肝素C5异构酶的表达优化及分子改造
王兵兵,周正雄,金学荣,李江华,史仲平,康振
·生物育种与工艺优化·
肝素C5异构酶的表达优化及分子改造
王兵兵1,2,周正雄1,2,金学荣1,2,李江华1,2,史仲平1,2,康振1,2
1 江南大学 生物工程学院 工业生物技术教育部重点实验室,江苏 无锡 214122 2 江南大学 生物工程学院 糖化学与生物技术教育部重点实验室,江苏 无锡 214122
肝素和硫酸乙酰肝素是一类应用于临床抗凝血的糖胺聚糖。肝素葡萄糖醛酸C5异构酶(Heparosan-N-sulfate-glucuronate 5-epimerase,C5,EC 5.1.3.17) 是肝素和硫酸乙酰肝素合成过程中重要的修饰酶,催化N-硫酸化肝素前体(N-sulfoheparosan) 的D-葡萄糖醛酸(D-GlcA) 上5号位羧基翻转生成L-艾杜糖醛酸(L-iduronic acid,L-IdoA)。文中以大肠杆菌为宿主对斑马鱼来源的肝素葡萄糖醛酸C5异构酶基因进行重组表达优化与分子改造。比较了3种不同的表达载体pET20b(+)、pET28a(+) 和pCold Ⅲ对C5表达的差异情况,其中以嗜冷启动型载体pCold Ⅲ表达酶活最高,达到(1 873.61±5.42) U/L。为了进一步提高C5的可溶表达量,在N端融合促溶标签SET2后,可溶蛋白表达量比对照提高了50%,酶活达到 (2 409.25±6.43) U/L。在此基础上,通过理性设计对底物结合口袋进行定点突变,获得最优突变体(V153R)的酶活和比酶活分别为 (5 804.32±5.63) U/L和(145.14±2.33) U/mg,是原始酶的2.41倍和2.28倍。肝素C5异构酶改造与表达优化为酶法催化合成肝素奠定了基础。
肝素,葡萄糖醛酸-C5-异构化酶,异源表达,理性设计,底物结合口袋
肝素是一种存在于细胞表面和胞外基质中的糖胺聚糖[1],由己糖醛酸和葡糖胺组成的二糖单位重复连接而成[2],在机体内作为细胞识别、信号传导和抗凝血等的生物学活性分子[3]。临床上主要用于治疗血栓栓塞性疾病、心肌梗死、心血管手术和术后抗凝血等。目前,肝素的生产方式主要包括生物提取[4]、化学合成[5]、酶法合 成[6]等。其中生物提取法获得的肝素中混有多种糖胺聚糖,对产物造成污染;化学合成法生产周期长,过程繁琐[7];而酶法合成产物单一,过程简单[8]。
在酶法合成肝素过程中,肝素前体先经过脱乙酰和硫酸化作用转变为N-硫酸化肝素前体[9],N-硫酸化肝素前体经肝素C5异构酶催化[10-12],其多糖链上的D-葡萄糖醛酸(D-glucuronic acid,D-GlcA) 异构化为L-艾杜糖醛酸(L-iduronic acid,L-IdoA),其中葡萄糖醛酸C5位的氢与介质中的质子发生交换[13],C5位的羧基翻转,导致分子构型发生变化[14]。含有艾杜糖醛酸的多糖链进一步通过后续不同硫酸化位点的修饰合成具有生物学功能的肝素[15]。C5异构化作用是肝素酶法合成过程中的一步关键反应[16],含有IdoA的GAGs (糖胺聚糖链) 才具有抗凝血和抗血脂等的功能[17],因此C5异构化作用是后续硫酸化修饰的前提。
随着药理学及临床医学的进展,肝素的应用不断扩大。但在体外酶法合成肝素[18]的过程中,途径所涉及的关键性异构酶酶活却很低[19-21],这严重限制了肝素的工业化生产,提高C5异构酶的催化活性对于肝素的合成及其临床应用具有重大意义。本研究通过优化表达载体、N端融合促溶标签以及理性改造酶结构等策略,获得一株催化活性显著提高的突变体V153R,其酶活与比酶活分别为 (5 804.32±5.63) U/L和 (145.14±2.33) U/mg,是野生型C5异构酶酶活的2.41倍、比酶活的2.28倍。C5异构酶的高活性表达为肝素的全酶法合成奠定了基础。
1 材料与方法
1.1 宿主和质粒
本实验所有的质粒构建均在大肠杆菌JM109中进行,重组菌株构建的出发菌株为BL21(DE3)。菌株JM109、BL21(DE3),质粒pET-28a (+)、pET-20b (+)、pCold Ⅲ均为本实验室保存。详情见表1。
1.2 菌株构建
将密码子优化后的斑马鱼来源的基因(NP_998014.1) 经PCR扩增(所用引物见表2) 后酶切连接至pCold Ⅲ、pET20b、pET28a,构建重组载体pCold Ⅲ-C5、pET20b-C5、pET28a-C5。将来源于大肠杆菌的麦芽糖蛋白(Maltose binding protein,MBP)、酿酒酵母的促溶标签SET2 (Solubility-enhancement tags) 及小泛素样蛋白(Small ubiquitin-related modifier,SUMO) 基因经PCR扩增(所用引物见表2) 后一步克隆连接至pCold Ⅲ-C5构建重组载体pCold Ⅲ-MBP-C5、pCold Ⅲ-SET2-C5、pCold Ⅲ-SUMO-C5。42 ℃热激90 s后,转化至JM109感受态细胞中,挑取转化子测序,测序正确则上述重组载体构建成功。按上述方法将测序成功的重组质粒转化至BL21(DE3) 感受态细胞。
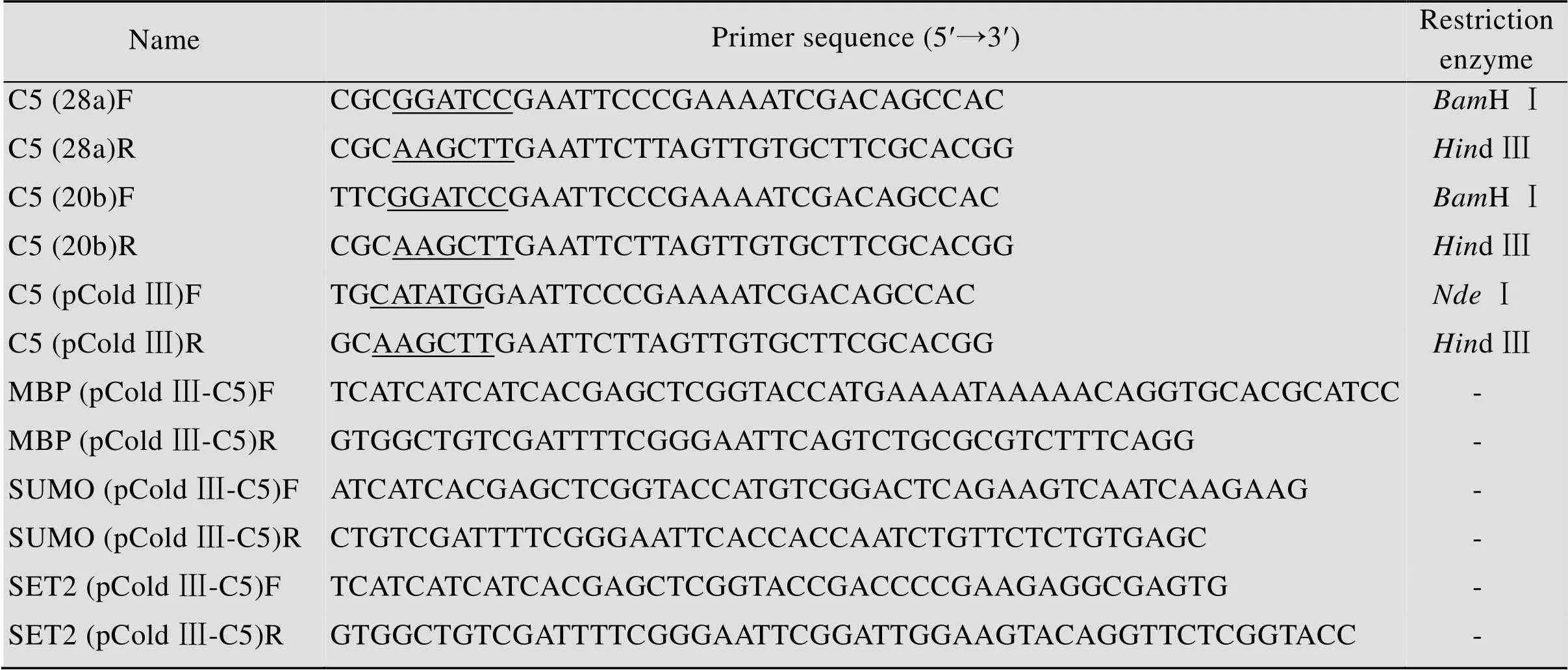
表2 本文所用引物
Underlines represent the restriction site.
1.3 突变位点的预测
本实验基于上述表达优化的基础,进一步对C5异构酶酶分子结构[22]理性改造提高C5异构酶的催化活性。根据在蛋白质结构数据库(Protein data bank,PDB) 下载的斑马鱼来源的蛋白结构(PDB: 4pxq),利用软件Discovery Studio的CDOCKER模块进行分子对接,具体操作方法和参数设置根据Discovery Studio软件的操作手册所述。
1.4 培养基组分
LB培养基:酵母粉5 g/L,胰蛋白胨10 g/L,NaCl 10 g/L (固体培养基添加2%琼脂粉)。
TB培养基:酵母粉24 g/L,胰蛋白胨12 g/L,K2HPO412.54 g/L,KH2PO42.31 g/L。
1.5 重组菌株培养
挑取单菌落接种于50 mg/L卡那霉素或者100 mg/L的氨苄霉素的3 mL液体LB培养基中,37 ℃、220 r/min过夜培养。按1% (/) 将种子液接种于50 mL TB培养基中,37 ℃、220 r/min培养至600为0.6–0.8,添加终浓度为0.05 mmol/L的异丙基-β-D-硫代半乳糖苷(isopropyl-β-D- thiogalactoside,IPTG),分别以30 ℃诱导培养pET系列重组菌株10 h、以15 ℃诱导培养pCold系列载体22 h,诱导异构酶的表达。
1.6 粗酶液的制备
将上述发酵结束后的菌液于4 ℃、7 000 r/min条件下离心10 min,弃上清收集菌体,用20 mmol/L Tris-HCl (pH 7.4) 洗涤2次,稀释菌体至600为8.0,冰水浴超声破碎后(功率300 W,工作4 s,间歇6 s,10 min),4 ℃、12 000 r/min离心30 min,分别收集上清和沉淀。上清液即为所需粗酶液。
1.7 目的蛋白的纯化
用25 mL溶液A (20 mmol/L Tris-HCl,pH 7.4) 平衡Ni-His Trap FF柱后上样已过0.22 µm滤膜的粗酶液,分别用10%、30%、100%的溶液B (20 mmol/L Tris-HCl,500 mmol/L咪唑,pH 7.4) 进行洗脱并收集相对应的洗脱液。对得到的洗脱液进行脱盐处理,所用脱盐缓冲液为20 mmol/L Tris-HCl (pH 7.4),脱盐柱为G10。经基质辅助激光解吸电离飞行时间质谱(Matrix-assisted laser desorption/ionization time of flight mass spectrometry,MALDI-TOF-MS) 鉴定所得条带为目的异构酶条带。
1.8 酶活性的检测
C5异构酶酶活测定:采用与肝素硫酸转移酶2-OST (2-O-硫酸转移酶,2-O-sulfotransferase) 偶联[23-26]测酶活方法。标准反应条件为向1.5 mL的Tris-HCl (20 mmol/L,pH 7.4) 中添加50 mmol/L PNPS (对硝基苯硫酸盐,para-nitrophenylsulfate),0.5 mmol/L PAP (3-磷酸腺苷-5-磷酰,3′-phosphoadenosine-5′-phospho),0.5 mg AST Ⅳ(芳香磺基转移酶,aryl sulfotransferase Ⅳ),0.4 mg N-sulfoheparosan,0.3 mg硫酸转移酶2-OST, 0.3 mg C5异构酶(对照组为基于上述条件下添加等量失活的C5异构酶酶液) 于37 ℃反应2 h后,100 ℃水浴煮沸5 min终止反应。10 000 r/min离心10 min去除沉淀,在400的吸光度测定对硝基苯酚(PNP) 的吸光值变化[27]。酶活单位定义为:在最适反应条件下(37 ℃,pH 7.4),每小时生成1 µmol/L的PNP所需的酶量。
1.9 动力学常数测定
C5异构酶的动力学常数测定反应液为1.5 mL Tris-HCl (20 mmol/L,pH 7.4),其中包括50 mmol/L PNPS,0.5 mmol/L PAP,0.5 mg AST IV,0.3 mg 2-OST,0.3 mg C5及1.6–4 700 mg/L等不同浓度的N-sulfoheparosan作为底物测定C5异构酶的酶活,根据测定值进行米氏常数拟合。
2 结果与分析
2.1 不同载体对C5异构酶表达的影响
将C5异构酶基因分别构建在pET20b (+)、pET28a (+) 和pCold Ⅲ 3种表达载体中,考察不同载体对C5异构酶表达差异性的影响。在液体培养基中接种重组菌pET20b-C5、pET28a-C5、pCold Ⅲ-C5及空载体作为对照菌,当600达到0.6时,加入IPTG诱导表达,收集pET系列10 h后的菌体及pCold系列22 h后的菌体,破碎细胞后利用SDS-PAGE分析胞内上清及沉淀。如图1A所示,C5异构酶在3种载体中均实现成功表达,其中pCold Ⅲ载体的上清可溶部分表达量最高,酶活最高为 (1 873.61±5.42) U/L。这可能是因为在低温条件下(15 ℃),嗜冷型启动子的转录强度高于T7启动子[28],且低温使得合成的C5异构酶可以正确折叠,可溶表达部分增加。
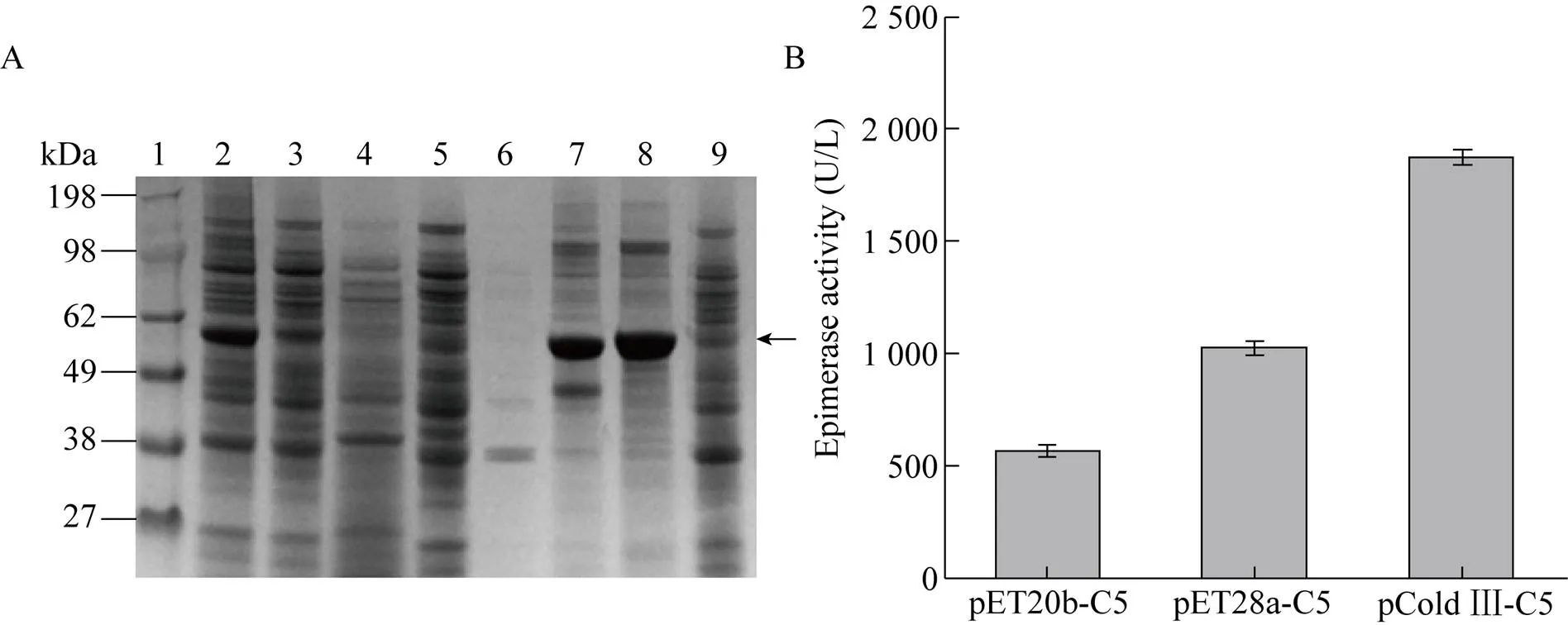
图1 不同载体对C5异构酶表达的影响
2.2 不同融合标签对C5异构酶表达的影响
以上3种载体均实现了C5异构酶的活性表达,其中以pCold Ⅲ载体表达的C5异构酶胞内可溶表达量最高。在此基础上,进一步研究了N端融合促溶标签MBP、SUMO、SET2对C5异构酶活性表达的影响。如图2所示,在N端融合SET2后C5异构酶可溶性表达量最高,酶活也最高,达到 (2 409.25±6.43) U/L,是融合前的1.28倍。结果表明促溶标签SET2可有效地促进异构酶的可溶表达[29]。
2.3 定点突变提高C5异构酶催化活性
根据已报道的酶晶体结构分析,经分子对接,选择距离底物结合口袋5 Å范围内的氨基酸位点,以pCold Ⅲ-SET2-C5为模板,对V153、D155、Q185、K397、G399、N478、D529、D545进行饱和突变。获得催化活性显著提高的突变体V153R,胞内上清酶液纯化后比酶活达到 (145.14±2.33) U/mg。反应动力学常数显示其m值由原来的(1.922±0.131) mmol/L降低至 (0.941±0.083) mmol/L,催化常数cat/m由(16.129±0.111) 增加至(44.633±0.547) L/(s·mol);突变体G399Em值降低至 (1.525±0.273) mmol/L,催化常数cat/m增加至 (22.951±0.146) L/(s·mol)、D545Ym值降低至 (1.366±0.196) mmol/L,催化常数cat/m增加至 (27.086±0.102) L/(s·mol),说明突变体与底物的亲和力增加。原因可能是由于底物带有较强的负电荷,当153位氨基酸由侧链不带电荷的缬氨酸变为带有正电荷的精氨酸时,异构酶底物结合口袋局部环境中的正电荷强度增加(如图4A、4B所示),且突变后(图4B) 相比突变前(图4A),侧链更长,距离底物更近,底物更容易进入结合口袋;399位氨基酸突变前后如图4C、4D所示,突变前399位甘氨酸(图4C) 与底物只有一个氢键作用力,突变为谷氨酸(图4D) 后相比突变前氢键作用力增加,但同时底物结合口袋负电荷强度也增加;545位氨基酸突变前后如图4E、4F所示,突变前545位天冬氨酸(图4E) 与底物没有作用,突变为酪氨酸后(图4F) 与底物增加了疏水作用力,同时突变后底物结合口袋局部负电荷强度变弱,因此以上3个氨基酸位点可能发生多重作用力的叠加,导致催化活性呈现不同程度的提高。基于以上结果对153R、399E、545Y 3个单点突变体进行了两两叠加突变,并未发现明显效果(数据未展示)。
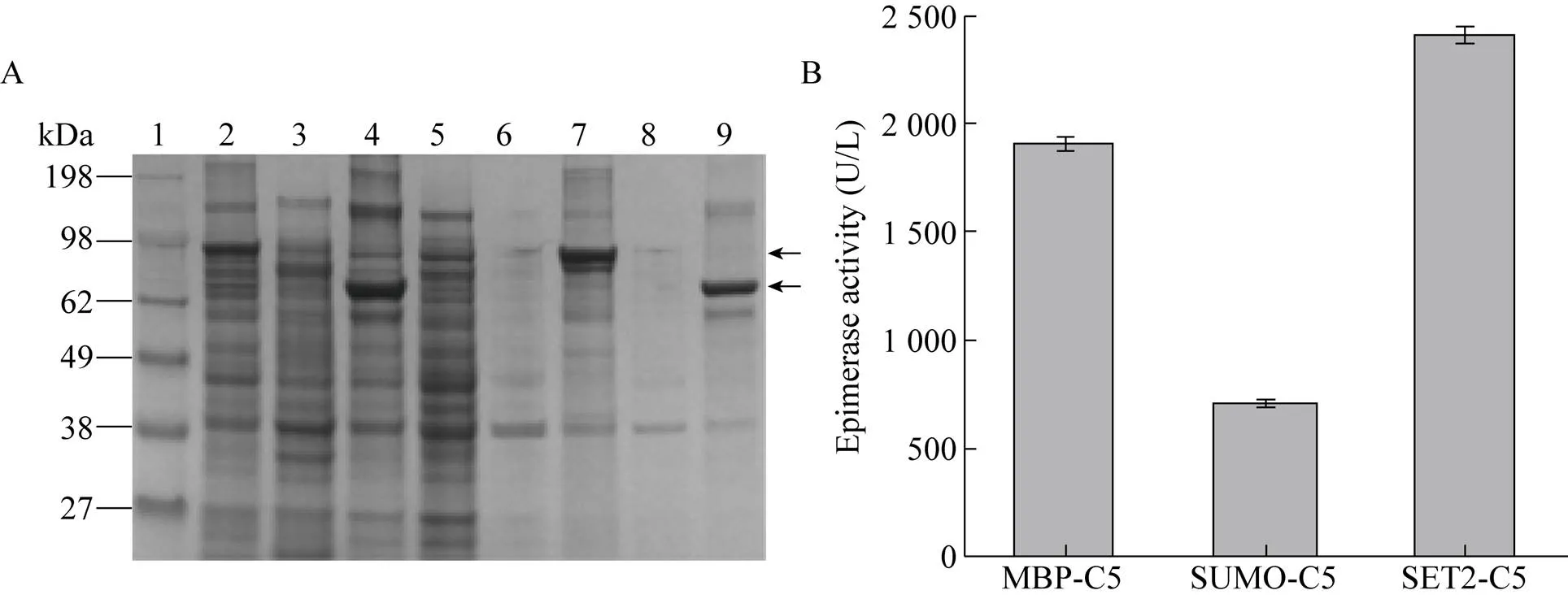
图2 不同促溶标签对C5异构酶表达的影响
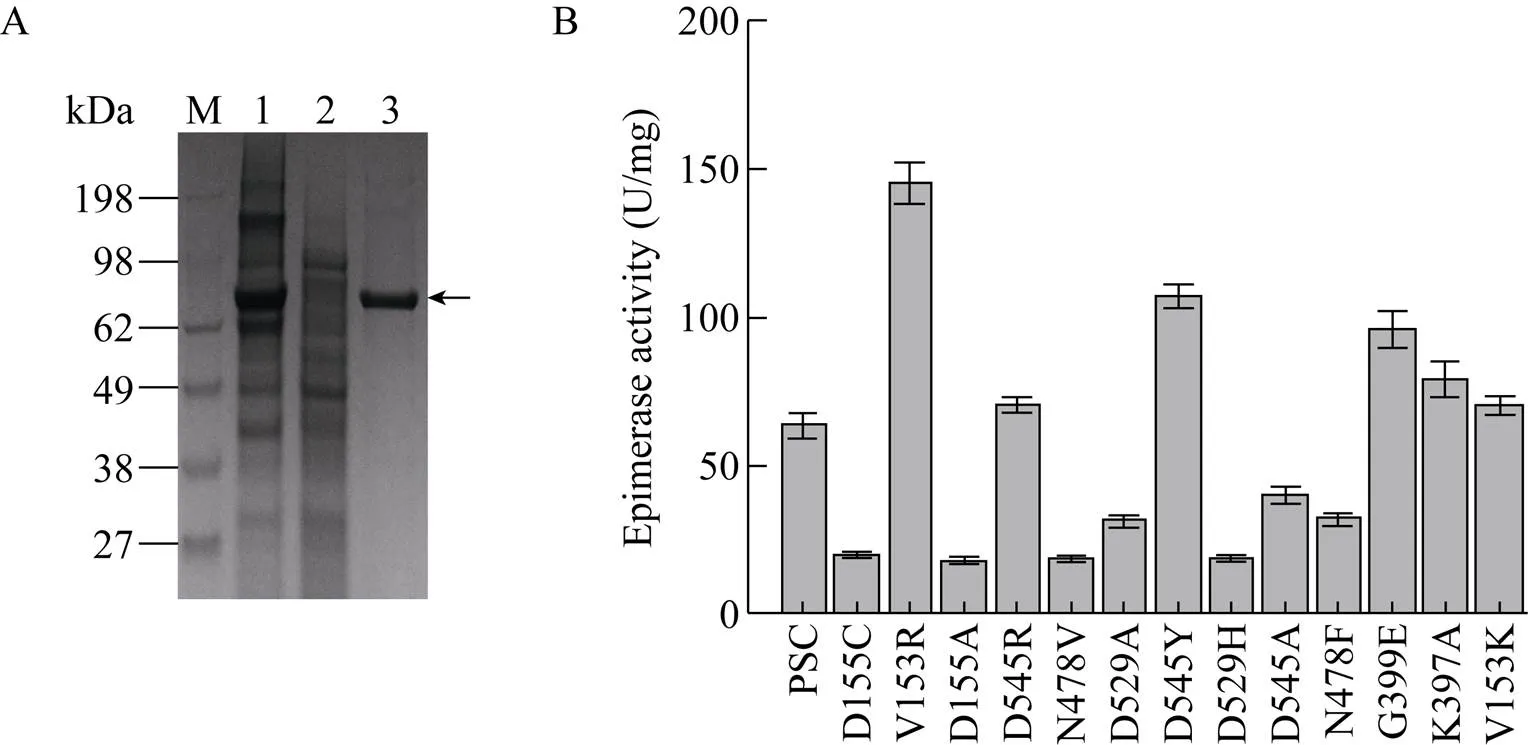
图3 肝素C5异构酶纯化及突变体酶活
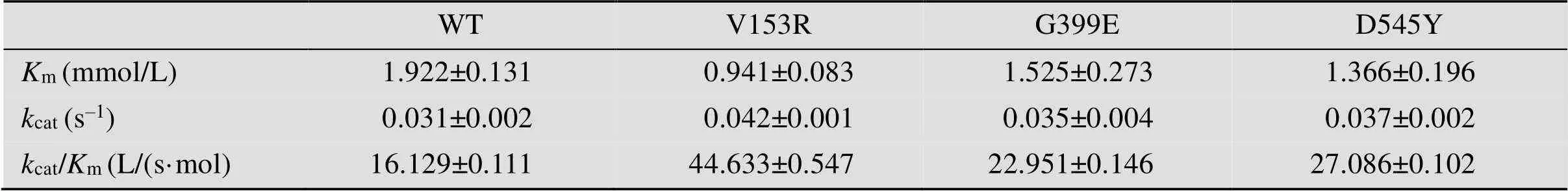
表3 肝素异构酶酶学性质表

图4 肝素C5异构酶突变体结构分析
3 结论
C5异构酶作为肝素酶法合成中的一个关键酶,催化葡萄糖醛酸异构化为艾杜糖醛酸。本研究通过表达载体优化、N端融合标签及蛋白质理性改造成功地在大肠杆菌中实现了斑马鱼来源的肝素C5异构酶的活性表达。研究表明3种载体pET28a、pET20b、pCold Ⅲ均实现了C5异构酶的胞内活性表达,相比嗜冷启动型载体pCold Ⅲ表达的C5异构酶活性最高。通过N端融合促溶标签SET2减少了无活性的包涵体的积累,进一步提高了C5异构酶的活性表达((2 409.25±6.43) U/L)。在此基础上,为了获得更高催化活性的C5异构酶,我们对C5异构酶的分子结构进行了理性改造,获得了突变体V153R,其酶活与比酶活分别为 (5 804.32±5.63) U/L和 (145.14±2.33) U/mg,是野生型C5异构酶的2.41倍和2.28倍。该研究为实现肝素的全酶法合成奠定了基础。
[1] Feyerabend TB, Li JP, Lindahl U, et al. Heparan sulfate C5-epimerase is essential for heparin biosynthesis in mast cells. Nat Chem Biol, 2006, 2(4): 195–196.
[2] Zhou ZX, Wang BB, Xu RR, et al. Optimized expression of heparin sulfotransferases and their application in sulfation of animal derived heparin. Chin J Biotech, 2018, 34(11): 1784–1793 (in Chinese).周正雄, 王兵兵, 胥睿睿, 等. 肝素硫酸转移酶优化表达及其在动物源肝素硫酸化中的应用. 生物工程学报, 2018, 34(11): 1784–1793.
[3] Linhardt RJ, Dordick JS, Deangelis PL, et al. Enzymatic synthesis of glycosaminoglycan heparin. Semin Thromb Hemost, 2007, 33(5): 453–465.
[4] Middeldorp S. Heparin: from animal organ extract to designer drug. Thromb Res, 2008, 122(6): 753–762.
[5] Achour O, Bridiau N, Godhbani A, et al. Ultrasonic-assisted preparation of a low molecular weight heparin (LMWH) with anticoagulant activity. Carbohydr Polym, 2013, 97(2): 684–689.
[6] Schultz V, Suflita M, Liu XY, et al. Heparan sulfate domains required for fibroblast growth factor 1 and 2 Signaling through fibroblast growth factor receptor 1c. J Biol Chem, 2017, 292(6): 2495–2509.
[7] Cai C, Dickinson DM, Li LY, et al. Fluorous-assisted chemoenzymatic synthesis of heparan sulfate oligosaccharides. Org Lett, 2014, 16(8): 2240–2243.
[8] Xu YM, Cai C, Chandarajoti K, et al. Homogeneous low-molecular-weight heparins with reversible anticoagulant activity. Nat Chem Biol, 2014, 10(4): 248–250.
[9] Zhang X, Wang FS, Sheng JZ. “Coding” and “Decoding”: hypothesis for the regulatory mechanism involved in heparan sulfate biosynthesis. Carbohydr Res, 2016, 428: 1–7.
[10] Hagner-McWhirter Å, Hannesson HH, Campbell P, et al. Biosynthesis of heparin/heparan sulfate: kinetic studies of the glucuronyl C5-epimerase with N-sulfated derivatives of theK5 capsular polysaccharide as substrates. Glycobiology, 2000, 10(2): 159–171.
[11] Li JP, Gong F, El Darwish K, et al. Characterization of the D-glucuronyl C5-epimerase involved in the biosynthesis of heparin and heparan sulfate. J Biol Chem, 2001, 276(23): 20069–20077.
[12] Sheng JZ, Xu YM, Dulaney SB, et al. Uncovering biphasic catalytic mode of C5-epimerase in heparan sulfate biosynthesis. J Biol Chem, 2012, 287(25): 20996–21002.
[13] Jacobsso I, Lindahl U, Jensen JW, et al. Biosynthesis of heparin. Substrate specificity of heparosan-sulfate D-glucuronosyl 5-epimerase. J Biol Chem, 1984, 259(2): 1056–1063.
[14] Chappell EP, Liu J. Use of biosynthetic enzymes in heparin and heparan sulfate synthesis. Bioorg Med Chem, 2013, 21(16): 4786–4792.
[15] Wang ZY, Yang B, Zhang ZQ, et al. Control of the heparosan-deacetylation leads to an improved bioengineered heparin. Appl Microbiol Biotechnol, 2011, 91(1): 91–99.
[16] Jia J, Maccarana M, Zhang X, et al. Lack of L-iduronic acid in heparan sulfate affects interaction with growth factors and cell signaling. J Biol Chem, 2009, 284(23): 15942–15950.
[17] Kuberan B, Lech MZ, Beeler DL, et al. Enzymatic synthesis of antithrombin III-binding heparan sulfate pentasaccharide. Nat Biotechnol, 2003, 21(11): 1343–1346.
[18] Muñoz E, Xu D, Avci F, et al. Enzymatic synthesis of heparin related polysaccharides on sensor chips: rapid screening of heparin-protein interactions. Biochem Biophys Res Commun, 2006, 339(2): 597–602.
[19] Crawford BE, Olson SK, Esko JD, et al. Cloning, Golgi localization, and enzyme activity of the full-length heparin/heparan sulfate-glucuronic acid C5-epimerase. J Biol Chem, 2001, 276(24): 21538–21543.
[20] Grigorieva E, Eshchenko T, Rykova VI, et al. Decreased expression of human D-glucuronyl C5-epimerase in breast cancer. Int J Cancer, 2008, 122(5): 1172–1176.
[21] Raedts J, Lundgren M, Kengen SWM, et al. A novel bacterial enzyme with D-glucuronyl C5-epimerase activity. J Biol Chem, 2013, 288(34): 24332–24339.
[22] Qin Y, Ke JY, Gu X, et al. Structural and functional study of D-glucuronyl C5-epimerase. J Biol Chem, 2015, 290(8): 4620–4630.
[23] Li K, Bethea HN, Liu J. Using engineered 2--sulfotransferase to determine the activity of heparan sulfate C5-epimerase and its mutants. J Biol Chem, 2010, 285(15): 11106–11113.
[24] Zhang JH, Suflita M, Li GY, et al. High cell density cultivation of recombinantstrains expressing 2--sulfotransferase and C5-epimerase for the production of bioengineered heparin. Appl Biochem Biotechnol, 2015, 175(6): 2986–2995.
[25] Préchoux A, Halimi C, Simorre JP, et al. C5-epimerase and 2--sulfotransferase associate in vitro to generate contiguous epimerized and 2--sulfated heparan sulfate domains. ACS Chem Biol, 2015, 10(4): 1064–1071.
[26] Sterner E, Li LY, Paul P, et al. Assays for determining heparan sulfate and heparin-sulfotransferase activity and specificity. Anal Bioanal Chem, 2014, 406(2): 525–536.
[27] Paul P, Suwan J, Liu J, et al. Recent advances in sulfotransferase enzyme activity assays. Anal Bioanal Chem, 2012, 403(6): 1491–1500.
[28] Qing GL, Ma LC, Khorchid A, et al. Cold-shock induced high-yield protein production in. Nat Biotechnol, 2004, 22(7): 877–882.
[29] Klermund L, Riederer A, Groher A, et al. High-level soluble expression of a bacterial-acyl-D-glucosamine 2-epimerase in recombinant. Protein Expr Purif, 2015, 111: 36–41.
Expression optimization and molecular modification of heparin C5 epimerase
Bingbing Wang1,2, Zhengxiong Zhou1,2, Xuerong Jin1,2, Jianghua Li1,2, Zhongping Shi1,2, and Zhen Kang1,2
1 Key Laboratory of Industrial Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi 214122, Jiangsu, China 2 Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education,School of Biotechnology, Jiangnan University, Wuxi 214122, Jiangsu, China
Heparin and heparan sulfate are a class of glycosaminoglycans for clinical anticoagulation. Heparosan N-sulfate-glucuronate 5-epimerase (C5, EC 5.1.3.17) is a critical modifying enzyme in the synthesis of heparin and heparan sulfate, and catalyzes the inversion of carboxyl group at position 5 on D-glucuronic acid (D-GlcA) of N-sulfoheparosan to form L-iduronic acid (L-IdoA). In this study, the heparin C5 epimerase genefrom zebrafish was expressed and molecularly modified in. After comparing three expression vectors of pET-20b (+), pET-28a (+) and pCold Ⅲ, C5 activity reached the highest ((1 873.61±5.42) U/L) with the vector pCold Ⅲ. Then we fused the solution-promoting label SET2 at the N-terminal for increasing the soluble expression of C5. As a result, the soluble protein expression was increased by 50% compared with the control, and the enzyme activity reached (2 409±6.43) U/L. Based on this, site-directed mutations near the substrate binding pocket were performed through rational design, the optimal mutant (V153R) enzyme activity and specific enzyme activity were (5 804±5.63) U/L and (145.1±2.33) U/mg, respectively 2.41-fold and 2.28-fold of the original enzyme. Modification and expression optimization of heparin C5 epimerase has laid the foundation for heparin enzymatic catalytic biosynthesis.
heparin, glucuronic acid-C5-epimerase, heterologous expression, rational design, substrate binding pocket
10.13345/j.cjb.190516
November 18, 2019;
January 21, 2020
Supported by:National Natural Science Foundation of China (No. 31670092), National Key R&D Program of China (No. 2018YFA0901401), Fundamental Research Funds for the Central Universities (No. JUSRP51707A), National First-class Discipline Program of Light Industry Technology and Engineering (No. LITE2018-16).
Zhen Kang. Tel: +86-510-85918307; Fax: +86-510-85918309; E-mail: zkang@jiangnan.edu.cn
Zhongping Shi. Tel: +86-510-85329031; Fax: +86-510-85918309; E-mail: zpshi@jiangnan.edu.cn
国家自然科学基金(No. 31670092),国家重点研发计划 (No. 2018YFA0901401),江南大学自主科研计划重点项目(No. JUSRP51707A),国家轻工技术与工程一流学科自主课题(No. LITE2018-16) 资助。
2020-05-08
https://kns.cnki.net/kcms/detail/11.1998.Q.20200508.1434.004.html
王兵兵, 周正雄, 金学荣, 等. 肝素C5异构酶的表达优化及分子改造. 生物工程学报, 2020, 36(7): 1450–1458.
Wang BB, Zhou ZX, Jin XR, et al. Expression optimization and molecular modification of heparin C5 epimerase. Chin J Biotech, 2020, 36(7): 1450–1458.
(本文责编 郝丽芳)
——α-葡萄糖醛酸酶的研究进展*

