ad libitum were treated orally with a single dose of 50,500,2 000 mg/kg of CAB or normal saline (0.9% 10 mL/kg).The animals were observed closely for signs of toxicity,behavioural changes or death at 0,15,30,60,120 and 180 min,24 h and 14 days after extract administration.
2.5.Oral glucose tolerance test (OGTT)
Sprague Dawley rats were fasted (18 h) overnight and used in OGTT to first evaluate the hypoglycemic properties of the crude extracts[15].Rats were randomly chosen and assigned into groups of six animals;negative control group (vehicle: water),treatment group (crude extract: 125,250 and 500 mg/kg,p.o.) and positive control group (glibenclamide: 5 mg/kg,p.o.).Thirty minutes after administration of the test extracts and standard drug,the animals were given 2 g/kg glucose solution p.o.The plasma glucose levels of the animals were measured before glucose administration and at 30,60,90 and 120 minutes afterwards from blood collected from their tail veins after the application of lignocaine using OneTouch Select glucometer (LifeScan,Inc.Milpitas,CA 95035 USA).
2.6.STZ-induced diabetes in rats
Sprague Dawley rats that were fasted overnight were made diabetic by a single intraperitoneal (i.p.) injection of STZ (45 mg/kg) which was freshly prepared in 0.1 mol/L cold sodium citrate buffer (pH 4.5).The injected animals could drink a 5% glucose solution overnight.The diabetic state was confirmed in the rats by their elevated blood glucose levels which were measured 2 days after STZ administration.Rats with fasting blood glucose above 10 mmol/L were considered diabetic and used in the study[16].
Diabetic rats were randomly selected and divided into groups of six animals;negative control group (vehicle: water),treatment group(crude extract: 125,250 and 500 mg/kg) and positive control group(glibenclamide: 5 mg/kg p.o.).Another group that was made of normoglycemic rats and received distilled water served as normal control.The treatment started 3 days after diabetes induction and served as the first day of the study which continued until day 22.
2.7.Biochemical analysis
The plasma glucose levels of the experimental animals were measured every hour for the first 6 hours after the first treatment with the extract and glibenclamide.Fasting blood glucose level and body weight were measured every week for 21 d.On the last day of the study,the animals were sacrificed in the morning by decapitation under anaesthesia and blood was collected for determination of their biochemical parameters.Markers of hepatic and renal functions including aspartate aminotransferase (AST),alanine aminotransferase (ALT) and total protein (TP);and serum urea and serum creatinine levels were also determined using the Mindray BS-200 chemistry analyser (www.mindray.com).The lipid parameters of the animals which include the triglyceride (TG),total cholesterol(TC),low-density lipoprotein (LDL) and high-density lipoprotein(HDL) were also determined with the biochemistry analyser.
2.8.Inhibition of intestinal α-glucosidase
The inhibition of the α-glucosidase activity of the plant extract and fractions was evaluated using a previously described procedure with slight modifications[17].Homogenized rat intestinal acetone powder in 0.1 mol/L phosphate buffer (6.9) was centrifuged at 12 000 g for 30 min.Then,100 μL of the enzyme solution obtained was incubated with 50 μL of the test samples (extract,fractions,and acarbose) at various concentrations (from 3 μg/mL to 1 000 μg/mL) at 25 ℃ for 10 min in 96-well plates.Fifty μL of the 5 mmol/L p-nitrophenylα-D-glucopyranoside solution was then added to the mixtures and incubated for 30 min at 37 ℃.The absorbances of the mixtures were recorded at 405 nm on a microplate reader (Thermomax,Molecular Device Corp.,Sunnyvale,CA).Fifty μL of buffer solution was processed in a manner similar to the test samples and was used as the control.Inhibitory action was calculated according to the equation below:
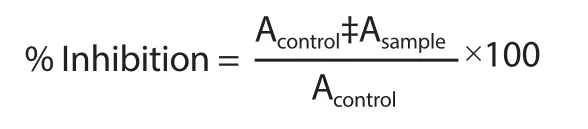
where Acontrol= the absorbance of buffer control;Asample= the absorbance of the test sample.IC50was determined through linear regression of plots,where the x-axis is the concentration of test sample and y-axis average percent of inhibition.
2.9.Porcine pancreatic α-amylase inhibition assay
The α-amylase inhibitory activity was performed according to methods previously described with slight modifications[18].Pancreatic α-amylase was dissolved in 0.02 mol/L phosphate buffer,pH 6.9 to obtain the enzyme solution (0.5 mg/mL) which was used for the assay.Five hundred μL of test samples [extract,fractions,and acarbose (1 μg/mL-1 000 μg/mL)]were added to 500 μL solutions containing 1% starch solution and incubated at 25 ℃ for 10 min.The reaction was initiated by adding 500 μL enzyme solution to the mixture.After 10 min of incubation at 25 ℃,the reaction was stopped with 1.0 mL dinitrosalicylic acid colour reagent to the mixture.The mixtures were heated at 100 ℃ for 10 min,cooled and their absorbances were recorded at 540 nm after dilution with 1.0 mL distilled de-ionised water.
The α-amylase inhibition activity was calculated using the equation below:
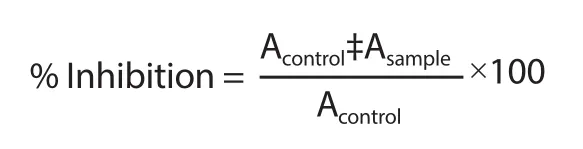
where Acontrol= the absorbance of negative control;Asample= the absorbance of the test sample.IC50was determined through linear regression of plots,where the x-axis is the concentration of the test sample and the y-axis is the average percent of inhibition.
2.10.Cell culture
C2C12 cells were cultured in DMEM supplemented with 10% FCS,2 mmol/L glutamine,2% horse serum and 1% antibiotics (penicillin 100 U/mL,streptomycin 100 μg/mL) in 5% CO2at 37 ℃.3T3-L1 pre-adipocytes were grown in DMEM augmented with 1% penicillin/streptomycin and 10% newborn calf serum in 5% CO2at 37 ℃ and were differentiated in DMEM containing 1 μmol/L dexamethasone,3-isobutyl-1-methylxanthine (0.5 mmol/L),1% penicillin/streptomycin,10 μmol/L human insulin and 10% FCS.After differentiation,they were kept in DMEM augmented with 1%penicillin/streptomycin and 10% FCS.
2.11.Glucose uptake bioassays
2.11.1.C2C12 myotubes
The effect of C.albidum extracts on the uptake of glucose in differentiated C2C12 myotubes was assessed using the3H 2-deoxyglucose uptake assay described previously with slight modifications[19].Briefly,C2C12 cells were starved for 4 h and treated with the test samples [extract and fractions (10 and 30 μg/mL);insulin-like growth factor (IGF-1,10 nmol/L)]for 60 min.The cells were rinsed twice with HBS-buffer and incubated with radioactive 2-deoxyglucose (2 μCi/mL) for 5 min.The reaction was halted with a CB-stop solution (HBS-buffer supplemented with 10 μmol/L cytochalasin B) and myotubes were iced for 15 min.Afterwards,the cells were washed 3× with cold PBS solution and lysed with 0.2 mol/L NaOH.Four mL of liquid scintillation cocktail was added to the lysate and the radioactive [3H]2-deoxyglucose associated with the samples measured with Packard TRI-CARB®2200CA scintillation counter in triplicate.The protein content of the cells was measured using the Pierce BCA-Protein Assay Kit.The uptake of glucose uptake was calculated as cpm/mg of protein and expressed as the percentage of control.Non-specific glucose uptake was determined using 10 μmol/L cytochalasin B.
2.11.2.Glucose uptake in 3T3-L1 adipocytes
Fully differentiated 3T3-L1 adipocytes were washed (3×),serum starved and incubated for 4 h.After treatment with test samples[extract and fractions (10 and 30 μg/mL);insulin (0.1 nmol/L)]for 60 min,the cells were washed twice with KRP-buffer and incubated with 1 μCi/mL radioactive 2-deoxyglucose for 10 min.The reaction was halted with a CB-stop solution and cells were iced for 10 min.Thereafter,they were rinsed (3×) with cold PBS and lysed in 0.2 mol/L NaOH.Lysed cells of each well were mixed with liquid scintillation cocktail (4 mL) and their radioactivity was measured with a scintillation counter.The protein contents of the cells were measured using the Pierce BCA-Protein Assay Kit.The uptake of glucose uptake was calculated as cpm/mg of protein and expressed as the percentage of control.Non-specific glucose uptake was determined using 10 μmol/L cytochalasin B[20].
2.12.In vitro antioxidant activity
2.12.1.DPPH radical scavenging assay
The DPPH anti-radical method previously described by Harley and co-workers was used[21].The reaction mixture consisted of a solution of DPPH in methanol (20 mg/L) and test extract.The mixture was incubated for 30 min in dark,after which the absorbance for each concentration was measured at 517 nm.Ascorbic acid was used as positive control and methanol was processed as the blank determinant.Each test was carried out in triplicate.
2.12.2.Nitric oxide (NO) radical scavenging assay
The NO scavenging activity of the test samples [crude extracts,fractions and α-tocopherol (reference drug)]was assessed using the Griess reagent[22].Two mL of sodium nitroprusside (10 mmol/L) in phosphate buffer was incubated with 150 μL of a methanol solution containing 50 μL of test samples at room temperature for 2 h.Five mL of Griess reagent was added afterwards and absorbance was measured at 540 nm using a spectrophotometer (Model Cecil CE 7200,Cecil instrument limited,Milton Technical Centre,England).The procedure was repeated with methanol as blank which served as control.All experiments were carried out in triplicate.
2.13.Phytochemical screening
The stem bark extract and fractions of C.albidum were subjected to screening for secondary metabolites present according to methods described[23].
2.14.Statistical analysis
Data for the in vivo experiments are presented as mean ± SEM whereas results for the in vitro assays are expressed as mean ± SD.Data were statistically analysed using one-way analysis of variance(ANOVA) and multiple comparisons between treatment groups were carried out using Dunnet's post hoc test with GraphPad for Windows version 6 (GraphPad Prism Software,San Diego,USA).
2.15.Ethical statement
All procedures and techniques employed in the study were done according to the Guidelines for the care and use of laboratory animals (Institute of laboratory animal resources) and approved by the College of Health Sciences Ethics Committee,KNUST,Kumasi,Ghana (REF: CHRPE/AP/204/14).
3.Results
3.1.Acute toxicity study
There were no changes in the behavioural pattern in the treated animals up to 2 000 mg/kg of the extract.Neither was there any observable difference in body weight and food consumption when compared to the animals that were given distilled water.No gross pathological differences were also observed between the extracttreated animals and the control.Treated animals at 2 000 mg/kg did not show any sign of toxicity 2 weeks after receiving the extract.
3.2.Effect of C.albidum extract on OGTT in normal rats
The ethanol stem bark extract and glibenclamide significantly(P<0.01) prevented a sudden elevation in blood glucose levels after the administration of glucose solution when compared to the control group (Figure 1).The activity of the extract was comparable to that of glibenclamide (Figure 1).
3.3.Effect of C.albidum extract on plasma glucose of diabetic rats
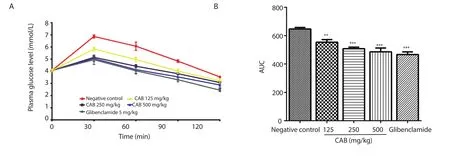
Figure 1.(A) Changes of glucose level in the presence of Chrysophyllum albidum stem bark extract and glibenclamide.(B) Total plasma glucose levels,calculated as AUCs;values are recorded as mean ± SEM (n=6);***P<0.001,**P<0.01 compared to the negative control group.CAB: Chrysophyllum albidum stem bark extract.AUC: Area under curve.
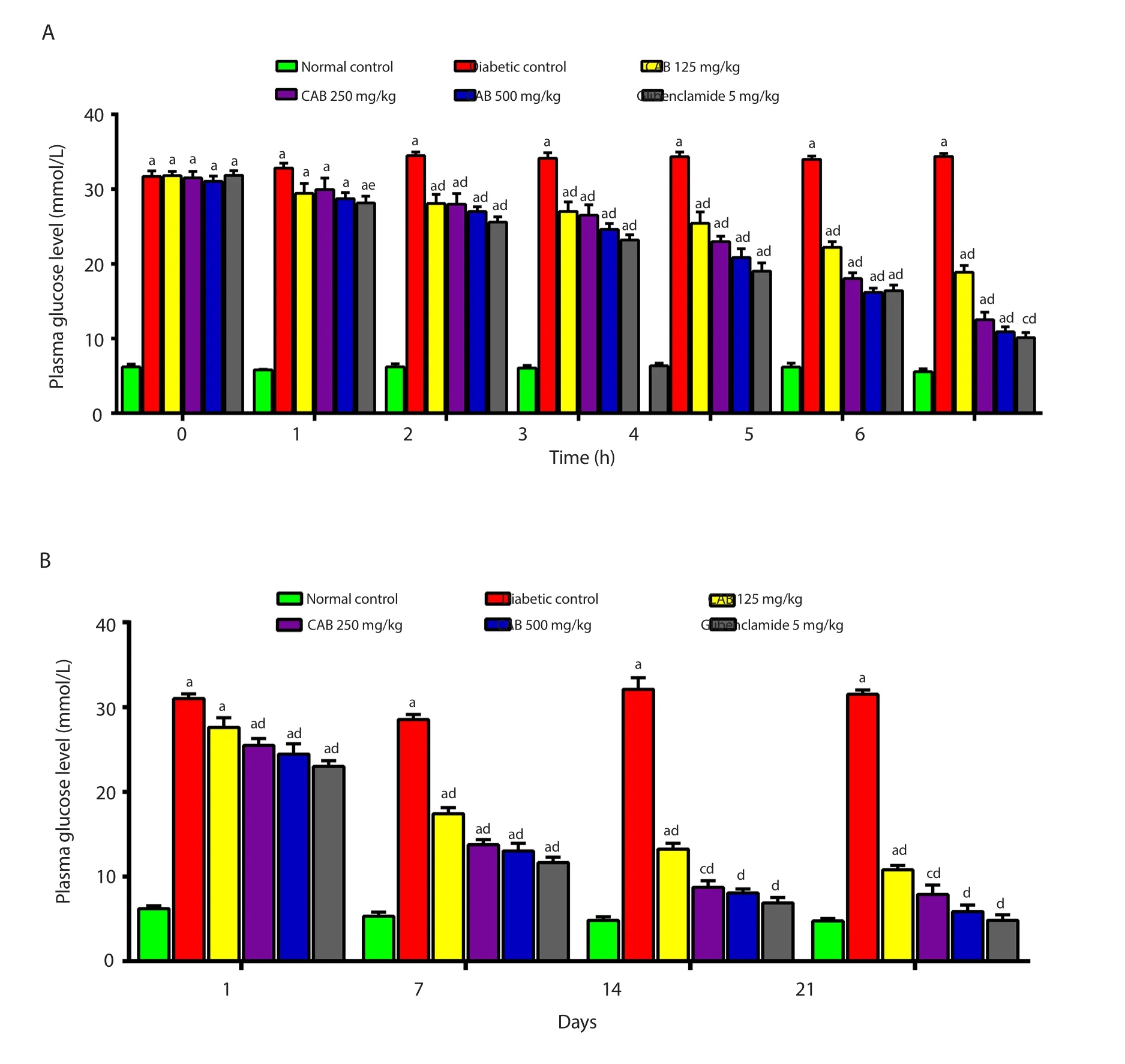
Figure 2.Effect of Chrysophyllum albidum stem bark extract and glibenclamide on plasma glucose of streptozotocin-induced diabetic rats after 6 h (A) and 21 days (B) of treatment.Values indicate mean ± SEM (n=6).aP<0.001,bP<0.01,cP<0.05 in comparison to the normal control.dP<0.001,eP<0.05 when compared to the diabetic control.
The blood glucose level of the untreated diabetic rats was increased by (8.77 ± 2.77)% 6 h after treatment and -(0.52 ± 2.62)% after 21 days,respectively.In contrast,the C.albidum stem bark extract dose-dependently reduced the plasma glucose levels of the diabetic animals by (64.73 ± 2.66)% and (81.10 ± 2.43)% (at 500 mg/kg body weight) after 6 h and 21 days of treatment,respectively (Figure 2).Glibenclamide group (5 mg/kg) also showed a decrease of (68.10± 2.56)% and (84.83 ± 1.88)% in the plasma glucose levels of the diabetic animals 6 h and 21 days after treatment,respectively.Thus,the fasting blood glucose levels of diabetic rats were significantly(P<0.001) lowered by the oral administration of the extract and glibenclamide to a very comparable extent (Figure 2).
3.4.Effect of C.albidum extract on the body weight of diabetic rats
The body weight of the experimental animals was decreased after STZ administration.Diabetic rats continued to lose weight till the end of the study.C.albidum extract and glibenclamide treated animals showed marked improvement in their body weight from day 7 till the end of the study (Figure 3).
3.5.Effect of C.albidum extract on lipid profile of diabetic rats
The levels of TC,TG,and LDL were significantly increased in the STZ-treated diabetic rats with a decrease in HDL (Table 1).In C.albidum stem bark extract and glibenclamide treated groups,the TG,LDL,and TC were significantly (P<0.01) reduced while HDL was significantly increased (P<0.01) (Table 1).
3.6.Effect of C.albidum extract on TP,AST,ALT,serum urea and creatinine in diabetic rats
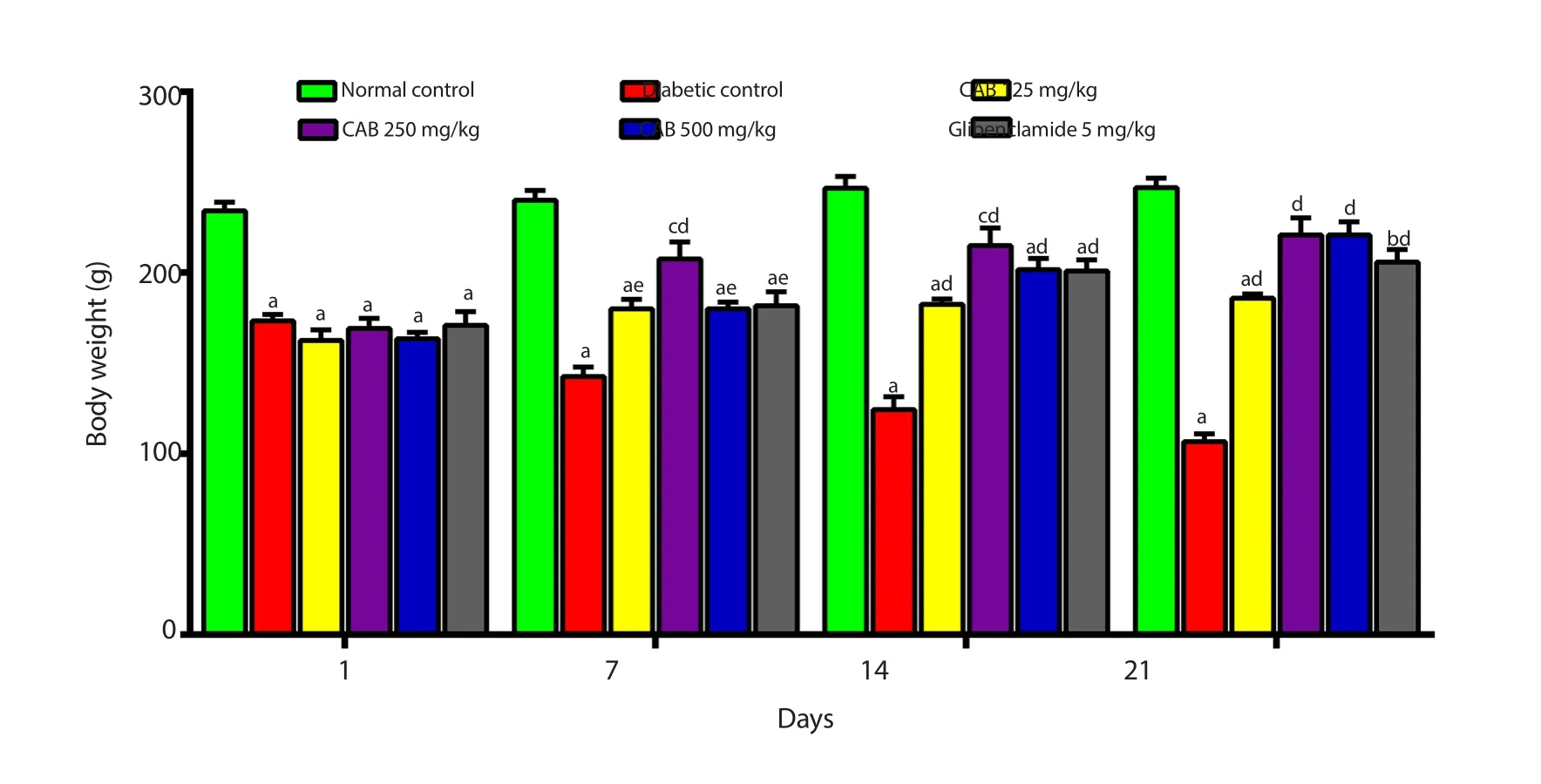
Figure 3.Effect of Chrysophyllum albidum stem bark extract and glibenclamide on body weight of streptozotocin-induced diabetic rats after 21 days of treatment.Values are presented as mean ± SEM (n=6).aP<0.001,bP<0.01,cP<0.05 when compared to the normal control.dP<0.001,eP<0.01 in comparison to the diabetic control.
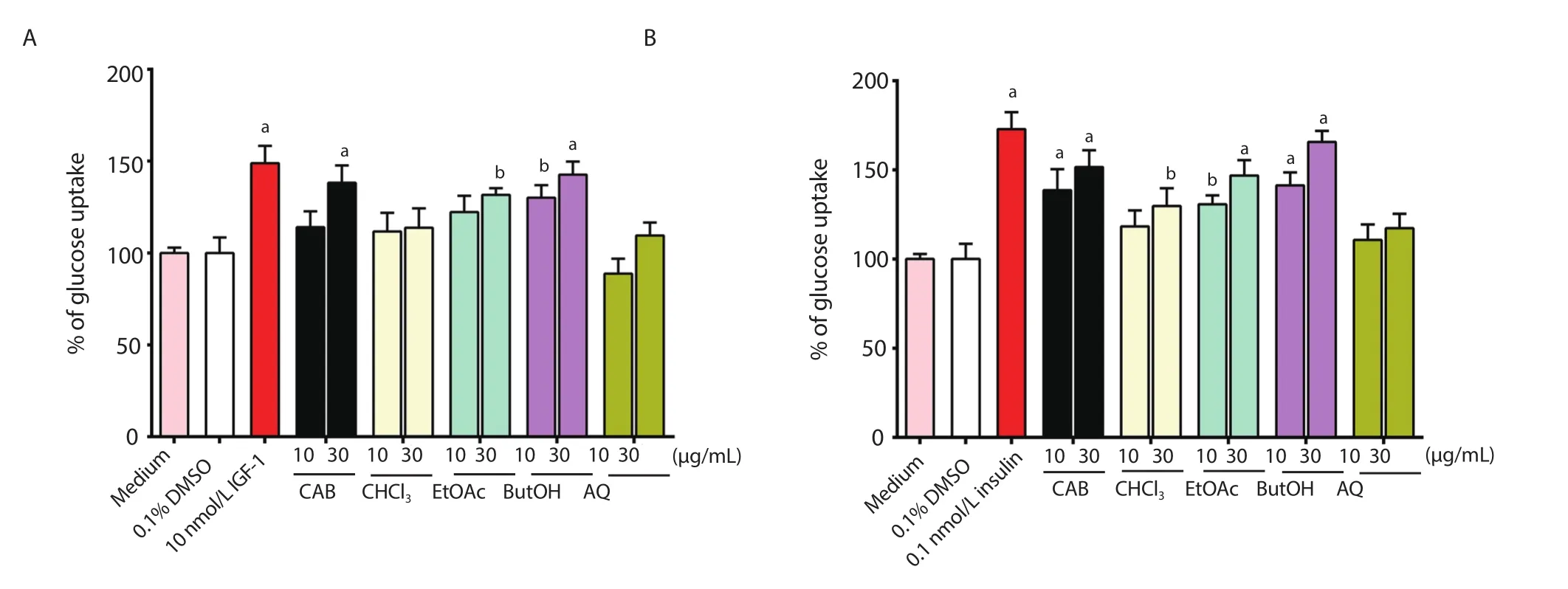
Figure 4.(A) Glucose uptake activity of Chrysophyllum albidum extract and fractions in C2C12 myotubes.and (B) 3T3-L1 adipocytes.Values are presented as mean ± SD (n=3).aP<0.001,bP<0.01 compared to the negative control.CAB: Extract of Chrysophyllum albidum stem bark,CHCl3,EtOAc,ButOH and AQ:chloroform,ethyl acetate,n-butanol,and aqueous fractions of CAB,respectively.

Table 1.Effect of Chrysophyllum albidum stem bark extract on the lipid profile of experimental rats after 21 days of treatment.
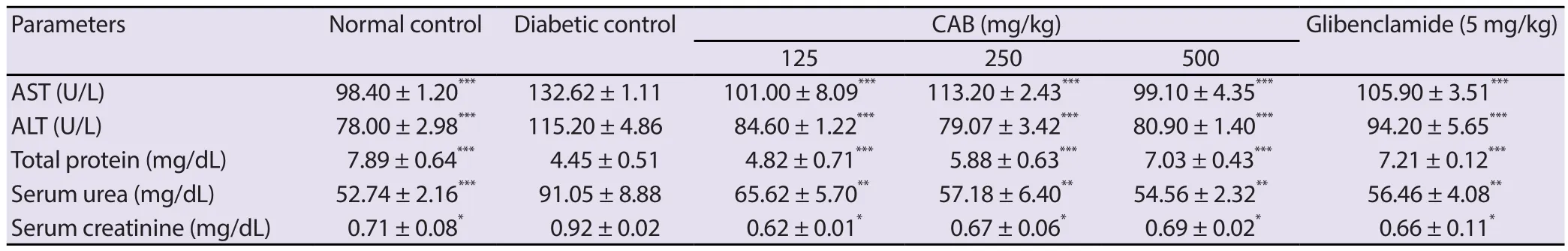
Table 2.Effect of Chrysophyllum albidum stem bark extract on levels of AST,ALT,plasma protein,serum urea,and serum creatinine in experimental rats after 21 days of treatment.
There were significant increases in the serum levels of AST and ALT in diabetic rats (Table 2).After treatment with C.albidum stem bark extract,the activities of ALT and AST were significantly(P<0.001) lowered.Similarly,C.albidum stem bark extract-treated animals showed considerable (P<0.001) increase in the plasma protein level with serum urea and creatinine levels significantly(P<0.05) reduced when compared to the diabetic control rats.Glibenclamide (5 mg/kg) treated rats also showed similar significant effects on blood levels of AST,ALT,TP,serum urea and serum creatinine in diabetic rats (Table 2).
3.7.Effect of C.albidum extract on α-glucosidase and α-amylase activities
The test extract and fractions exhibited inhibitory effects on α-glucosidase and α-amylase in a dose-dependent manner.The crude extract showed considerable enzyme inhibition activities by exhibiting 2-fold and 3.7-fold better inhibitory activity than acarbose on the α-glucosidase and α-amylase enzymes,respectively.The n-butanol fraction of C.albidum extract showed higher activity than the crude extract (Table 3).
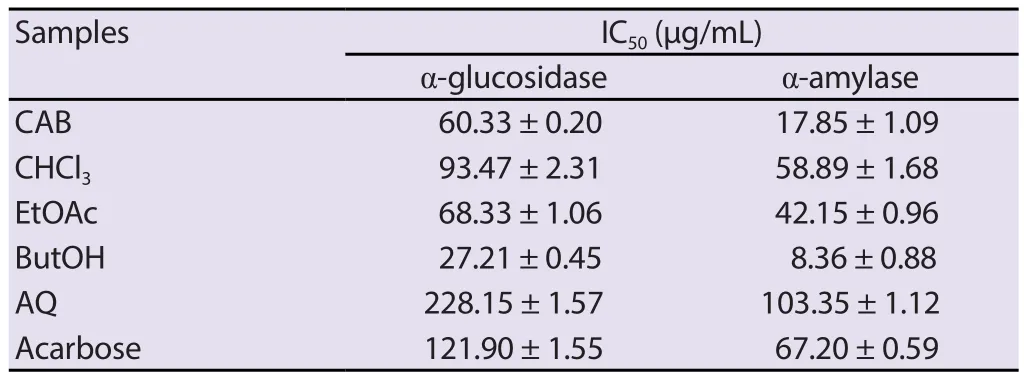
Table 3.α-Glucosidase and α-amylase inhibitory effects of crude fractions obtained from the ethanol extract of Chrysophyllum albidum.
3.8.Effect of C.albidum extract on glucose uptake in C2C12 myotubes and 3T3-L1 adipocytes
The effects of C.albidum stem bark extracts on glucose uptake in C2C12 myotubes and 3T3-L1 adipocytes are depicted in Figure 4.The cells were incubated with the extracts in the presence of DMSO(0.1%) as the negative control.IGF-1 (10 nmol/L) and insulin (0.1 nmol/L),used as positive controls,significantly enhanced glucose uptake to 148.86% and 172.35% of control values in C2C12 myotubes and 3T3-L1 adipocytes,respectively.The crude extract at 30 μg/mL increased glucose uptake in C2C12 cells by 138.24%.Its n-butanol fraction showed the highest increase in glucose uptake in the skeletal muscle,up to 142.66% at 30 μg/mL,which was followed by the ethyl acetate,chloroform and aqueous fractions (Figure 4).Moreover,the crude extract at 10 and 30 μg/mL enhanced the uptake of glucose in the adipocytes by 138.62% and 151.58% of control,respectively.The highest uptake was observed with the n-butanol fraction which promoted glucose transport to 165.72% of control (P<0.001),whereas the aqueous fraction did not exhibit any significant glucose uptake activity in either C2C12 myotubes or 3T3-L1 adipocytes (Figure 4).The extract and fractions demonstrated higher glucose uptake in 3T3-L1 adipocytes than in the C2C12 myotubes.
3.9.In vitro antioxidant activity
3.9.1.DPPH radical scavenging activity
The crude ethanol extract of C.albidum and the standard drug,ascorbic acid demonstrated a concentration-dependent scavenging effect on the DPPH radical with IC50values of (2.52 ± 0.01) μg/mL and(2.29 ± 0.01) μg/mL,respectively.Similarly,the fractions demonstrated good radical scavenging activity with the n-butanol fraction being the most potent with an IC50of (2.65 ± 0.01) μg/mL (Table 4).
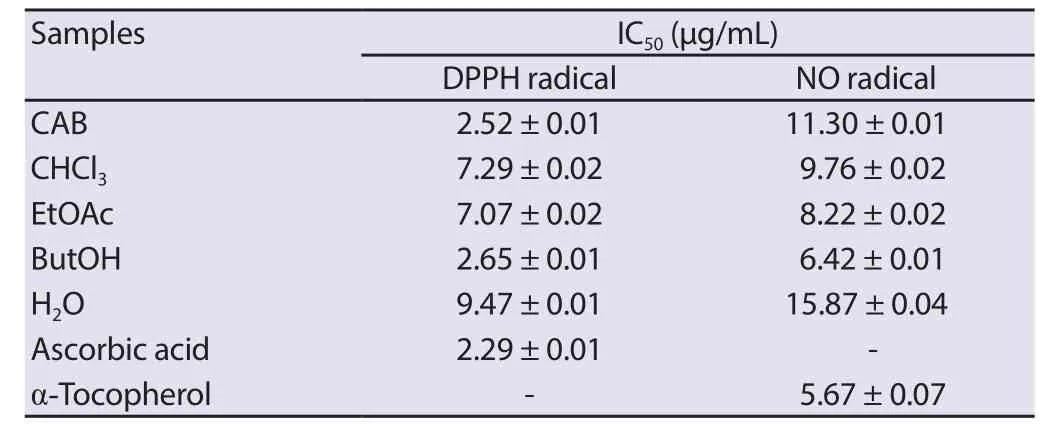
Table 4.DPPH and NO radical scavenging effects of crude extract and fractions of Chrysophyllum albidum stem bark.
3.9.2.NO radical scavenging activity
The IC50values of C.albidum crude extract and α-tocopherol were found to be (11.30 ± 0.01) μg/mL and (5.67 ± 0.07) μg/mL,respectively in the NO scavenging assay.The n-butanol fraction demonstrated a higher anti-radical scavenging effect on the NO than the other fractions (Table 4).
3.10.Phytochemical screening
Phytochemical investigation of the extract and different fractions of C.albidum revealed the presence of alkaloids,phenolics,terpenoids,sterols,glycosides,and flavonoids.The percentage yields of the chloroform,ethyl acetate,n-butanol,and aqueous fractions were found to be 11.32%,17.10%,24.60% and 27.45% by weight of the hydro-alcoholic crude extract,respectively.The chloroform and ethyl acetate fractions predominantly contained phytosterols,triterpenoids and alkaloids.Phenolics including flavonoids and saponins were detected in the n-butanol and aqueous fractions.
4.Discussion
The present study investigated the antidiabetic potential of the hydro-ethanolic extract of the stem bark of C.albidum.The intraperitoneal administration of STZ (45 mg/kg) selectively targeted and destroyed insulin-producing pancreatic islet β-cells by damaging their DNA strands,which reduced insulin secretion and eventually resulted in diabetes mellitus in the rats.
Elevated levels of glucose,observed in the STZ-induced diabetic rats,were lowered by treating with the extract.Within 6 h of the first dose,C.albidum extract-treated rats demonstrated a significant reduction in their plasma glucose levels compared to untreated diabetic group,highlighting the rapid onset of action of the extract.Daily supplementation of the extract reduced considerably the fasting plasma glucose levels of the diabetic animals comparable to glibenclamide.This could have occurred through stimulation of insulin from surviving and regenerated β-cells of the islets of Langerhans by the extract and/or promotion of the uptake of glucose into the peripheral tissues[24,25].The transport of glucose into the periphery as mechanism by which the extract exerted its glucoselowering activity was established using in vitro assays.The extract demonstrated the ability to promote glucose uptake in C2C12 myotubes and 3T3-L1 adipocytes.The capacity of the various solvent-fractions of C.albidum extract to enhance glucose transport in skeletal muscles and adipose tissues was also investigated.The n-butanol (10 and 30 μg/mL) and ethyl acetate (30 μg/mL)fractions stimulated considerably glucose transport into the skeletal muscles.In the 3T3-L1 adipocytes,glucose transport occurred with all the fractions except the aqueous fraction.The tendency of the ethyl acetate and n-butanol fractions to stimulate glucose uptake in both C2C12 myotubes and 3T3-L1 adipocytes is indicative of the presence of insulin-like phytoconstituents contributing to the observed in vivo hypoglycemic action of the plant.The capacity of C.albidum extract to promote glucose utilization was evident in OGTT.Extract-treated animals showed better glucose utilization capacity compared to the untreated animals,which undoubtedly affirmed the potential of the extract to increase glucose tolerance by stimulating glucose homeostasis mechanisms like those mediated by insulin secretion.
C.albidum extract also inhibited considerably α-glucosidase and α-amylase in vitro,suggesting that its hypoglycemic effect could also be mediated by its interference with the metabolism of dietary carbohydrates through the inhibition of pancreatic α-amylase and intestinal α-glucosidase.The inhibition of these enzymes hinders the digestion of carbohydrates to glucose,leading to reduced postprandial blood glucose levels[26].This has been linked to a reduction in glycated haemoglobin (HbA1c) and the prevention of diabetic cardiovascular complications especially in type 2 diabetes mellitus[27].The n-butanol fraction exhibited the highest inhibitory effects with IC50of 27.21 μg/mL and 8.36 μg/mL for α-glucosidase and α-amylase,respectively.
Diabetic cardiovascular complications such as hypertension,ischaemic heart disease,and atherosclerosis occur as a result of the alterations in the lipid profile of diabetic patients[28].Dyslipidaemia occurs as a result of increased lipolysis on fat depots due to defects in insulin action[29].Treatment of the diabetic rats with the extract significantly improved alterations in the lipid profile of the animals by decreasing the elevated serum levels of TC,TG,and LDL and at the same time increasing the serum HDL levels.Therefore,the improved lipid parameters of the diabetic animals could be ascribed to the improved insulin secretion and action due to the administration of the C.albidum extract.
The diminished body weight observed in diabetic patients is due to increased muscle wasting by excessive catabolism of proteins to provide amino acids for gluconeogenesis during insulin deficiency[30].Treated animals showed improvements in their body weight in the course of the study suggesting the ability of the C.albidum extract to restore glycemic control through insulin production and release,thereby promoting structural protein synthesis.
Serum activities of AST and ALT are used in the assessment of hepatic disorders.These enzymes are found in high concentrations in the liver and increases in their serum activities suggest liver damage[31].Their elevated serum activities in diabetic animals are due to the necrotic action of STZ on the liver.C.albidum extract treated diabetic rats showed reduced activities of these enzymes compared to the diabetic control group.This result confirms the study conducted on the root bark of C.albidum which showed the ability of the plant to decrease the activities of serum liver enzymes in alloxan-induced diabetic rats[11].
Serum urea and creatinine levels are used as indicators of renal function[32].The elevation of these parameters in the blood indicates renal dysfunction.Increased serum urea levels are associated with enhanced protein degradation resulting in a decline in plasma total proteins as seen in diabetic animals[33].Treatment of diabetic rats with the C.albidum extract significantly decreased the elevated levels of serum urea and creatinine whilst increasing the total protein concentrations.Thus,C.albidum may potentially avert the occurrence of nephropathy in diabetic rats.Similarly,the ethanol extract of C.albidum stem bark did not exert any toxic effects in healthy experimental animals up to 2 000 mg/kg,indicating that it was safe at that dose and that the lethal dose was above 2 000 mg/kg.Oxidative stress has been shown to play critical roles in the development of many diseases including diabetes mellitus.There is evidence to suggest that diabetic micro- and macro-vascular complications are also mediated by oxidative stress[7].This occurs as a result of excessive production of reactive oxygen species due to prolonged hyperglycemia overpowering the body's antioxidant mechanisms.The increase in the generation of reactive oxygen species in the diabetic state occurs through several metabolic pathways that include increased flux of glucose through the polyol pathway,increased formation of advanced glycation end products and their receptors,and activation of protein kinase C isoforms-β,δ,and α[34].
In the present study,the antioxidant activity of the stem bark extract of C.albidum was evaluated using radical scavenging assays.NO,although an important molecule in the body,involved in the regulation of blood vessels,reacts with superoxides (O2.) to form a highly reactive nitrite radical which can induce oxidative damage in cells and on cellular function[35].The competitive scavenging of this radical is an important step in the prevention of the oxidative damage of nitrite radicals in lipids and proteins.The result of the study indicates that the extract could effectively scavenge NO radicals and prevent cellular oxidative damage.The radical scavenging activities of the extract and fractions were also confirmed in the DPPH radical assay.
Several plant metabolites have been shown to possess hypoglycemic and antioxidant activities.The presence of one or more of these metabolites may be responsible for the observed biological activities recorded by the C.albidum extract in this study.Some triterpenoids,glycosides,alkaloids,and flavonoids have been identified to inhibit the actions of α-amylase and α-glucosidase while others have shown glucose uptake stimulatory effects in adipocytes and skeletal muscles[36].These metabolites acting additively or synergistically could contribute to the antidiabetic activity of the stem bark of C.albidum.
The results of the in vitro studies indicated that the hypoglycemic activity of C.albidum is strongly associated with its flavonoid-rich n-butanol fraction and ethyl acetate fraction enriched with alkaloids and triterpenoids.Further studies will,therefore,focus on the isolation and identification of these bioactive compounds.
In conclusion,the stem bark extract of C.albidum showed considerable hypoglycemic and anti-hyperlipidemic activities in diabetic rats.The extract demonstrated significant ability to enhance the uptake of glucose in C2C12 myotubes and 3T3-L1 adipocytes as well as significant inhibitory activities against α-glucosidase and α-amylase,suggesting that its hypoglycemic action may be mediated by these mechanisms.The present study thus provides a rationale for the use of the ethanol extract of C.albidum stem bark in the management of diabetes mellitus.
Conflict of interest statement
The authors declare no competing interest.
Acknowlegdments
The supports of the technicians in the Department of Pharmacology in the Faculty of Pharmacy and Pharmaceutical Sciences,KNUST,Kumasi are greatly appreciated.
 Asian Pacific Journal of Tropical Biomedicine2020年9期
Asian Pacific Journal of Tropical Biomedicine2020年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Opuntia humifusa aqueous extract alleviates ethanol-induced gastric ulcer in a mouse model
- Allolobophora caliginosa coelomic fluid ameliorates gentamicin-induced hepatorenal toxicity in rats
- Immunomodulatory and anticancer activity of Bombax ceiba Linn leaf extract
- Resveratrol downregulates TGF-β1 and Smad3 expression and attenuates oxidative stress in CCl4-induced kidney damage in rats
