Perioperative chemotherapy for advanced gastric cancer-results from a tertiary-care hospital in Germany
Katrin Bauer,Giulia Manzini,Doris Henne-Bruns,Peter Buechler
Katrin Bauer,Peter Buechler,Department for General,Visceral,Thoracic and Paediatric Surgery,Clinic of Kempten,Kempten 87439,Germany
Giulia Manzini,Doris Henne-Bruns,Department of General and Visceral Surgery,University Hospital of Ulm,Ulm 89081,Germany
Abstract
Key words:Gastric cancer;Perioperative;Chemotherapy;Tumour localisation;Age distribution;Validity
INTRODUCTION
In contrast to Asian countries,where prophylactic gastroscopy is implemented in the national healthcare system due to the high incidence of gastric cancer,gastric cancer in European countries is often only detected in an advanced stage due to lateappearing symptoms.Administration of perioperative chemotherapy has been recommended in the European guidelines to improve the prognosis of adenocarcinomas of the stomach and the gastro-oesophageal junction from stage >T2/N+ for many years[1-3].Mainly patients younger than 75 years were included in the randomized controlled trials (RCTs),which build the basis for these guideline recommendations (in Germany,Great Britain,and Europe).Therefore,there is no convincing evidence concerning the benefit of perioperative chemotherapy for elderly patients.The guidelines also do not mention the effectiveness of perioperative chemotherapy in relation to the tumour site (proximal or distal stomach).At congresses and in tumour conferences,the question of whether perioperative chemotherapy of antrum and pyloric carcinomas is just as effective as in the proximal sections of the stomach is much debated.Another unclear question is whether elderly individuals (> 75 years),who make up the majority of patients in the everyday European hospital routine,profit just as much from the recommended therapy as younger patients who are regularly included in the RCTs.These questions are of growing importance because the incidence of distal gastric tumours has decreased in the last decades,whereby proximal tumours are increasingly diagnosed.
Considering our retrospective data and the resulting survival times,it should be investigated whether the patient age and tumour location should influence the decision for a perioperative chemotherapy,and what prognostic differences exist for the treated groups.
MATERIALS AND METHODS
Patient selection
One hundred and fifty-eight patients who underwent resection of adenocarcinomas of the stomach or the gastro-oesophageal junction in our clinic between 2008 and 2016 were analysed.One hundred and twenty-nine of these patients presented with an advanced tumour stage [Union internationale contre le cancer (UICC) > Stage II].
As a tertiary-care hospital,the clinic of Kempten is certified by the German Cancer Society for the treatment of carcinomas of the stomach,colorectum,and pancreas.
The date of birth,gender,month of diagnosis,extent of the operation,application of perioperative chemotherapy,TNM-classification,and UICC-stage,including all relevant histologic criteria and eventual date of death,were recorded for each patient.
An endoscopic examination with histological confirmation of diagnosis,as well as a computed tomography of the abdomen and the thorax for staging,was performed to rule out distant metastases and to assess the preoperative tumour stage.Endosonography was not routinely performed in all patients.
Application of perioperative chemotherapy was recommended by the interdisciplinary tumour conference depending on the preoperative suspected TNM stage,the patient's general condition,and the urgency of the tumour operation (e.g.,bleeding of the tumour).According to the German guidelines,perioperative chemotherapy was usually recommended for tumours > T2 and/or N+.Upfront surgery was preferred in patients with poor performance status and/or severe comorbidity.
Subtotal gastric resection,gastrectomy,expanded gastrectomy,transhiatal distal oesophageal resection (Merendino),or abdomino-thoracal oesophageal resection with gastric endo-sleeve was performed,depending on the tumour site and the Lauren classification.A D2-lymphadenectomy was the standard procedure.
In order to analyse comparable groups of patients regarding the TNM-Status,we excluded the patients of the surgery-only group with the postoperative stadium of pT1/N0 and pT2/N0.Therefore,from an initial 158 patients with curatively-resected gastric cancer,129 patients with advanced tumour stages remained.
Two young patients of the surgery-only group,in whom the preoperative diagnostic tools had led to an under-staging,received adjuvant chemotherapy.
Perioperative chemotherapy was administered according to the epirubicin,oxaliplatin,and capecitabine schema from 2008 to 2014:Each 3-wk cycle consisted of epirubicin (50 mg/m2) intravenously on d 1,oxaliplatin (130 mg/m2) intravenously on d 1,and capecitabine (625 mg/m2,twice/d) orally administered from d 1 to d 21.
From 2014,the 5-FU,oxaliplatin,und docetaxel (FLOT) schema was used:5-floururacil (2600 mg/m2) + natriumfolinat (200 mg/m2) intravenously on d 1,oxaliplatin (85 mg/m2) intravenously on d 1,and docetaxel (50 mg/m2) intravenously on d 1.
Both the epirubicin,oxaliplatin,and capecitabine schema,and the FLOT schema consisted of three preoperative cycles and three postoperative cycles of chemotherapy,so for this study the term perioperative chemotherapy is used.
It was not necessary to obtain a decision by the Ethics Commission for this retrospective analysis of our internal hospital data according to a consultation with the Federal Medical Association.
Statistical analysis
Values are presented as the mean ± SD and median (range) for continuous variables.Dichotomic variables are presented as absolute number as well as percent.A twosidedPvalue < 0.05 was considered statistically significant.Survival curves were obtained using the Kaplan-Meier method according to chemotherapy (yes or no),localisation of the gastric tumour (proximal or distal),and age (more or less 75 years).Additionally,a subgroup survival analysis was performed in order to investigate the role of chemotherapy selectively on both distal and proximal gastric cancer.Missing values were < 5% in the dataset,and no imputation strategies were used.All calculations were conducted using R Project for Statistical Computing (The R Foundation,Version 3.1.0,Vienna,Austria).All patients have been followed up for at least 24 mo.
RESULTS
Fifty-three of the 129 above-mentioned patients (41%) were 75 years of age or older when diagnosed.Forty-five of all patients (35%) received perioperative chemotherapy.Patient characteristics are listed in Table 1.A tumour located in the cardia,fundus,or the gastro-oesophageal junction (AEG) was defined as proximal,and a tumour in the pylorus,antrum,or corpus was defined as distal.
Table2 shows the operative procedures undertaken on the 129 patients with gastric adenocarcinoma and the histological results.Seventeen patients with intraoperativedetected liver metastasis and/or peritoneal tumour spread (UICC IV) and macroscopic R0-resection were included in the analysis.
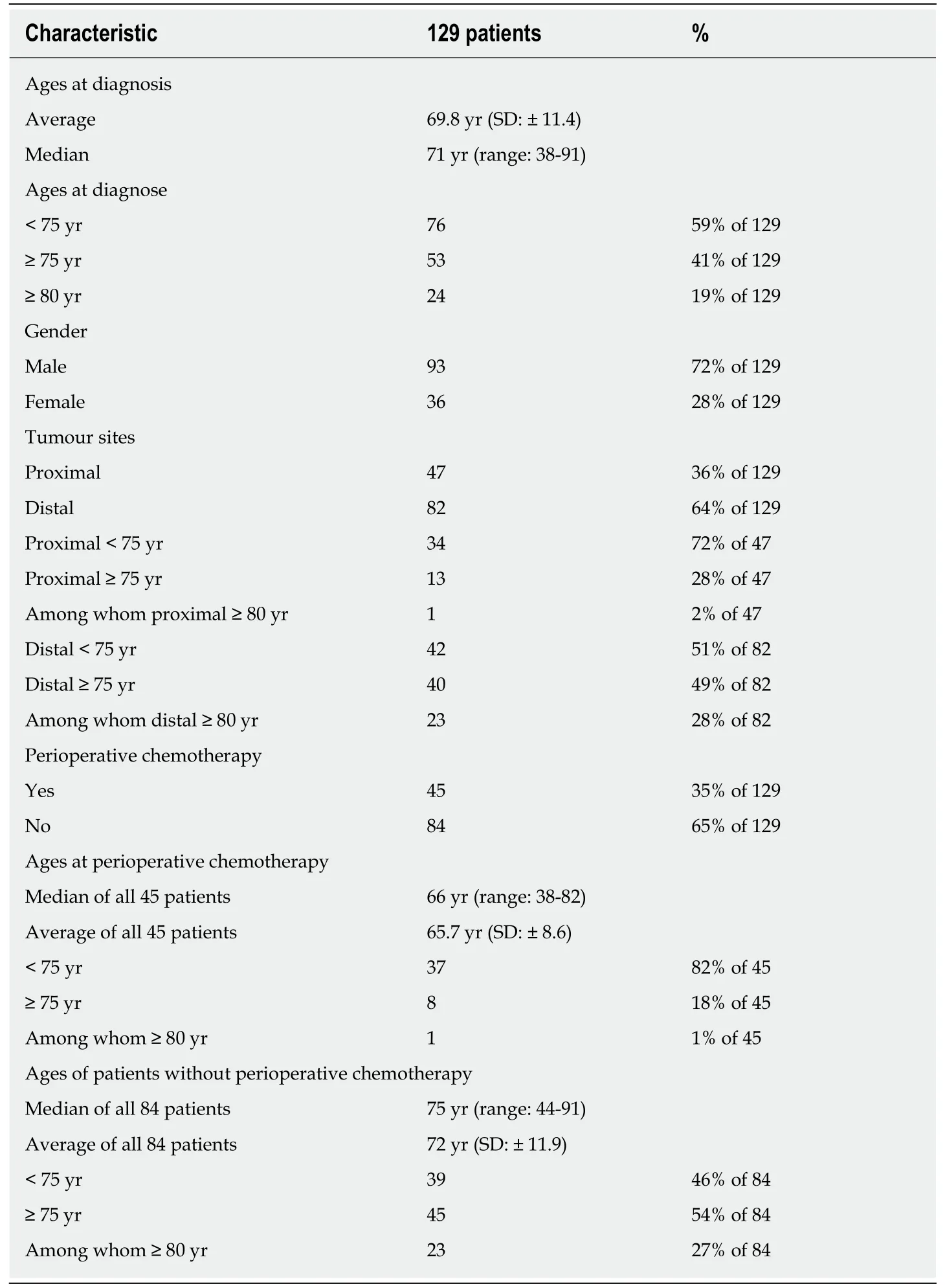
Table1 Patient characteristics before therapy
The part of patients who received perioperative chemotherapy demonstrates a clear dependence on tumour location.The percentage of pre-treated patients increased from 10% for distal tumours (pylorus,antrum,and distal corpus) to 78% for tumours located in the gastro-oesophageal junction (AEG I/II),which required a two-cavity intervention.Merendino's procedure constituted an exception,and was usually only offered to patients who,due to their physical constitution,did not qualify for a twocavity procedure.
On the basis of the recorded data,survival curves were obtained with the Kaplan-Meier method.Figure 1A shows the effects of perioperative chemotherapy,regardless of tumour localisation.Although the survival interval seemed to be prolonged for the whole group of patients receiving preoperative chemotherapy,a statistically significant difference (P= 0.125) could not be detected.The 5-year survival rate was 33% for all patients (42 of 129);for the group of patients who received perioperative chemotherapy,the 5-year survival rate was 40% (18 of 45 patients) and 29% for the group without perioperative chemotherapy (24 of 84 patients).
Further,we evaluated whether the tumour localization,independently of perioperative chemotherapy,determined the prognosis.Survival did not differ significantly between the patient group with a proximalvsthat with a distalcarcinoma (P= 0.782).
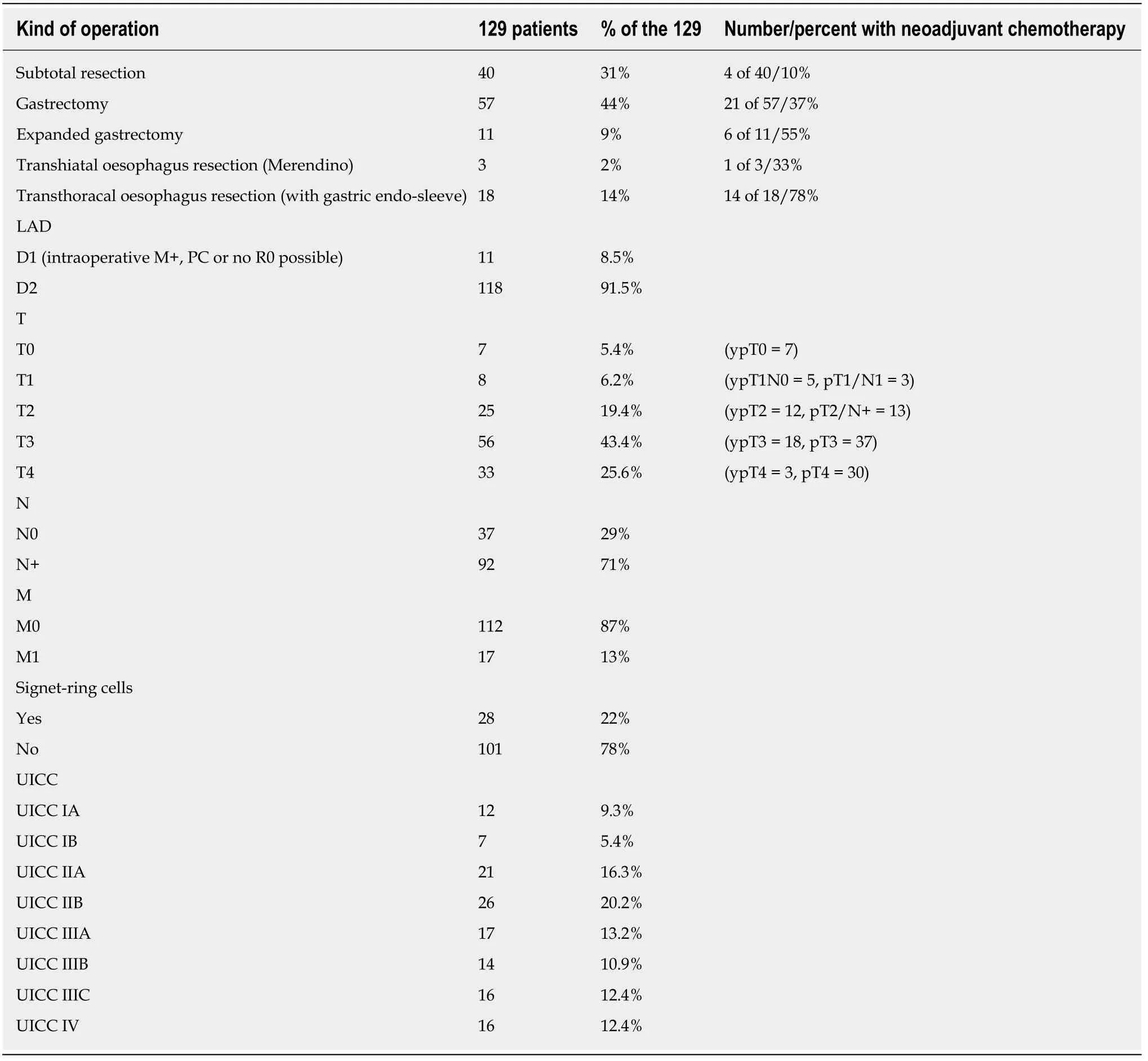
Table2 Postoperative patient characteristics
In subgroups,we examined whether there were differences in outcomes between the patient groups with distal and with proximal tumours depending on the administration of perioperative chemotherapy.There was no significant difference in median survival between the patients (Figure 1B) with proximal tumours who had received perioperative chemotherapy and those who had not (P= 0.614).
The subgroup analysis also showed no significant difference in survival times in the patients with distal tumours (Figure 1C) who received perioperative chemotherapy and those who did not (P= 0.134).
Finally,we examined if there was a difference in survival time between the elderly patients subgroup (≥ 75 years) and the younger ones (< 75 years).Figure 1D shows no significant difference in survival (P= 0.855) regarding the age of the patient.
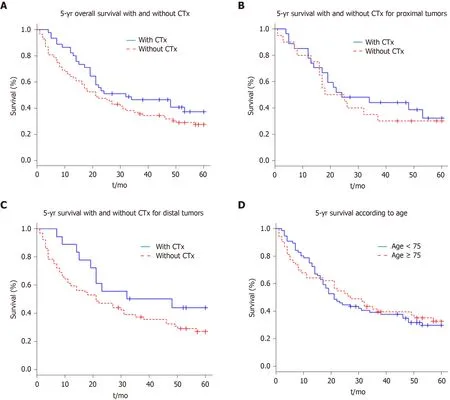
Figure1 Kaplan-Meier survival curve.
Summarizing the results from our retrospective analysis,we found that perioperative chemotherapy does not significantly improve survival.In detail,we could observe that:(1) The patients with gastric carcinoma in our hospital in Kempten(Germany) are on average (70 years) older than the patients in the RCTs (4,5,6) that analysed the effectiveness of perioperative chemotherapy for adenocarcinoma of the stomach;(2) Elderly patients (≥ 75 years) receive perioperative chemotherapy far less often than younger patients with the same preoperatively-determined tumour stage;(3) The group with distal,non-pre-treated gastric carcinoma contained an aboveaverage number of elderly patients (≥ 75 years of age);(4) The incidence of proximal gastric carcinomas decreases with increasing age of the patient from 72% in the patients group younger than 75 years to 28% in the patients group older than 75 years;(5) Our hospital-intern tumour conference recommends perioperative chemotherapy significantly more often for patients with proximal tumours (e.g.,from 10% for carcinomas of the pylorus up to 78% for carcinomas of the gastroesophageal junction);(6) The 5-year survival time of patients with distal tumours (36%) did not significantly differ from that of patients with proximal tumours (33%),regardless of whether they had received perioperative chemotherapy or not;and (7) The subgroup analysis(distal or proximal tumours) also showed no significant difference in survival times for patients with or without perioperative chemotherapy and for elder and younger patients.
DISCUSSION
Based on the results of some RCTs[4-6],perioperative/neoadjuvant chemotherapy has been recommended in the German guidelines for advanced adenocarcinoma of the stomach and the gastro-oesophageal junction zone[1]since 2010.Perioperative chemoradiotherapy (AEG I) is also recommended in the revised S3 guidelines for tumours >T2 and/or N+.The guideline does not comment on the patient's age or on tumour localisation.
Our former analysis[7-9]showed that the few RCTs that examined the effectiveness of perioperative chemotherapy for advanced gastric cancer had some grave shortcomings in their validity.We concluded that perioperative chemotherapy cannot be generally recommended for advanced gastric cancer based on these RTCs.In addition,part of our working group analysed the validity of RCTs on the subject of adjuvant chemotherapy after resecting gastric cancer[10].Several of the RCTs also showed substantial deficits in their validity.
We therefore wanted to examine the effectiveness of perioperative chemotherapy in a tertiary-care hospital,which is certified by the German Cancer Society as an oncosurgical centre for the treatment of carcinomas of the stomach,the colon/rectum,and the pancreas.Patients with advanced gastric cancer have been treated with perioperative chemotherapy in Kempten,Germany since 2007 according to the national guidelines.The analysis of our patients,which was conducted and evaluated over 9 years (2008-2016),showed that perioperative chemotherapy produced no significant advantage in the 5-year survival time:40% for the perioperative chemotherapy group and 29% for the surgery-only group (P= 0.125).Cunninghamet al[4]report a 5-year survival rate of 36% for the perioperative chemotherapy groupvs23% for the surgery-only group;Ychouet al[6]could detect a 5-year survival rate of 38% for the perioperative chemotherapy groupvs24% for the surgery-only group.Compared to the two RCTs of Cunninghamet al[4]and Ychouet al[6],which had built the basis of the guideline recommendations,the patients of our analysis had a slightly better 5-year survival time.This might be caused by the high D2-lymphadenectomy rate of about 91.5% compared with 41% in the study of Cunninghamet al[4].In our analysis,we excluded the postoperative staged pT1/N0 and pT2/N0-tumours of the surgery-only group to avoid a positive effect on the 5-year survival rate of these patients.Cunninghamet al[4]included 16 patients (8.3% of 193 surgery-only patients)with a pT1 stadium,and 55 patients of the same group with pT2 (28.5% of 193).Unfortunately,the N-stage is reported for only 291 of the 503 patients (57.8%) and the TNM stage is not mentioned.
A recent Asian study from Eomet al[11]matched 43 patients who were treated with perioperative chemotherapy (S-1 and docetaxel) and 86 patients who received surgery only.The neoadjuvant group had a significantly higher 5-year overall survival rate(73.3%vs51.1%,P= 0.005) and a trend towards a higher 5-year progression-free survival rate (62.8%vs49.9%,P= 0.145).The authors concluded that perioperative chemotherapy was associated with better long-term survival without increasing postoperative complications in the setting of D2 surgery,suggesting that perioperative chemotherapy can also be a therapeutic option in East Asian countries.
In 2018,Kanajiet al[12]published a review that summarizes recent evidence of the benefits of (neo-)adjuvant therapy for locally advanced gastric cancer according to the tumour stage and the histological subtype in Asia,the United States and Europe.They concluded that FLOT can be considered the new standard care in perioperative treatment for European patients with resectable gastric and gastroesophageal junction adenocarcinoma.In Asia,the perioperative chemotherapy combination S1 and oxaliplatin is considered to reduce both hematogenous and peritoneal recurrence for Stage III adenocarcinoma,while for bulky lymph node metastasis and scirrhous carcinomas,additional neoadjuvant chemotherapy with S1/cisplatin followed by postadjuvant treatment with S1 is not recommended.
The subgroup analysis,in which patients with distal and proximal tumours were separately evaluated,also showed no significant difference in survival between the preoperatively-treated patients and those who only underwent resection.
Nevertheless,in our hospital,perioperative chemotherapy is offered to patients with proximal tumours far more often by our tumour conference.This may be explained on one side by the persisting opinion that chemotherapy is less effective for distal carcinomas,and by the fact that the patient group with proximal tumours is the younger one.Indeed,Ychouet al[6]were able to detect a significant treatment effect of perioperative chemotherapy exclusively for the patient group with tumours of the gastroesophageal junction.
Fifty-three of 129 patients (41%) with gastric cancer in our hospital were older than 75 years at the time of diagnosis.These patients are not represented in most of the RCTs in relation to the effectiveness and tolerance of perioperative chemotherapy for advanced gastric cancer.The MAGIC trial by Cunninghamet al[4]had no age limit in its inclusion criteria,while the two other large studies on perioperative therapy for gastric cancer by Schuhmacheret al[5]and Ychouet al[6]were limited to patients younger than 75 years.The median age of patients in our hospital was 71 years,and thus consequently our population was distinctly older that the ones that were included in the large RCTs on which guidelines are based.Patients included in the MAGIC trial by Cunninghamet al[4]had a median age of 57 years (range:23 to 85);patients in the EORTC study by Schuhmacheret al[5]had a median age of 57 years(range:26 to 70);patients in the ACCORD study by Ychouet al[6]had a median age of 63 years (range:36 to 75).
Since our group of patients with gastric cancer contained an above-average number of patients older than 75 with distal carcinomas and without application of perioperative chemotherapy,we expected a worse prognosis for this patient group.Only 8 patients (18%) who received perioperative chemotherapy were older than 75 years and only one patient (1%) was over 80 years.These were of course biologically fit patients.Surprisingly,the age of the patient had no influence on the median survival.In contrast to our data,Ciesielskiet al[13]could detect an extremely high mortality rate after a successful gastrectomy for cancer in elderly patients.
Our data show that treatment of advanced gastric cancer still needs further improvement and reflects the aggressive biology of gastric cancer.All patients seem to have a similar bad prognosis regardless of age,tumour localisation,and tentative donation of perioperative chemotherapy.It is still necessary to continue research on the detection of tools that help to identify the patients that really benefit from perioperative chemotherapy and those who do not.
A limitation of our study is the small sample size,consequently a Beta error could be present,and power could not be enough to detect the effect of chemotherapy on survival.For subgroup analysis concerning the effectiveness of the selective application of chemotherapy in distal and proximal carcinomas depending on patient age,the different groups would have been too small to receive a significant result.However,the data reflect the reality in German hospitals.In the last three years,between 30 and 45 patients were annually treated operatively in our department by two surgeons.This provides much more homogenous treatment for our patients,compared to the 503 patients of the MAGIC study[4]who were treated by 129 surgeons from four continents over a period of 8 years without a standardized operative procedure and a D2-lymphadenectomy rate of only 41%.There are studies,such as pilot studies and RCTs,that offer a smaller sample size than our analysis[5,14-16].Our study should be considered a basis to investigate the role of chemotherapy in a setting of RCT in the future.
Another limitation is the acquisition of retrospective data.In contrast to a randomized controlled study where patient characteristics should be well balanced in the different groups,in a retrospective study a selection bias concerning the decision whether to recommend perioperative chemotherapy or not cannot be excluded.It is likely that an interdisciplinary tumour conference prefers upfront surgery in the case of patients with poor performance status or comorbidity.This would signify that the surgery-only group probably had a poorer prognosis per se,and would support the result that even on the basis of two slightly different groups of patients,there was no statistically significant difference in the five-year survival.
On the basis of the present analysis of patients from a German tertiary-care hospital certified for gastric cancer treatment by the German Cancer Society,we have to conclude that the effectiveness of perioperative chemotherapy in advanced gastric cancer referring to the prolongation of survival has to be challenged again.
Consequently,our previous statements demonstrating that the quality of the existing RCTs is not sufficient to justify perioperative chemotherapy in patients with advanced gastric cancer could be confirmed by this study.
ARTICLE HIGHLIGHTS
Research background
For ten years,European guidelines have recom mended perioperative chemotherapy for advanced gastric cancer.The recommendation is based on a few randomized controlled trials(RCTs) of poor validity.Decisions regarding therapy often differ between selected patients included in an RCT and elderly patients with comorbidities in regular healthcare.Oftentimes,latter patients have to break up perioperative chemotherapy because of adverse effects.Considering the increasing incidence of proximal gastric cancer and the fact that gastric cancer is one of the most frequent reasons of tumour-associated deaths worldwide,it is particularly important to study this topi c.
Research motivation
Guidelines should be applicable for daily patient treatment,but European guidelines do not mention tumour localization nor the age of the patient.In order to find out which patients will have a real benefit from perioperative chemotherapy,we wanted to analyse the effect of perioperative chemotherapy in patients from our clinic with respect to tumour localization and age.This is important to save resources and to protect patients who will not benefit from perioperative chemotherapy from experiencing adverse effects.
Research objectives
The aim of this study was to analyse the efficacy of perioperative chemotherapy in our total patient population as well as in subgroups with respect to tumour localization and age.
Research methods
Patient characteristics before and after therapy of every resected patient with advanced gastric adenocarcinoma between 2008 and 2016 were added to our database.Survival curves were obtained using the Kaplan-Meier method according to chemotherapy (yes or no),localisation of the gastric tumour (proximal or distal) as well as age (more or less than 75 years).
Research results
Administration of perioperative chemotherapy did not lead to a significant survival advantage in our study population.Thus,our research could not confirm the data of RCTs,which are the basis of the European guidelines.
Research conclusions
Fifty-three patients of the above-mentioned 129 (41%) were 75 years of age or older when diagnosed.The lack of a significant survival benefit due to perioperative chemotherapy was independent of tumour localization and age.Gastric cancer is not very sensitive to chemotherapy.Therefore,all efforts have to be done to detect it earlier or to identify tumour characteristics whose treatment offers more personalized medicine.The treatment of advanced gastric cancer differs substantially in different parts of the world.Individual tumour stage depends on genetic diversity,prophylactic gastroscopy,quality of surgical treatment (e.g.,D2 lymphadenectomy or not),and other differences in particular healthcare systems.The few RCTs that analysed perioperative chemotherapy in gastric cancer are known all over the world but led to different guideline recommendations on different continents.The study wanted to prove the hypothesis that perioperative chemotherapy is effective and that this efficacy is dependent on tumour localization or patient age.The retrospective analysis of our database does not provide any new methods.We found that patients treated due to advanced gastric cancer are on average much older than cohorts of RCTs on this topic.Elder patients (> 75 years) do not have worse prognoses compared to younger ones.The incidence of proximal gastric carcinomas decreases with increasing patient age.Elderly patients (≥ 75 years) receive perioperative chemotherapy far less often than younger.The 5-year survival time of patients with distal tumours did not significantly differ from that of patients with proximal tumours,regardless of whether they had received perioperative chemotherapy or not.The study could refute the hypotheses that perioperative chemotherapy is more effective in patients with proximal gastric cancer.The decision of whether or not to apply perioperative chemotherapy in future research is necessary.Until new insights arise,tumour conferences concerning the decision of perioperative chemotherapy should not be influenced by tumour localization but only by tumour stage.
Research perspectives
It is necessary to prove the applicability of guidelines to daily patient treatment,particularly if the guideline recommendations are based on few RCTs with poor validity.Stratification according to defined risk factors,such as tumour characteristics,should be introduced to identify possible responders to therapy and thereby reduce the number of unnecessary treatments,particularly because the clinical approach to oncological patients has switched from standardized to personalized medicine.For example,MSI status should be evaluated,as in recent studies it was shown that patients with mismatch repair deficiency should not be treated with perioperative chemotherapy because of severe adverse effects and missing survival benefits.Instead of new RCTs,which often fail due to the difficult recruitment of highly selected patients,we recommend analysing big cohorts of registered patients in order to better understand the real situation in a particular country.
 World Journal of Gastrointestinal Oncology2020年5期
World Journal of Gastrointestinal Oncology2020年5期
- World Journal of Gastrointestinal Oncology的其它文章
- Espresso coffee,caffeine and colon cancer
- Perineural invasion as a prognostic factor in patients with stage l-lll rectal cancer-5-year follow up
- Association of Helicobacter pylori infection with colorectal polyps and malignancy in China
- Gastric cancer with peritoneal metastases:Efficiency of standard treatment methods
- Neutropenia in colorectal cancer treated with oxaliplatin-based hyperthermic intraperitoneal chemotherapy:An observational cohort study
- Characterization and strong risk association of TLR2 del-196 to-174 polymorphism and Helicobacter pylori and their influence on mRNA expression in gastric cancer
