高硅SSZ-13分子筛膜二次水热生长过程
王贺礼 朱美华 吴 婷 张 飞 吴亚芬 陈祥树*,
(1江西师范大学化学化工学院,先进材料研究院,分子筛膜材料国家地方联合工程实验室,南昌 330022)
(2江西省科学院能源研究所,南昌 330096)
(3江西中医药大学,南昌 330004)
0 Introduction
The separation of CO2from CH4is important in natural gas industry for CO2is corrosive in the presence of water and incombustible CO2decreases the energy content of natural gas[1].The conventional CO2removal technologies including cryogenic distillation and amine scrubbing suffer from high energy consumption and environmental impact.Separation of CO2using membrane is regarded as an alternative technology for low-energy and environmental friendly[2].Polymeric membranes are used in industrial facilities for good processibility and lower operational cost,but easy to plasticize and lose selectivity at high temperature or pressure[3-4].Inorganic zeolite membranes such as MFI[5],CHA[6-7],AEI[8],DDR[9],ERI[10]could endure high temperature and pressure,and have been researched intensively in recent years.
Since Kalipcilar et al[6]firstly prepared the inorganic SSZ-13 zeolite membranes on the inside surface of porous stainless steel tubes and got an ideal separation selectivity of CO2/CH4of 11 at 298 K,SSZ-13 zeolite membrane has been regarded as an attractive material for CO2removal from natural gas and has been investigated intensively[11-17].SSZ-13 zeolite belongs to the CHA topology and has a 3-dimensional structure with 8-membered rings,the intersecting channels with a ring diameter of 0.38 nm×0.38 nm.High-silica SSZ-13 zeolite was reported for possessing high adsorption selectivity of CO2over other light gas such as N2and CH4[18],with the moderate window size of channel and the outstanding chemical stability and hydrophobicity,and it has been expected one of the best choice to separate CO2/CH4in natural gas.
The gas separation performance of SSZ-13 membranes was characterized as gas permeation flux and separation selectivity,which affected significantly by the quality of the membrane layer and the supports′property.A high quality membrane refers to a dense layer without defects,and it should be as thin as possible.Yu et al[19]prepared CHA membranes with a depth ofca.1.3 µm used 20~200 nm nanocrystal seeds,and the single gas CO2permeance was as high as 1.72×10−5mol·m−2·s−1·Pa−1at room temperature.A conflict of preparing thin membranes is the membrane layer usually not dense enough when their depth less than a certain value,thus,a sensible way is reducing the depth of membrane prepared without defect.Besides the porosity and average pore size,the surface roughness of supports affected the thermal stability of membranes and very smooth surface supports were prone to crack formation upon detemplation[20].Extra preparation procedure as ultrasound pretreatment[21]and post-treatment[22-23]can also improve the quality of SSZ-13 membrane and mend defects for a better gas separation performance.
SSZ-13 membranes were prepared byin-situcrystallization[11],hydrothermal secondary growth[12-17]or inter-zeolite conversion[24-26].Maghsoudi et al[11]prepared high-silica SSZ-13 membranes onα-alumina porous disks byin-situcrystallization.To reduce defects generated,thein-situcrystallization process was repeated three times and each for 40 hours,which delayed the preparation period and increased the depth of membrane and resulted a decreased gas flux permeated.The hydrothermal secondary growth method was generally used for preparing SSZ-13 membranes by controlling the quality conveniently[12-17].Different to thein-situcrystallization,a seeding procedure is carried out before the hydrothermal synthesis.The according zeolite seeds were synthesized and the zeolite seeds were coated on the supports by rub coating[13]or dip coating[7].Then,the membranes were synthesized at fixed temperature and time.Inter-zeolite conversion crystallization from FAU[24],P[25],or LTA[26]to synthesize SSZ-13 membranes is another method for the secondary growth and the zeolite seeds are different with the membrane material.The advantage of inter-zeolite conversion is that it could reduce synthesis time markedly[27].For secondary growth method,intergrowth of crystals is enhanced for reducing defects and getting a dense membrane;however,it results an increased depth of membrane and decreased gas separation performance.
As mentioned above,the secondary growth method is very important for preparing SSZ-13 membranes in laboratory.In order to optimize the synthesis conditions and get high gas separation performance,a fundamental understanding of the formation process on the SSZ-13 membrane is significant.To our best knowledge,the growth process of SSZ-13 membranes by secondary growth method has not discussed yet.In the current work,we prepared SSZ-13 membranes on tubular mullite supports used a diluted organic template gel composition.The SSZ-13 membranes and powders together with the membranes were characterized by X-ray powder diffraction(XRD)and field emission scanning election microscopy(FE-SEM),and the possible growth process of SSZ-13 membrane was discussed.
1 Experimental
1.1 Synthesis procedure for SSZ-13 seeds
High-silica SSZ-13 seed crystals were synthesized according to the procedure of Zones[28],but the synthesis conditions were modified[13,29].The molar composition of this gelwas 1.0∶0.10∶0.025∶0.20∶44,where TMAdaOH isN,N,N-trimethtyl-1-adamantammonium hydroxide.The gel was formed by adding 0.89 g of NaOH(96%,Sinopharm),0.43 g of Al(OH)3powder(99%,Wako)and 18.68 g of TMAdaOH(25%(w/w)in water)to 20 g deionized water(DI water),and stirred at 150 r·min−1with heating till Al(OH)3was dissolved.After cooled down to room temperature,the above solution was added to a PP bottle drop by drop with 16.63 g colloidal silica(Ludox TM-40,Sigma-Aldrich).DI water for evaporation was considered and supplied.The formed gel was aged for 12 h with stirring at 300 r·min−1,then,transferred to a Teflon-lined autoclave for crystallization at 433 K for 144 h.After synthesis,the SSZ-13 zeolite crystals were recovered by high speed centrifugation,washed several times with DI water to neutral and dried 24 h at 373 K.The organic template of TMAdaOH was removed by calcination in the air at 823 K for 10 h and cooling rates of 10 K·min−1.
1.2 Synthesis procedure for SSZ-13 membranes
The supports used in this study were porous mullite tubes as in our previous work[13],but without polishing procedure.The composition of gelfor membrane preparation was 1.0∶0.10∶0.005∶0.05∶80,noted that the single and less amount(a half of proportion)of TMAdaOH template used in this study,which aimed to decrease intergrowth ofcrystalsand wasdifferentfrom ourprevious work[13,15].The mullite tubes were seeded by rub-coating,then placed vertically in an autoclave and filled with synthesis gel about 70%in volume.The hydrothermal reaction was carried out in a conventional oven at 433 K from 2 to 48 h.To remove the organic template,the membranes were calcined in a muffle furnace at 773 K for 10 h with heating and cooling rate of 0.5 K·min−1,and then stored at 393 K in an oven before gas separation test.
1.3 Characterization and separation measurements
The morphology of high-silica SSZ-13 seeds and membranes were viewed with a field emission scanning electron microscopy(FE-SEM,Hitachi SU8020)at acceleration voltages from 3 to 10 kV,and all samples were coated by Pt before ion sputtering.The crystal phase was identified by X-ray diffraction(XRD,Rigaku UltimaⅣ)using CuKαradiation(λ=0.154 06 nm)operated at 40 kV and 40 mA in air with 2θat 5°~45°and a step size of 0.05°.The Fourier transform infrared spectrometry(FTIR,BRUKER TENSORⅡ)in the wavenumber range of 4 000~400 cm−1were recorded and the concentration of the solid sample in the KBr pellets was kept constant at 0.01 gram per gram KBr,it was accumulated with 256 scans to obtain the IR spectra.
Single-gas permeation of SSZ-13 membranes was tested with a dead-end and retentate stream blocked system without sweep gas according to our previous study[13,15].The synthesized membranes were mounted in a stainless steel module and sealed at each end with stainless steel rings and silicone O-rings in turn,and the effective separation area of membrane was about 19 cm2.The ideal selectivity of membrane is the ratio of two single-gas permeances,and each data point was obtained at stabilization status with changes in permeances lower than 3%in an hour.
2 Results and discussion
2.1 Characterization of SSZ-13 seeds
High-silica SSZ-13 seeds were synthesized and calcined before characterization.The XRD patterns of SSZ-13 seeds and seeded support are shown in Fig.1a.The characteristic peaks of SSZ-13 seeds are corresponding to CHA structure without other phase and indicated the pure CHA phase of seeds.The average size of SSZ-13 seed crystals was about 1µm viewed on SEM images as Fig.1b,which is larger than our previous study[13]and matches the pore size of mullite supports better.The Si/Al ratio(nSi/nAl)of seeds was 77 by inductively coupled plasma(ICP),which is much higher than our previous study[13].SSZ-13 seeds were rubcoated on the outer surface of tubular mullite supports by fingers,and the SEM images of seeded supports were shown in Fig.1c and 1d.
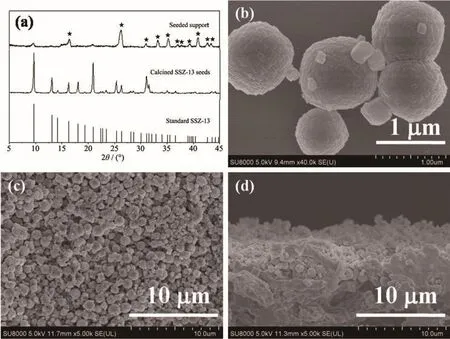
Fig.1 (a)XRD patterns of standard SSZ-13,calcined SSZ-13 seeds and the seeded support and SEM images of(b)SSZ-13 seeds,(c)surface view and(d)cross-section of seeded support
2.2 Characterization of SSZ-13 membranes
Fig.2 shows the XRD patterns of high-silica SSZ-13 membranes prepared with various synthesis times at 433 K.No characteristic CHA peaks appeared on XRD patterns after 2 and 4 h hydrothermal synthesis,indicating that membrane layer had not yet formed on supports.Characteristic CHA peaks appeared until 6 h crystallization,indicating that the SSZ-13 membrane layer had formed and 6 h was the lower time limit for SSZ-13 membrane preparation.The intensity of typical CHA peaks increased with the increase of synthesis time except 72 h due to crystals dissolution,suggesting the crystallinity of SSZ-13 crystals composed of the membrane increased as well.The Si/Al ratio of the SSZ-13 crystals on membranes surface was 36 according to EDX analysis and higher than our previous study[13].
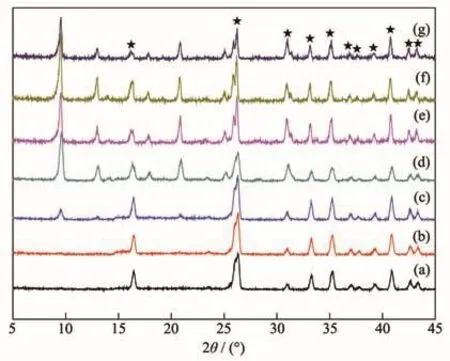
Fig.2 XRD patterns of SSZ-13 membranes prepared with synthesis times of(a)2 h,(b)4 h,(c)6 h,(d)12 h,(e)24 h,(f)48 h,and(g)72 h
The surface morphologies of SSZ-13 membranes prepared with various synthesis times are shown in Fig.3.The bared mullite supports with no membrane layer except of amorphous residue after 2 and 4 h hydrothermal synthesis in Fig.3a and 3b,respectively.After 6 h synthesis,the mass of SSZ-13 crystals verified by XRD were distributed on the outside surface of the support,noted that some tiny crystals dispersed.Spherical and cubic crystals packed loosely on the surface of mullite supports as in Fig.3c and 3d.More crystals packed and intergrowth coexisted on the surface of supports from 24 to 72 h as shown in Fig.3e~g.The densest membrane was prepared for 48 h crystallization in Fig.3f but it′s ideal selectivity of CO2/CH4was lower than the membrane prepared for 72 h in Fig.3g.
Fig.4 shows the cross-sectional SEM images of membranes prepared with various synthesis times at 433 K.Generally,the thickness of membrane increased as the crystallization time.The membranes synthesized for 2 and 4 h almost have no membrane layer,and the 6 and 12 h membranes have a quite thin layer.The thickness of membrane synthesized for 24,48 and 72 h are about 5,6 and 12µm,respectively.
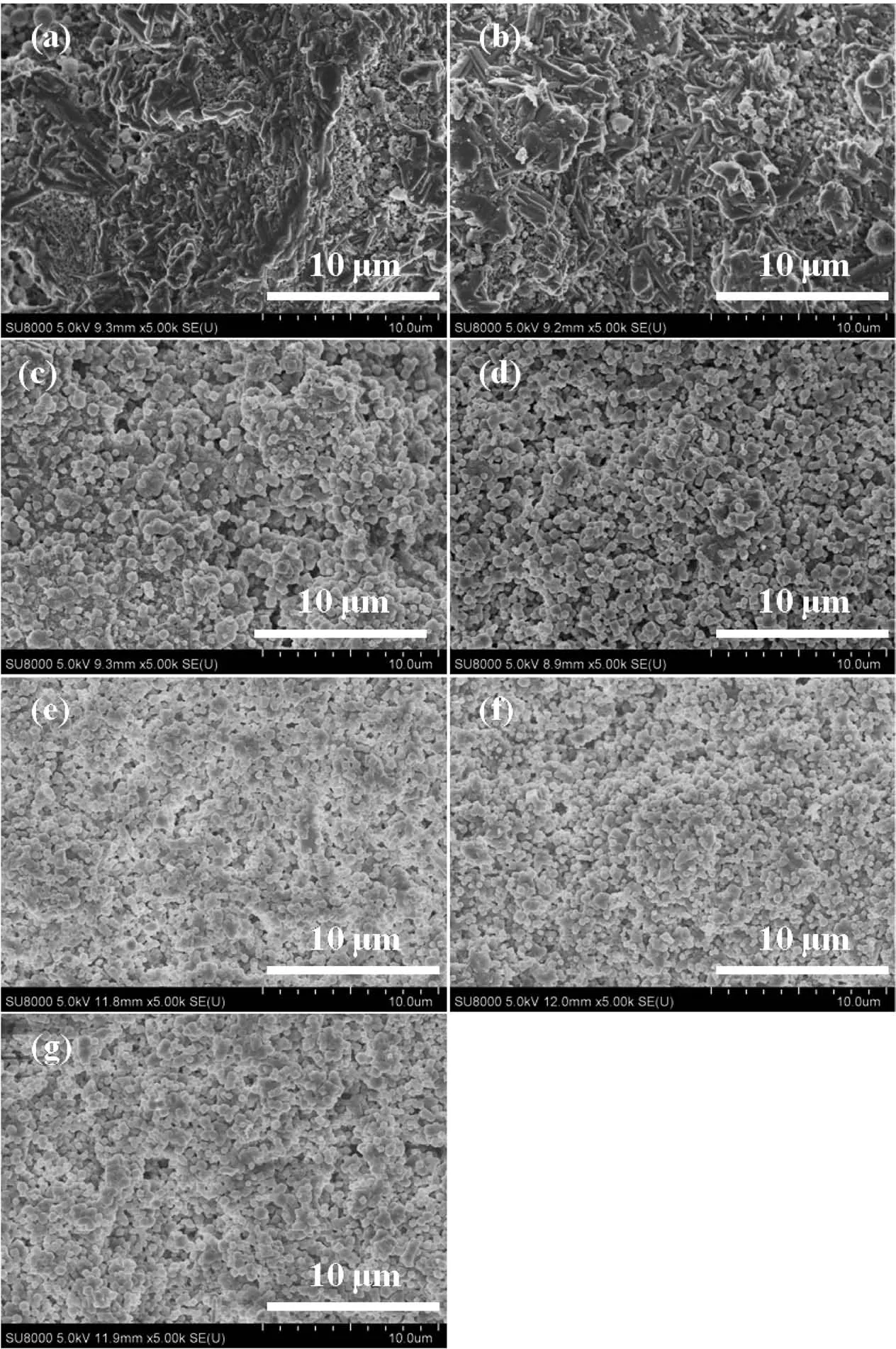
Fig.3 Surface SEM images of membranes prepared for(a)2 h,(b)4 h,(c)6 h,(d)12 h,(e)24 h,(f)48 h and(g)72 h
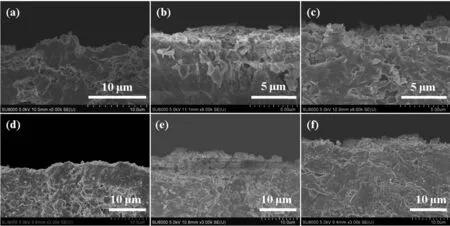
Fig.4 Cross-sectional SEM images of membranes prepared for(a)4 h,(b)6 h,(c)12 h,(d)24 h,(e)48 h and(f)72 h
2.3 Characterization of powders crystallized together with the membrane
Fig.5 shows the XRD patterns of powders crystallized together with the high-silica SSZ-13 membranes.After the membrane synthesis,the powers from the bottom of autoclave were collected,dried and characterized.Similar to the SSZ-13 membranes,no characteristic CHA peaks appeared after 2 and 4 h crystallization.Only after 6 h crystallization,the typical peaks of powders were corresponded to CHA phase.The crystallinity of powders increased with the synthesis time from 6 to 48 h except for 72 h,which was consistent with the SSZ-13 membranes.
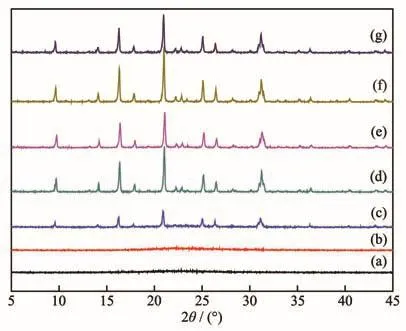
Fig.5 XRD patterns of powders crystallized together with the SSZ-13 membranes with synthesis time of(a)2 h,(b)4 h,(c)6 h,(d)12 h,(e)24 h,(f)48 h,and(g)72 h
The morphologies of powders crystallized together with the SSZ-13 membranes are shown in Fig.6.After 2 and 4 h synthesis,no SSZ-13 crystals and only amorphous was observed by SEM images.Some spherical or cubic SSZ-13 crystals(verified by XRD patterns)appeared along with amorphous in the powders after 6 h crystallization.
From 6 to 72 h crystallization,the largest SSZ-13 crystals in the powders almost kept the same level in size.Considering the XRD patterns of powders(Fig.5)do not change between 12 and 72 h of synthesis time,it might mean that the largest size CHA crystals was reached after 12 h and then one should not even expect further increase of crystal size.
From the XRD patterns(Fig.5)and SEM images(Fig.6)of the powders crystallized together with the SSZ-13 membranes,a phenomenon was noted that SSZ-13 crystals could be observed in the powder only after 6 h crystallization,indicating a drastic change occurred to generate SSZ-13 crystals in the hydrogel during the first 6 hour.As well-known,SSZ-13 zeolite is CHA topology,andd6runit is critical to form the CHA structure,which accords to an IR band at 637 cm−1in the spectra[30-31].Fig.7 shows the IR spectra of powders crystallized together with the SSZ-13 membranes from 2 to 12 h crystallization.An IR band corresponding tod6runits appeared after 6 h crystallization.
2.4 Single gas permeation
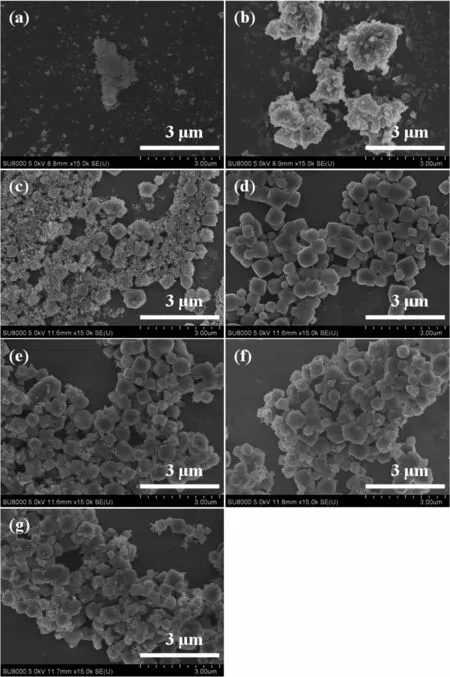
Fig.6 SEM images of powders crystallized together with the SSZ-13 membranes prepared for(a)2 h,(b)4 h,(c)6 h,(d)12 h,(e)24 h,(f)48 h and(g)72 h
CO2and CH4single-gas permeance through the SSZ-13 membranes at 298 K and 0.4 MPa pressure drop are shown in Table 1.The permeance of CO2and CH4decreased and the CO2/CH4selectivity increased with the delaying of crystallization time,which resulted from the increased depth for prolonged synthesis time.The ideal selectivity of CO2/CH4of M7 was 24 and the CO2permeance was 2.08×10−7mol·m−2·s−1·Pa−1,which was higher than the best membrane in our previous work[13].
2.5 Growth process of SSZ-13 membrane
It is generally acknowledged that rubbing seeds onto supports could embellish the defects and roughness of the surface,and a high quality seeding layer is benefit for growing of defect-free membranes[32].But according to our experimental results,the seeds rubbedon supports dissolved and left from the support before the SSZ-13 zeolite layer had grown on because of the weak and unstable Van der Waals′force.Hence,we deduced rubbing seeds embellish the surface of supports was temporary,and the induction effect of nucleation to accelerate the growth of membrane was pivotal for the membrane preparation.
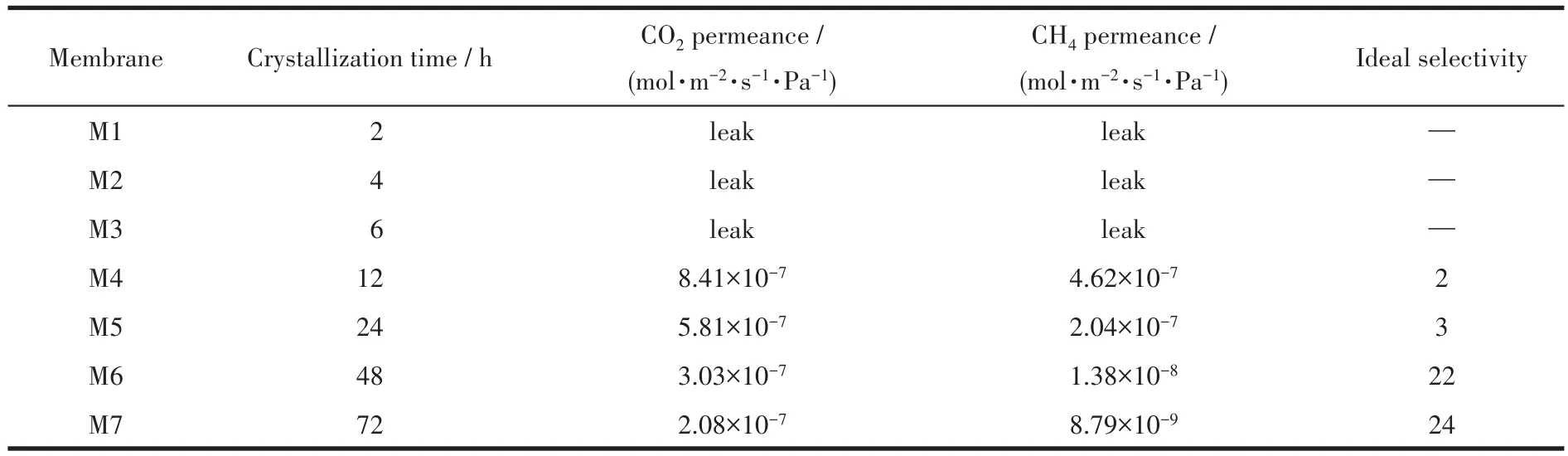
Table 1 Selectivities of CO2/CH4of SSZ-13 membranes at 298 K and pressure drop of 0.4 MPa
The growth of the SSZ-13 zeolite on the mullite supports(as membrane layer)and the powder together with the membrane(as SSZ-13 zeolite)was competing.The SSZ-13 seed crystals rubbed on the mullite supports dissolved in the gel and induced a rapid formation of orderly structure units.Due to concentration gradients and interfacial effects,the orderly structure units deposited on the surface of supports in priority.The SSZ-13 zeolite crystals in the gel were grown to the maximization in quantity and size without interfering with each other for adequate mass transfer.This was the reason why the powders collected at the autoclave bottom had almost no difference in size by SEM images in Fig.6.In contrast to the growth of crystals in the gel,a different situation occurred on the surface of support.The limited space on the surface of supports compelled the SSZ-13 zeolites intergrowth,when the crystals number and scale reached a certain level,a dense and defect-free membrane layer with high gas separation performance was obtained as in Fig.8.
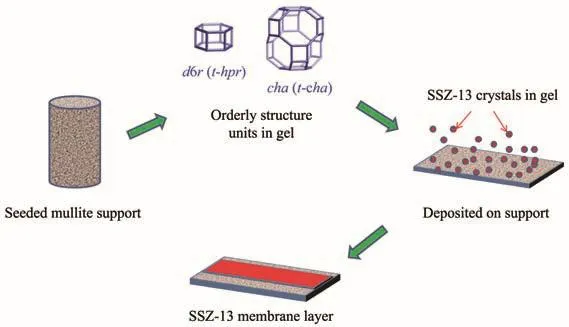
Fig.8 Growth process of SSZ-13 membrane by hydrothermal secondary growth
3 Conclusions
High-silica SSZ-13 membranes were prepared by secondary growth method with diluted organic template concentration gel on mullite supports.The membrane and powders together with the membranes simultaneously corresponded to CHA phase after 6 h of hydrothermal synthesis at 433 K.The crystalline of zeolite crystals increased with the crystallization time until 48 h,but the size of powder together with the membranes kept the same level due to nutrient exhausted in solution.The membrane prepared at 433 K and 72 h had a CO2permeance of 2.08×10−7mol·m−2·s−1·Pa−1with an ideal selectivity of CO2/CH4of 24 at 298 K and 0.4 MPa.The possible growth process of SSZ-13 membrane was as follows:firstly,the seed crystals dissolved in solution and induced to nucleation and small crystals;then,the SSZ-13 zeolite crystals grown on the surface of support,had to intergrowth for limited space,and formed a highly selective membrane with dense and fewer defects.
Acknowledgements:We gratefully acknowledge the financial support of this work from National Natural Science Foundation of China(Grants No.21968009,21476099,21766010 and 21868012),International Science,Technology Cooperation Program of China(Grant No.2015DFA50190),Jiangxi Provincial Department of Science and Technology (Grant No.20171BCB24005).