Syntheses, structures and magnetic properties of two 2,4-dichlorobenzoate-based samarium coordination polymers tuned by 4,4′-bipyridine
WANG Cen-Ru,LIU Wen-Hua,XU Shuang,ZHANG Ju-Wen
(College of Chemistry and Chemical Engineering,Bohai University,Jinzhou 121013,Liaoning,China)
Abstract:By reacting 2,4-dichlorobenzoic acid (2,4-HDCB) with Sm(NO3)3·6H2O and NaOH, the lanthanide-organic coordination polymer (CP) [Sm(2,4-DCB)3(CH3CH2OH)2] (1) has been solvothermally synthesized. When the second ligand 4,4′-bipyridine (4,4′-bpy) was introduced, the new lanthanide-organic CP [Sm2(2,4-DCB)6(4,4′-bpy)(H2O)2] (2) was obtained. CPs complexes 1 and 2 have been characterized by single-crystal X-ray diffraction, IR, PXRD and TG analyses. In complex 1, the Sm(III) ions are bridged by the 2,4-DCB anions to form a one-dimensional (1D) chain. In complex 2, two Sm(III) ions are linked by six 2,4-DCB anions to form a binuclear Sm2(2,4-DCB)6 unit, and then the 4,4′-bpy co-ligands connect the Sm2(2,4-DCB)6 units to generate a 1D chain. The magnetic properties of complexes 1 and 2 were investigated.
Key words:samarium;2,4-dichlorobenzoic acid;4,4′-bipyridine;magnetic property
In recent years, the synthesis of lanthanide-organic complexes is one of the hottest fields in coordination chemistry and crystal engineering due to their unique properties including magnetism[1-4], catalysis[5-7], gas adsorption[8-10]and luminescence[11-13]. Lanthanide-organic complexes have distinctive magnetic properties due to the large magnetic moment and magnetic anisotropy of the lanthanide ions[14]. On the other hand, a large number of lanthanide-organic complexes have been prepared by considering some factors such as the variable coordination behavior of lanthanide ions, the flexibility and function of organic ligands. Organic carboxylic acid ligands are frequently chosen to construct lanthanide-organic complexes considering their rich coordination modes[15-16].
Dichlorobenzoic acids, as a kind of organic carboxylic acid ligands, have been widely used to construct lanthanide carboxylate complexes. In particular, the dichlorobenzoic acid has halogen substituents, which can effectively change the steric hindrance of the carboxylic acid, and can be capable of linking several metal centers through different coordination modes[17]. For example, five zero-dimensional (0D) lanthanide dichlorobenzate complexes {[Ln2(2,6-DCB)6(H2O)8]·2H2O} (Ln = Ce, Pr, Nd, Tb, Dy, 2,6-HDCB = 2,6-dichlorobenzoic acid) have been reported by Brzyska and co-workers[18]. A 1D lanthanide dichlorobenzate complex [Dy(3,5-DCB)3(EtOH)] (3,5-HDCB=3,5-dichlorobenzoic acid) has been reported by Zheng and co-works[19], and its magnetic properties have been investigated. However, to date, no research group has used 2,4-dichlorobenzoic acid (2,4-HDCB) to synthesize lanthanide dichlorobenzoate complexes. Furthermore, some 0D 2,4-dichlorobenzoate-based lanthanide complexes including the second ligands have been reported by Zhang and co-workers[20-24]. However, 4,4′-bipyridine (4,4′-bpy) as the bridging linker has not been introduced into the above systems, which may expand the dimension of the complexes compared with the terminal ligands. Therefore, we synthesized successfully a lanthanide 2,4-dichlorobenzoate complex [Sm(2,4-DCB)3(CH3CH2OH)2] (1) by reacting Sm(NO3)3·6H2O with 2,4-HDCB and NaOH. When 4,4′-bpy was introduced, a new 2,4-dichlorobenzoate-based lanthanide complex [Sm2(2,4-DCB)6(4,4′-bpy)(H2O)2] (2) was obtained. At the same time, we investigated the magnetic properties of1and2.
1 Experimental
1.1 Materials and measurements
All raw materials were commercially available and were used as received. The IR data were obtained with KBr pellets by using a Magna FT-IR 560 spectrophotometer. Powder X-ray diffraction (PXRD) data were collected on a Bruker AXS D8-Advanced diffractometer using Cu Kα radiation (λ=0.154 06 nm). Thermogravimetric analyses (TGA) were performed on a Pyris-Diamond thermal instrument from 25 to 900 ℃ under N2atmosphere at a heating rate of 10 ℃ min-1. Magnetic measurement was made on a Quantum Design MPMS XL-7 SQUID-VSM magnetometer.
1.2 Synthesis of complex 1
A mixture of Sm(NO3)3·6H2O (0.2 mmol), 2,4-dichlorobenzoic acid (0.6 mmol), water solution of NaOH (6 mL, 0.1 mol·L-1), H2O (4 mL) and ethanol (0.5 mL) was sealed in a Teflon-lined stainless steel vessel (25 mL) and heated at 150 ℃ for 96 hours under autogenous pressure. Colorless needle-shaped crystal was obtained. IR (KBr, cm-1): 3 568 (w), 3 553 (w), 3 433 (m), 2 374 (w), 2 345 (w), 1 589 (s), 1 572 (s), 1 547 (s), 1 474 (w), 1 458 (w), 1 416 (s), 1 379 (m), 1 155 (w), 1 140 (w), 1 103 (w), 1 051 (w), 870 (m), 839 (w), 546 (m).
1.3 Synthesis of complex 2
A mixture of Sm(NO3)3·6H2O (0.2 mmol), 2,4-dichlorobenzoic acid (0.6 mmol), 4,4′-bpy (0.3 mmol), water solution of NaOH (6 mL, 0.1 mol·L-1), H2O (4 mL) and ethanol (0.5 mL) was sealed in a 25 mL Teflon-lined stainless steel vessel and heated at 150 ℃ for 96 hours. Colorless block-shaped crystal was collected. IR (KBr, cm-1): 3 422 (m), 3 288 (m), 3 086 (w), 2 972 (w), 2 378 (w), 2 345 (w), 2 308 (w), 1 604 (s), 1 587 (s), 1 541 (m), 1 490 (w), 1 408 (s), 1 228 (w), 1 103 (w), 1 053 (w), 868 (m), 783 (w), 669 (w), 542 (w).
1.4 X-ray crystallography
Single-crystal X-ray diffraction data of1and2were collected on a Bruker Smart APEX II diffractometer equipped with Mo Kα radiation (λ= 0.071 073 nm). The structures of1and2were solved by direct methods and refined onF2by the full-matrix least squares using the SHELXTL[25]. Selected crystallographic data and refinement parameters for1and2are given in Table 1. Selected bond lengths for1and2are given in Table 2. CCDC 1965256 and 1965265 contain the supplementary crystallographic data for1and2. These data can be obtained for free from The Cambridge Crystallographic Data Centre via www. ccdc.cam.ac.uk/data_request/cif.

Table 1 Crystallographic data and refinement parameters for 1 and 2
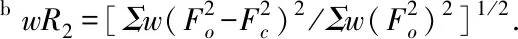
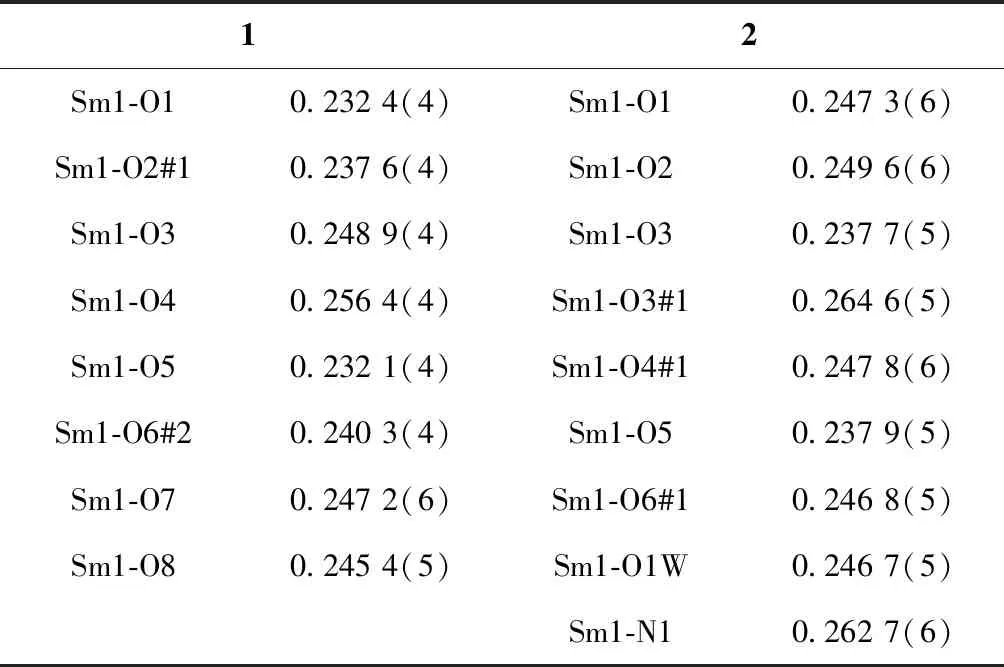
Table 2 Selected bond lengths (nm) for 1 and 2
Symmetry codes: for 1: #1 -x + 1, -y + 1, -z. #2 -x + 2, -y + 1, -z. For 2: #1 -x, -y +2, -z + 3.
2 Results and discussion
2.1 Crystal structure of complex 1
The asymmetric unit of1contains one Sm(III) ion, three 2,4-DCB anions and two CH3CH2OH molecules (Fig.1(a)). The Sm(III) ion is eight-coordinated by six oxygen atoms from five 2,4-DCB anions and two oxygen atoms from two CH3CH2OH molecules, featuring a distorted square-antiprism coordination geometry (Fig.1(b)), in which four corners of the upper plane are occupied by O1, O2#1, O4, O7 and four corners of the lower plane are occupied by O3, O5, O6#2, O8. The Sm-O bond lengths vary from 0.232 1(4) to 0.256 4(4) nm (Table 2). In1, two types of coordination modes a and b for the 2,4-DCB anion are observed (Fig.2). The Sm(III) ions are bridged by the 2,4-DCB anions with the coordination mode b to form a 1D chain (Fig.1(c)).
2.2 Crystal structure of complex 2
The asymmetric unit of2contains one Sm(III) ion, three 2,4-DCB anions, half a 4,4′-bpy co-ligand and one water molecule (Fig.3(a)). The Sm(III) ion is coordinated by eight oxygen atoms from five 2,4-DCB anions and one water molecule as well as one nitrogen atom from one 4,4′-bpy co-ligand. The coordination polyhedron of Sm(III) is a muffin-shaped geometry (Fig.3(b)). The O3 atom occupies the vertex of muffin. The bottom triangle is constructed by the O1W, O4#1, N1 atoms and the equatorial pentagonal plane is constructed by the O1, O2, O3#1, O5, O6#1 atoms. The Sm-O bond lengths are in the range of 0.237 7(5) to 0.264 6(5) nm and the Sm-N bond length is 0.262 7(6) nm (Table 2). In2, the 2,4-DCB anion shows three types of coordination modes (a, b, and c) (Fig.2). The 2,4-DCB anions bridge two Sm(III) ions by the coordination modes b and c into a binuclear Sm2(2,4-DCB)6unit with a Sm…Sm distance of 0.403 69(6) nm (Fig.3(a)). The neighboring Sm2(2,4-DCB)6units are linked by the 4,4′-bpy co-ligands into a 1D chain (Fig.3(c)). The adjacent 1D chains are further connected by the intermolecular hydrogen bonding interactions to form a two-dimensional (2D) supramolecular layer (Fig.3(d)).

Fig.2 Three types of coordination modes of 2,4-DCB with Sm(III) in 1 and 2 ( for 1: a and b, for 2: a, b and c)
Complex2was prepared under the similar reaction conditions with1except the introduction of 4,4′-bpy. Complex2shows a completely distinct structure from1.Meanwhile, the structure of2is also different from those of the 2,4-dichlorobenzoate-based lanthanide complexes reported by Zhang and co-workers[20-24]. Therefore, the 4,4′-bpy co-ligand has an important influence on the formation and structure of2.
2.3 PXRD and thermal analyses
The experimental and simulated PXRD patterns of1and2are illustrated in Fig.4. The experimental PXRD pattern of2is consistent with the simulated pattern based on the single-crystal data of2, indicating the phase purity of the as-synthesized product. However, the experimental PXRD pattern of1is slightly different from the simulated PXRD pattern based on the single-crystal data of1.This may be attributed to the release of the solvent molecules of the as-synthesized product for1in air before preforming PXRD measurement. Similar behavior was also observed in the previous work[26-28].
The TG curves of1and2are shown in Fig.5. CP1exhibits two main weight losses. The weight loss of 2.33% at the first step is much lower than the theoretical value of 11.32%. This further implies that the as-synthesized product for1has lost the solvent molecules before preforming TG measurement. The second weight loss is attributed to the decomposition of 2,4-DCB. CP2shows three main weight losses. The first weight loss corresponds to the release of the solvent molecules, and the next two weight losses are assigned to the decomposition of two kinds of organic ligands.
2.4 Magnetic properties
The temperature-dependent direct current (dc) magnetic susceptibilities of1and2were measured under an applied magnetic field of 1 000 Oe at 2-300 K. As shown in Fig.6, at 300 K, the χmT values of 0.123 (1) and 0.124 (2) emu mol-1K are slightly higher than the expected value of 0.09 emu mol-1K for one spin-only Sm(III) ion[29]. Upon cooling, the χmT values for1and2decrease linearly to the minimum values of 0.003 (1) and 0.003 (2) emu mol-1K at 2 K, respectively. For1and2, the decrease of χmT may be attributed to either the antiferromagnetic interactions between the Sm(III) ions or the thermal depopulation of stark excited energy levels of Sm(III)[30-31].

Fig.3 (a) Coordination environment of the Sm(III) ion in 2. All hydrogen atoms have been omitted for clarity. Symmetry code: #1 -x, -y +2, -z + 3. (b) Coordination geometry of the Sm(III) ion in 2. (c) 1D chain of 2. (d) 2D supramolecular layer of 2
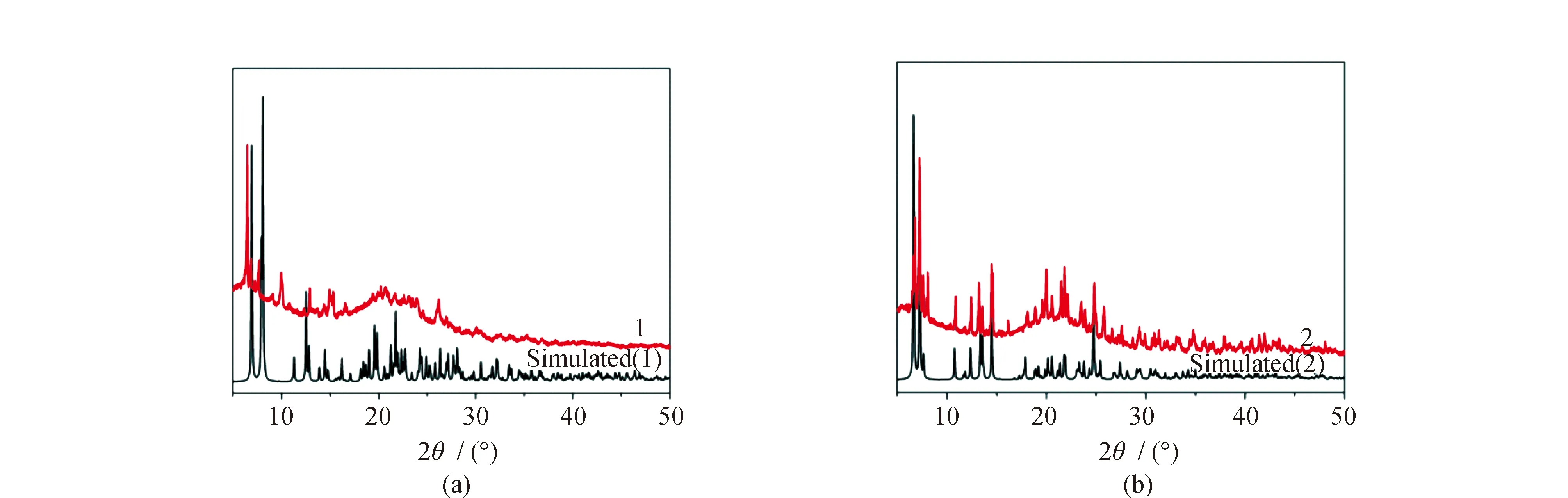
Fig.4 Experimental and simulated PXRD patterns of 1 (a) and 2 (b)
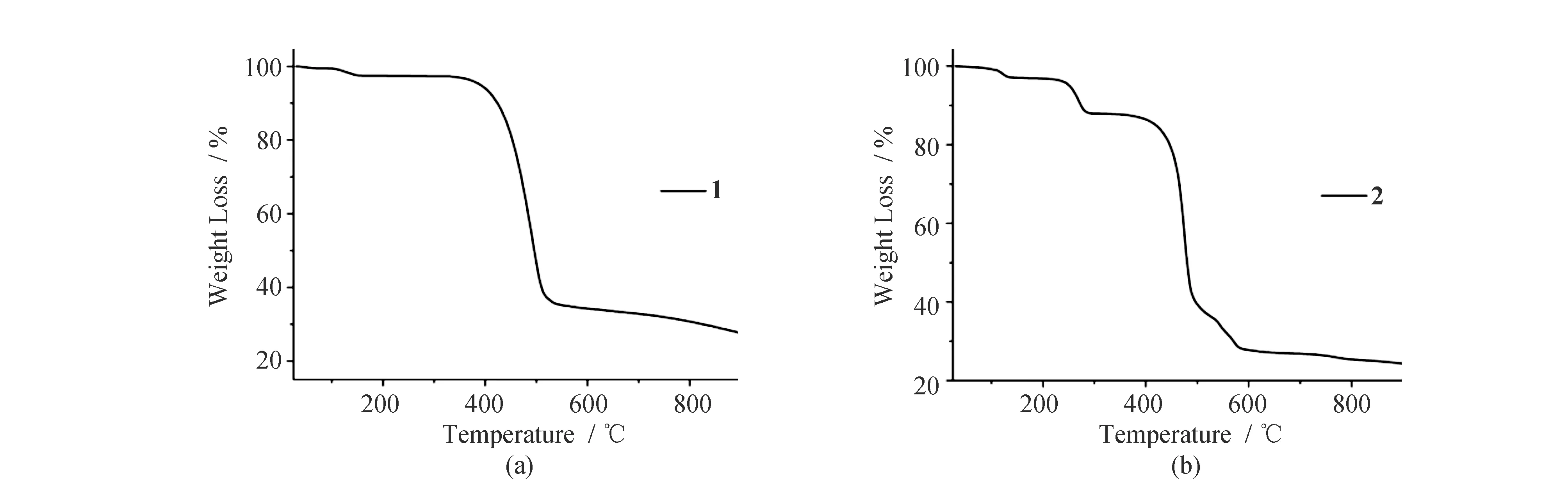
Fig.5 TG curves of 1 (a) and 2 (b)
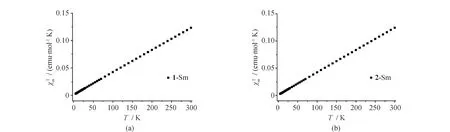
Fig.6 Temperature dependence of χmT for 1 (a) and 2 (b)
3 Conclusions
Two 2,4-dichlorobenzoate-based samarium CPs modulated by 4,4′-bpy have been successfully prepared under solvothermal conditions. The introduction of the second ligand has a significant effect on the formation and structures of these two 2,4-dichlorobenzoate-based samarium CPs. This work provides a useful example for the construction of dichlorobenzoate-based lanthanide complexes by introducing the second ligand.

