Characterization and strong risk association of TLR2 del-196 to-174 polymorphism and Helicobacter pylori and their influence on mRNA expression in gastric cancer
Caroline de Matos Lourenço,Manoela Dias Susi,Mariah Cristina Antunes do Nascimento,Vilson Serafim Junior,Ana Paula Simedan Vila,Gabriela Helena Rodrigues-Flemming,Eny Maria Goloni-Bertollo,Ana Elizabete Silva,Juliana Garcia de Oliveira-Cucolo
Caroline de Matos Lourenço,Manoela Dias Susi,Department of Graduate-Level Research,Sacred Heart University,Bauru,São Paulo 17011-970,Brazil
Mariah Cristina Antunes do Nascimento,Vilson Serafim Junior,Ana Paula Simedan Vila,Gabriela Helena Rodrigues-Flemming,Eny Maria Goloni-Bertollo,Juliana Garcia de Oliveira-Cucolo,Department of Molecular Biology,São José do Rio Preto School of Medicine,São José do Rio Preto,São Paulo 15090-000,Brazil
Ana Elizabete Silva,Department of Biology,São Paulo State University,São José do Rio Preto,São Paulo 15054-000,Brazil
Abstract
Key words:Toll-like receptor 2;Helicobacter pylori;Gastric cancer;Chronic gastritis;Polymorphisms;Gene expression
INTRODUCTION
The cascade of gastric carcinogenesis described by Correaet al[1]describes the role ofHelicobacter pylori(H.pylori) infection in the development of chronic gastritis (CG),considered the initial stage of tumor progression,and that ends with the development of gastric cancer (GC).Thus,H.pyloriinfection represents the main cause of CG,and infected patients have a 10-fold higher chance of developing GC[2].Thus,this bacterium is widely known as a class I carcinogen in gastric diseases[3].
Pattern recognition receptors (PRRs),including toll-like receptors (TLRs) 2 and 4,recognize different pathogen-associated molecular patterns (PAMPs) shared by most microorganisms,includingH.pylori[4].TLR2 is involved in the recognition of bacterial lipopolysaccharide (LPS);TLR2 activation results in the activation of nuclear factor-κB(NF-κB) and functions as an innate immune response,but a Th1 adaptive immune response is also triggered by binding toH.pylorineutrophil-activating protein[5].
The presence ofH.pyloridisrupts gastric mucosa homeostasis and initiates an inflammatory response,stimulating the production and secretion of proinflammatory mediators,such as interleukin (IL)-1β,IL-2,IL-6,IL-8,IL-12,and reactive oxygen and nitrogen species that cause DNA damage[6].This proinflammatory microenvironment may promote the development of precancerous lesions,such as chronic gastritis,gastric atrophy,intestinal metaplasia,and dysplasia,that eventually progress to gastric cancer[7].
TLR2polymorphisms are associated with the pathogenesis of GC,vary among different populations and ethnic groups,and can modulate the immune response toH.pyloriinfection and its persistence.For example,TLR2-196to-174 ins/del,a 22-bp deletion in the promoter region,is associated with protection against GC in the Chinese population[8,9]but has no association with gastric diseases in the Japanese population[10,11].However,studies in the Caucasian population demonstrated an increased risk for gastric cancer[12]and other types of cancer,such as breast[13],colorectal[14],and head and neck cancer[15].
ForTLR2 19216T/C(rs3804099),a synonymous variant located on chromosome 4,it is not yet clear which allele (CorT) is associated with the susceptibility to disease.Some studies in the Asian population have indicated a protective association between theTCorCCgenotype and different types of cancer,such as colorectal[16],breast[17],and hepatocellular carcinoma[18].Conversely,in the Russian population,theCCgenotype was closely associated with a risk of severe coronary atherosclerosis[19].
Thus,given the contradictory results and genetic heterogeneity of the Brazilian population,it is important to evaluate the role of these polymorphisms in the susceptibility to gastric carcinogenesis.In addition,we evaluated the influence ofTLR2polymorphisms andH.pyloriinfection onTLR2mRNA expression.Our findings showed that theTLR2-196 to-174ins/delandTLR2 19216T/Cpolymorphisms are associated with an increased risk of and protection against gastric cancer development,respectively.TLR2mRNA expression levels were upregulated in gastric cancer tissues and were influenced by both the presence ofH.pyloriand variant genotypes.
MATERIALS AND METHODS
Ethics statement
The Research Ethics Committee of Universidade do Sagrado Coração (USC) in Bauru,São Paulo,Brazil approved this study (Registration Number 382.514),and written informed consent for the collection of biological material (peripheral blood and gastric tissues) was obtained from all individuals.
Subjects and samples
This was a case-control study on CG and GC patients and healthy individuals.DNA was obtained from a total of 852 peripheral blood leukocyte or gastric tissue samples and genotyped forTLR2polymorphisms [TLR2 del-196to-174(rs111200466) andTLR2 19216T/C(rs3804099)].All samples included in this study were obtained from patients with gastric complaints who underwent an upper digestive endoscopy between January 2010 and March 2016 in the Gastroenterology Department,State Hospital of Bauru,São Paulo,Southeastern Region,Brazil.Patients treated with antibiotics,anti-inflammatory agents,chemotherapy drugs,radiotherapy or proton pump inhibitors within 30 days before endoscopy were not included in the study.
The case groups included 269 patients (123 men and 146 women;147H.pyloripositive and 122H.pylori-negative;mean age,50.89 ± 23.03 years) with a confirmed histopathological diagnosis of CG per the Sidney System[20]and 202 patients (152 men and 50 women;86H.pylori-positive and 116H.pylori-negative;mean age,66.26 ±16.32 years) with a confirmed histopathological diagnosis of GC per Lauren's classification[21].The gastric disease-free control group (C) consisted of 381 patients(176 men and 205 women;mean age,51.26 ± 16.77 years) who underwent endoscopy by medical indication,gave up gastric biopsy exclusively for this study,and were histopathologically confirmed to be negative for any gastric disease and forH.pyloriinfection by a trained professional of Sacred Heart University-Bauru-SP following the hospital standard (Table 1).
H.pyloriinfection was histologically established by Giemsa staining or the urease test performed by the Pathology Services of the State Hospital of Bauru,and the results were subsequently confirmed using PCR,as described in a previous study[22].
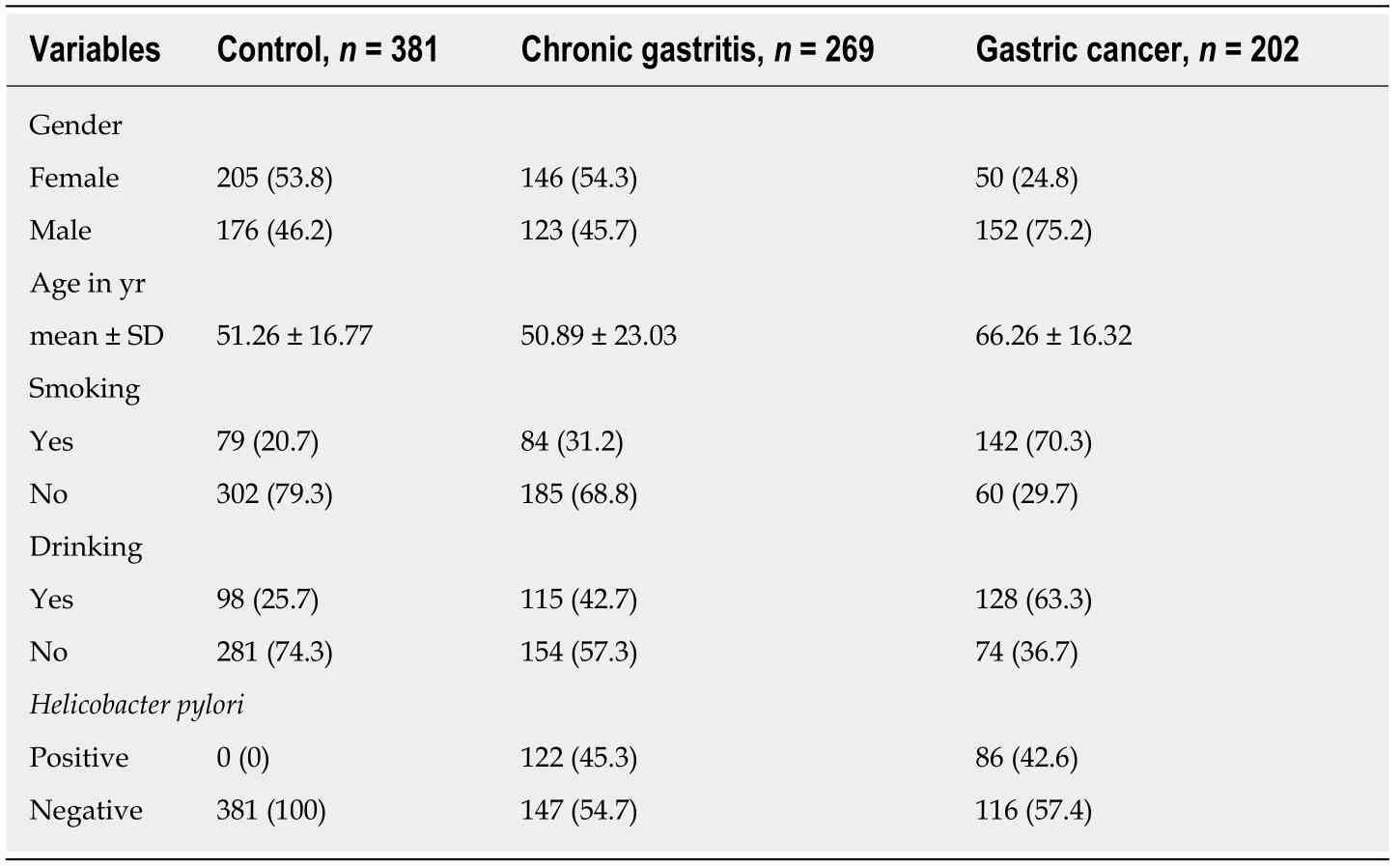
Table1 Epidemiological data of individuals with normal gastric mucosa (control group,chronic gastritis,and gastric cancer patients),n (%)
In addition,to quantifyTLR2mRNA levels,biopsies were collected during the endoscopic evaluation (gastric antrum and corpus regions) from 48 patients (29 men and 19 women;mean age,53.10 ± 9.41 years;16H.pylori-positive and 32H.pylorinegative) in the CG group,36 patients (25 men and 11 women;mean age,62.32 ± 14.66 years;21H.pylori-positive and 15H.pylori-negative) in the GC group,and 14 individuals in the C group who were free of gastric cancer andH.pyloriinfection (9 men and 5 women;mean age,49.58 ± 21.01 years).
Polymorphism genotyping
DNA was extracted from peripheral blood following a previously published protocol[23]with modifications (using Ficoll-Paque™ PLUS to separate blood components),while total RNA and DNA were simultaneously extracted from tissue samples using the QIAamp®tissue kit (Qiagen,Germany) according to the reagent protocol and stored at-20 °C.
TheTLR2-196 to-174 delpolymorphism (rs111200466) was detected by allelespecific PCR,and restriction fragment length polymorphism (RFLP)-PCR was used to assess theTLR2 19216 T/C(rs3804099) polymorphism.For both PCR techniques,the reaction solution contained the following:1 × buffer,15.3 μL of ultrapure H2O,2.0 μL(0.10 μmol/L) of dNTPs,0.5 μL (25 mmol/L) of MgCl2,1.25 μL of each primer (25 mmol/L),0.2 μL (1 U) ofTaqDNA polymerase,and 200 ng of genomic DNA.The amplification products of theTLR2-196 to-174 delanalysis and the digestion products of theTLR2 19216 T/Canalysis were visualized on a 3% agarose-1000 gel (Invitrogen®)with ethidium bromide in the presence of a 100 bp molecular marker.To ensure greater genotyping reliability,a positive control (a heterozygous sample for the evaluated polymorphism) was included in all reactions.Approximately 10% of the samples were processed in duplicate for quality control purposes.Table 2 summarizes the location of both polymorphisms,minor allele frequency (MAF),PCR conditions,primer sets,and enzymes used in each assay.
RNA extraction,reverse transcription,and real-time quantitative PCR
Total RNA was extracted using an RNeasy Mini Kit (Qiagen,Germany) according to the manufacturer's protocol.RNA concentration and quality were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific,United States) and a Bioanalyzer (Agilent,United States).A reverse transcription reaction was performed using a High Capacity cDNA kit (Applied Biosystems,Foster City,CA,United States)according to the protocol instructions.
Quantitative PCR was performed with aTaqMan®System (Life Technologies,United States) and the StepOne Plus Real-Time PCR system 2.2.3 (Applied Biosystems,United States) using aTaqMan probe specific for the TLR2 gene(Hs00610101_m1) and two reference genes,GUSB (Hs00187320_m1) and TBP(Hs00187332_m1).The reactions were performed in triplicate and included a negative control.Relative quantification (RQ) was performed using the 2-ΔΔCtmethod[24]after normalization to both reference genes,and 14 normalH.pylori-negative gastric tissue samples (C group) were used as a calibrator (RQ = 1.0).RQ was also performed forthe samples stratified by polymorphism genotype (at least one polymorphic allele vs wild-type homozygote) andH.pyloriinfection (negativevspositive).The data were expressed as median values.
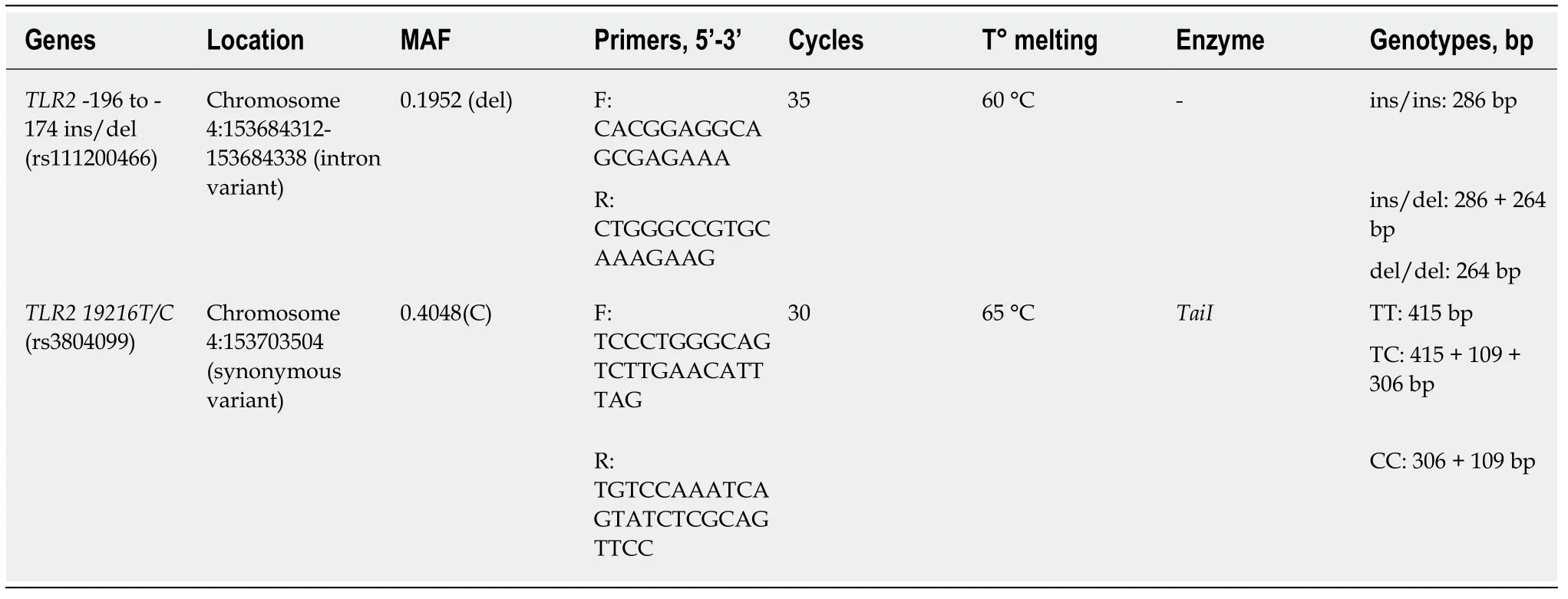
Table2 Nucleotide primer sequences,PCR conditions,and minor allele frequency for polymorphisms TLR2-196 to -174 ins/del(rs111200466) and TLR2 19216T/C (rs3804099)
Statistical analysis
SNPStats software was used to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the risk associations between polymorphisms and gastric diseases.The multiple logistic regression models were adjusted for age,gender,andH.pyloriinfection.The effect of the polymorphisms was evaluated in the models as (1)codominant (heterozygousvswild-type homozygous,and polymorphic homozygousvswild-type homozygous);(2) dominant (heterozygous + polymorphic homozygousvswild-type homozygous);(3) recessive (polymorphic homozygousvswild-type homozygous + heterozygous);(4) overdominant (heterozygousvswild-type homozygous + polymorphic homozygous);or (5) log-additive (polymorphic homozygous with 2 + heterozygousvswild-type homozygous).
The RQ values forTLR2mRNA were statistically analyzed.The continuous data distribution was evaluated using the D'Agostino-Pearson omnibus test for normality.The Mann-Whitney test and Wilcoxon's signed rank test were used for comparisons between groups (GC,CG and C) to analyze the influence ofH.pyloriinfection and polymorphisms onTLR2mRNA expression.Statistical analyses were performed using GraphPad Prism 5 software and SNPstats online tool (https://www.snpstats.net/start.htm).A probability level (P) of < 0.05 was considered to indicate statistical significance.MAF and Hardy-Weinberg equilibrium were evaluated using OEGE software[25].
RESULTS
TLR2-196 to-174 ins/del and TLR2 19216T/C polymorphisms,and risk of gastric lesions and H.pylori infection
The genotype and allele frequency distributions of the two polymorphisms complied with Hardy-Weinberg equilibrium in both the case and control groups (data not shown).The genotype frequencies of theTLR2-196 to-174 ins/delandTLR2 19216 T/Cpolymorphisms in all three groups (GCvsC,CGvsC,and GCvsCG) are shown in Table 3.
TLR2-196 to-174 del was associated with a higher risk of GC by comparison with the C group in the codominant [odds ratio (OR) = 3.70,95%CI:2.41-5.70 for TLR2-196 to-174 ins/del;OR = 5.73,95%CI:1.80-18.21 for TLR2-196 to-174 del/del;P< 0.0001],dominant (OR = 3.87,95%CI:2.55-5.86;P< 0.0001),recessive (OR = 4.00,95%CI:1.27-12.62;P= 0.0130),overdominant (OR = 3.44,95%CI:2.24-5.27;P< 0.0001),and logadditive models (OR = 3.23,95%CI:2.23-4.69;P< 0.0001).Similarly,this polymorphism was associated with an increased risk when comparing the GC and CG groups in the codominant (OR = 2.68,95%CI:1.71-4.20 for TLR2-196 to-174 ins/del;OR = 5.06,95%CI:1.45-17.70 for TLR2-196 to-174 del/del;P < 0.0001),dominant (OR= 2.84,95%CI:1.84-4.39;P< 0.0001),overdominant (OR = 2.49,95%CI:1.59-3.88;P<0.0001),recessive (OR = 3.77,95%CI:1.08-13.12;P= 0.0260),and log-additive models(OR = 2.54,95%CI:1.72-3.74;P< 0.0001).However,no association was found with this polymorphism in the comparison of the CG and C groups (Table 3).
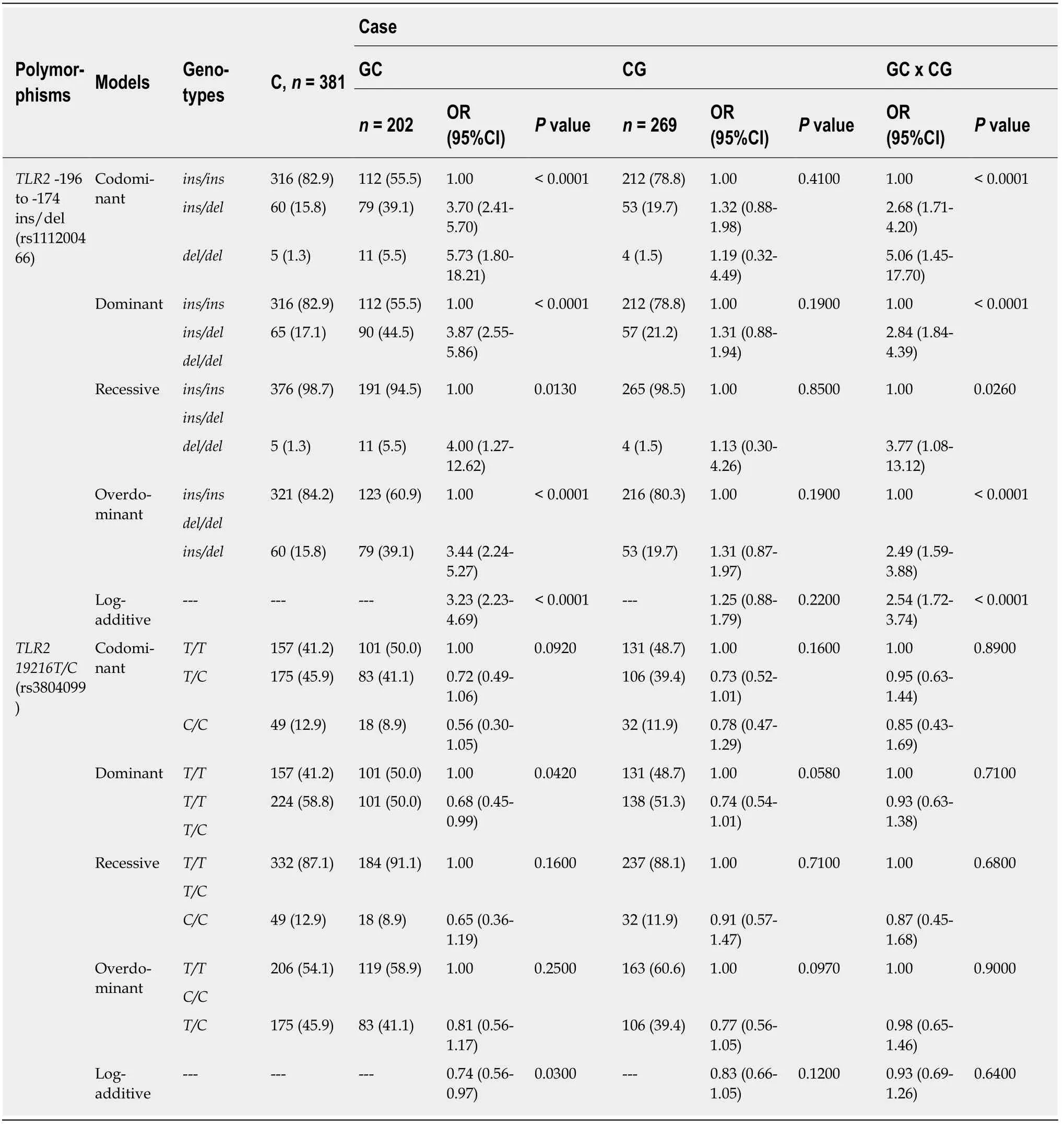
Table3 Genotype frequencies of TLR2-196 to -174 ins/del and TLR2 19216 T/C polymorphisms in both case and control groups (GC vs C,CG vs C and GC vs CG) ,n (%)
TLR2 19216T/Cwas associated with a protective effect against GC development compared to the C group by the dominant (OR = 0.68,95%CI:0.47-0.99;P= 0.0420)and log-additive models (OR = 0.74,95%CI:0.56-0.97;P= 0.0300).However,no association was found for the CGvsC and CGvsGC comparisons (Table 3).
Both polymorphisms were also investigated to evaluate their association withH.pyloriinfection.Thus,all the samples,including those in the case and control groups,were divided intoH.pylori-negative cases (n= 619,73%) andH.pylori-positive cases(n= 233,27%).For the TLR2-196 to-174 ins/del polymorphism,an association was observed withH.pylori-positive individuals in the codominant (OR = 1.55,95%CI:1.09-2.19 for TLR2-196 to-174 ins/del;OR = 2.48,95%CI:1.01-6.08 for TLR2-196 to-174 del/del;P= 0.0120),dominant (OR = 1.62,95%CI:1.16-2.27;P= 0.0051),overdominant (OR = 1.50,95%CI:1.06-2.12;P= 0.0240),and log-additive models (OR= 1.56,95%CI:1.17-2.08;P= 0.0030).In contrast,theTLR2 19216T/Cpolymorphism was associated with protection againstH.pyloriinfection in the codominant (OR =0.60,95%CI:0.44-0.83 forTLR2 19216T/C;OR = 0.58,95%CI:0.35-0.98 forTLR2 19216C/C;P= 0.0039),dominant (OR = 0.60,95%CI:0.44-0.81;P< 0.0001);overdominant (OR = 0.67,95%CI:0.49-0.91;P= 0.0097),and log-additive models (OR= 0.70,95%CI:0.55-0.88;P= 0.0021) (Table 4).
TLR2 mRNA expression in gastric lesions is influenced by H.pylori infection
The relative expression ofTLR2mRNA in the GC and CG groups is shown in Figure 1.We observed significantly increasedTLR2mRNA expression in the GC group(median RQ = 6.95) compared to CG group (median RQ = 0.84,P< 0.0001) and C group (RQ = 1;P< 0.0001).However,we did not find a statistically significant difference between the CG and C groups (P= 0.9387).
Another analysis was performed to determine whetherH.pyloriinfection influencesTLR2mRNA levels.Samples from the GC and CG groups were separated according to the presence or absence ofH.pyloriinfection and compared.The results showed significantly higherTLR2mRNA expression inH.pylori-positive cases(median RQ = 6.38) than inH.pylori-negative cases (median RQ = 0.79;P< 0.0001;Figure 2).We observed significantly increasedTLR2mRNA expression in theH.pylori-positive cases group compared to C group (median RQ = 1,P< 0.0001),but not betweenH.pylori-negative casesvsC group (RQ = 1;P= 0.4305).
Stratification of TLR2 polymorphisms and their influence on mRNA expression in gastric cancer and chronic gastritis
To evaluate the influence of theTLR2-196to-174 ins/delandTLR2 19216T/Cpolymorphisms on mRNA expression,the samples were grouped based on the presence of at least one polymorphic allele or a wild-type genotype (Table 5 and Figure 3).
In the GC group,individuals with at least one polymorphicTLR2-196to-174 delallele had greater than four-fold higherTLR2mRNA expression in tumor tissue(median RQ = 8.74) than those with the wild-type genotype (median RQ = 2.58,P=0.0010).In contrast,carriers ofTLR2-19216TC + CC polymorphic variants showed reduced expression (median RQ = 3.63) compared to those harboring the wild-typeTLR2-19216 TTallele (median RQ = 18.54,P= 0.0004).In the CG group,when the individuals were grouped asTLR2-196 to-174 ins/del+del/delorTLR2-19216 TC + CCpolymorphic allele carriers and homozygous wild-type allele carriers,the differences between the groups were not statistically significant (P= 0.5334 andP= 0.8827,respectively).
DISCUSSION
In our analyses,we demonstrated an association between theTLR2-196 to-174 ins/delandTLR2 19216 T/C polymorphisms and gastric cancer (an increased risk and a protective effect,respectively),as well as greater susceptibility toH.pyloripresence.Infection of the gastric mucosa byH.pylorileads to increased inflammation that contributes to cancer development,and factors that promote an exacerbated immune response may interfere with this process[26].
Regarding theTLR2-196to-174 ins/del(rs111200466) polymorphism,two metaanalyses have shown no association with gastric cancer risk,but in the Chinese population,an increased risk for gastric carcinogenesis was reported inH.pyloriinfected individuals,reinforcing the importance of this microorganism in disease pathogenesis[27,28].In contrast,a recent study in a southern Chinese population showed a risk association for gastric cancer;however,no association withH.pyloriinfection was observed[8].
Other studies have reported a risk association between theTLR2-196to-174 delvariant and head and neck cancer[15,29],cervical cancer in the Tunisian population[30],breast cancer in the Greek population[13],and prostate[31]and bladder cancer in the Indian population[32].In the Brazilian population,previous studies demonstrated a risk association for gastric and colorectal cancer[14,33].
For TLR219216T/C(rs3804099),our results demonstrated a protective association between theTCandCCgenotypes and gastric cancer.This protective association has been previously reported for some types of cancer,such as colorectal,breast,gastric,and hepatocellular carcinoma,but all these studies were in the Asian population[16-18,34].Two recent studies evaluated this polymorphism in the Thai population but failed todemonstrate any association with the development of gastric lesions or withH.pyloriinfection[35,36].Thus,our study shows this protective association ofTLR2 19216T/Cwith gastric cancer in the Brazilian population.
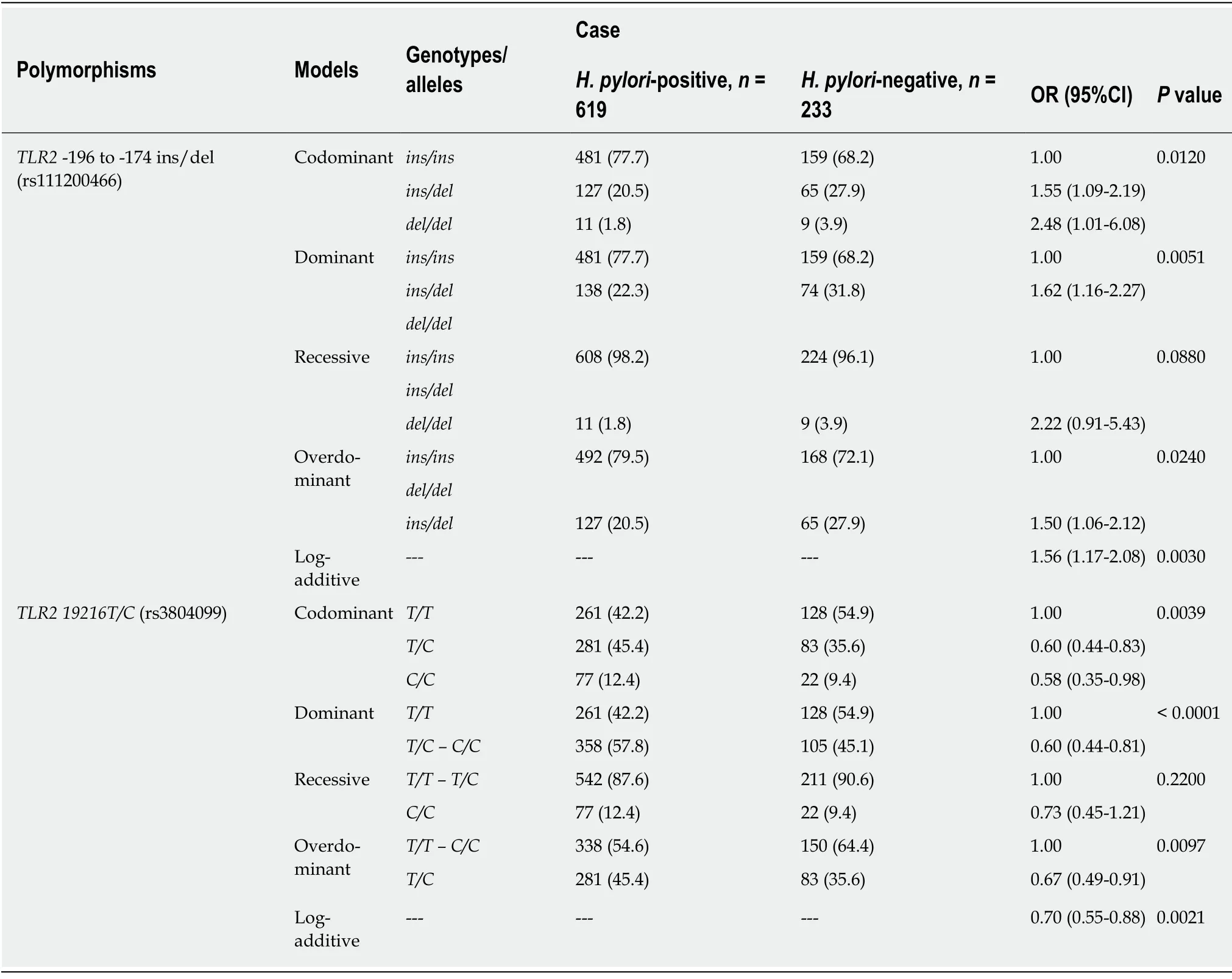
Table4 Genotype frequencies of TLR2-196 to -174 ins/del and TLR2 19216T/C polymorphisms in Helicobacter pylori-positive and Helicobacter pylori-negative groups,n (%)
When we analyzedTLR2mRNA expression,we found significantly higher levels ofTLR2mRNA in gastric cancer tissues than in chronic gastritis tissues.In the literature,a study in gastric cancer indicates that theTLR2mRNA expression levels were significantly increased in tumor tissues compared to either adjacent non-tumor tissues or normal tissues from GC-free individuals,regardless of risk factors orH.pyloriinfection[37].Similarly,a previous study from our research group also evaluated TLR2 receptor expression in different premalignant lesions (chronic gastritis,gastric atrophy,metaplasia) and observed a slight increase in TLR2 gene and protein expression in relation to normal gastric tissues[38].
Therefore,these studies showed thatTLR2mRNA expression levels are mainly increased in tumor tissue,and not in the chronic gastritis group,as this is a benign lesion that likely does not yet present significant alterations in genes associated with carcinogenesis.
Similar results were observed in colorectal cancer in the Brazilian population,where the relative mRNA expression in tumor tissues was 2.36-fold higher than that in adjacent normal tissues,and a strong immunostaining pattern was observed in the epithelium of tumor tissues[14].In 2018,Semlaliet al[16]analyzedTLR2mRNA expression in the Saudi Arabian population and showed a decrease inTLR2mRNA in colon cancer tissues compared to normal colon tissues,and this result was confirmed by immunohistochemistry.
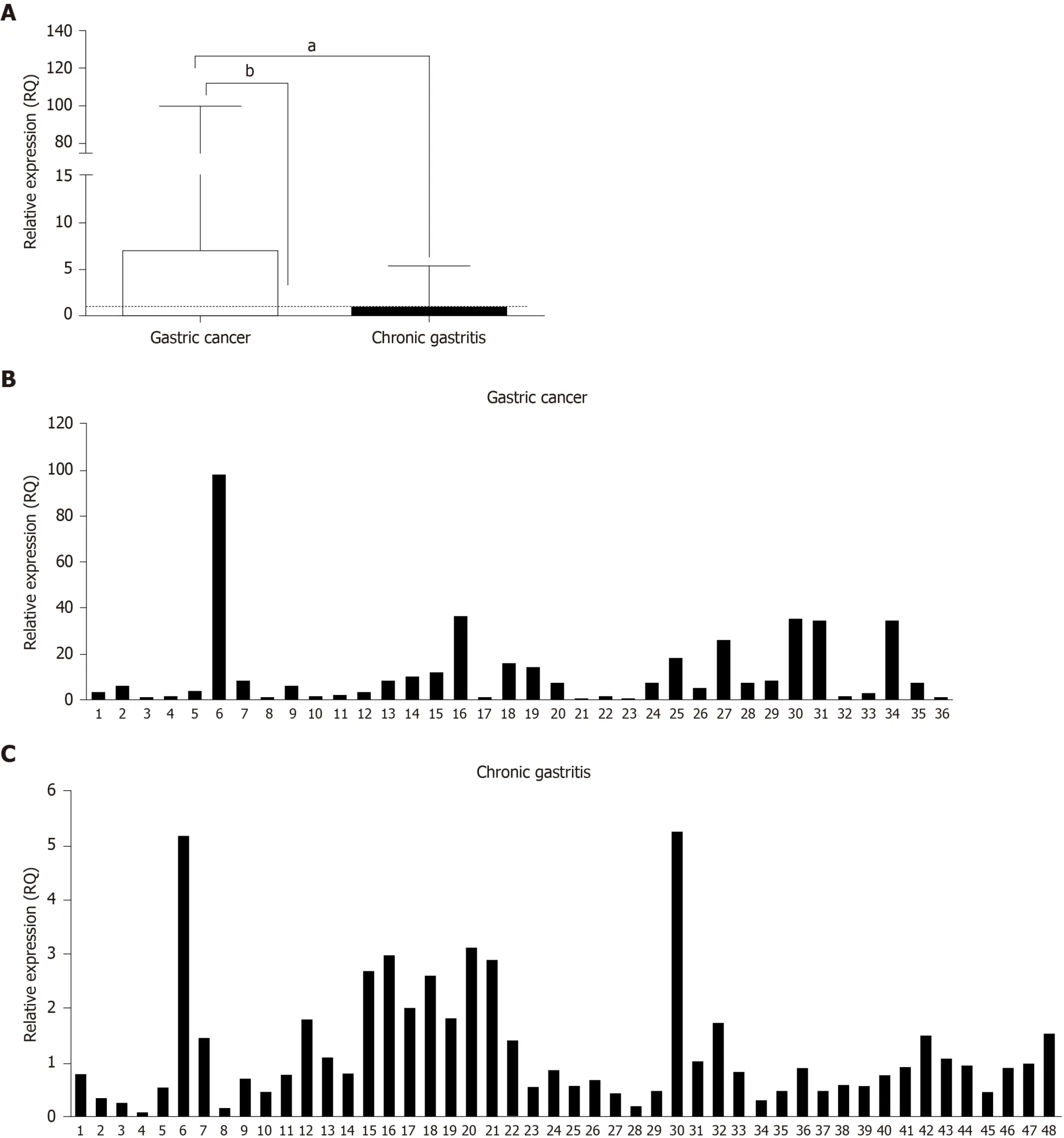
Figure1 Relative gene expression levels of TLR2.
This is the first study in gastric lesions to demonstrate the regulation of gene expression in the presence of variant genotypes (TLR2-196to-174 ins/del + del/delandTLR2 19216 T/C+C/C) andH.pyloriinfection,regardless of the type of lesion (cancer or gastritis).Thus,when we stratified gastric cancer tissue samples according to wildtype or variant genotypes,carriers of theTLR2-196 to-174 ins/del + del/delgenotypes had higherTLR2mRNA expression levels than carriers of theins/insgenotype.This finding supports the hypothesis that gene expression may be affected by allele variants in the promoter region and thatH.pyloriinfection can influence the inflammatory profile mediated by TLRs.Proençaet al[14]observed similar results in colorectal cancer,whereTLR2mRNA expression was 2.19-fold higher inTLR2-196to-174 delvariant carriers than in wild-type genotype carriers[14].
Conversely,the opposite was observed forTLR2 19216 T/C+C/Cvariant carriers(this polymorphism has a protective effect in gastric cancer),who had reducedTLR2expression levels with respect toTTwild-type genotype carriers.Thus,these data corroborate the results regarding the protective effect of theTLR2 19216 T/C+C/Cgenotype,which leads to the negative regulation of gene expression.Anin silicoanalysis of theTLR2nucleotide substitution in rs3804099 predicted a 70% probability that this SNP affectsTLR2mRNA splicing due to the creation of an additional splice site.Thus,this variant may alter protein expression,receptor conformation,and function[16].
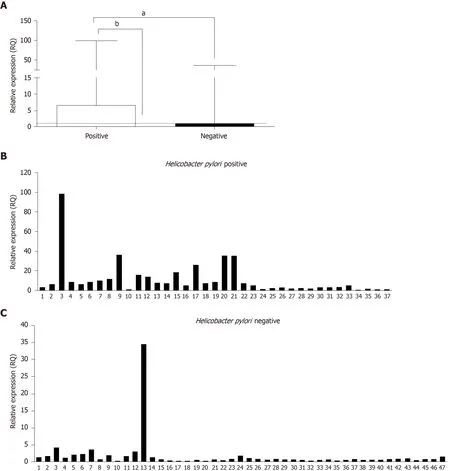
Figure2 Relative gene expression levels of TLR2 according to Helicobacter pylori infection.
Although the TLR2 19216T/C polymorphism results in a synonymous alteration(Asn199Asn),some genetic studies have shown a contribution of synonymous mutations to the risk of human diseases,including cancer[39].A polymorphism in the epidermal growth factor receptor (EGFR) gene (rs2293347) has been identified as a potential predictor of the clinical outcome of treatment for advanced non-small-cell lung carcinoma[40].Similarly,nonsynonymous SNPs in the Nijmegen breakage syndrome 1 (NBS1) gene (rs709816 and rs1061302) were associated with smokingrelated cancers[41],and the tumor suppressor p53 (TP53) gene (rs111287251) was associated with overall tumor susceptibility[42].
The high expression ofTLR2results in the recruitment of MyD88 to the TLR/TIR domain and in the production of inflammatory response cytokines by a classic signaling pathway,theNF-κBpathway,that exacerbates inflammation and thus facilitates tumor progression[5].

Figure3 Relative gene expression levels of TLR2 according to polymorphic genotypes.
In conclusion,our findings show that theTLR2-196to-174 ins/delandTLR2 19216T/Cpolymorphisms are associated with gastric cancer (an increased risk and a protective effect,respectively) andH.pyloriinfection in the Brazilian population.Additionally,our results indicate thatTLR2mRNA levels are upregulated in gastric cancer tissue,mainly in the presence of theTLR2-196to-174 delvariant allele,theTLR2 19216 Twild-type allele,and H.pyloriinfection.Thus,genetic polymorphisms may change gene expression,such asTLR2polymorphisms,alter the immune response profile,and consequently increase the risk and clinical manifestations of gastric cancer.
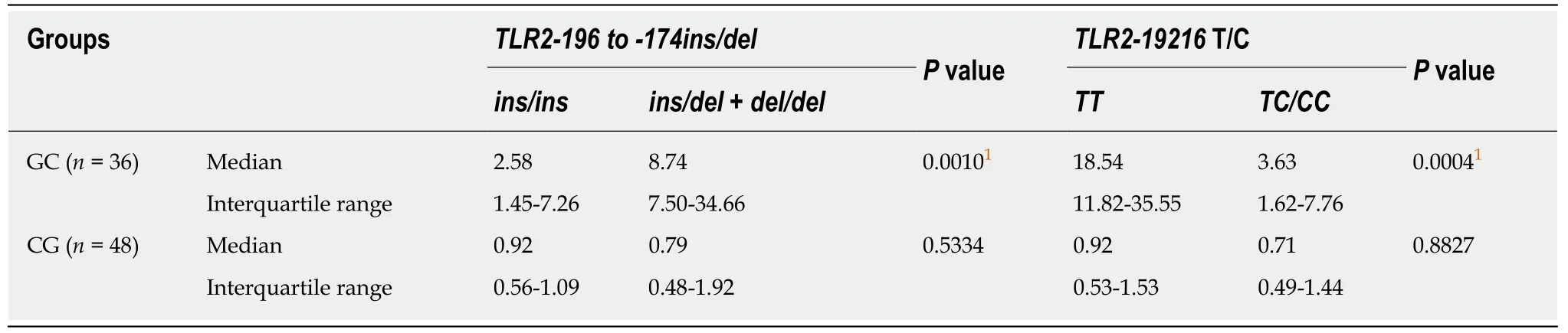
Table5 TLR2 gene expression level groups to assess influence of polymorphic genotypes in gastric cancer and chronic gastritis
ARTICLE HIGHLIGHTS
Research background
Helicobacter pylori (H.pylori) infection is a carcinogen for gastric cancer (GC),and Toll-like receptors are involved in recognition and activation of the inflammatory response for this bacterium.The presence of single nucleotide polymorphism (SNP) genes responsible for activating the innate immunity may influence the risk of precancerous lesions and GC,among them of which include TLR2 polymorphisms.GC is the fifth most common cancer worldwide,mortality rates are still high.In Brazil,GC is the fourth most frequent type of cancer in men,and the sixth in women,with an estimated incidence of 21230 new cases in 2020.
Research motivation
Considering the inconsistent results in the literature,as well as the importance of these receptors in immune response and for the susceptibility to inflammatory diseases and cancer,new studies are needed.The Brazilian population is highly mixed;thus it becomes important to confirm the real role among the factors that influence changes in the recognition of H.pylori and gastric carcinogenesis.
Research objectives
The aim of this study was to evaluate whether the TLR2 19216T/C (rs3804099) and TLR2-196 to-174 ins/del (rs111200466) polymorphisms contribute to gastric carcinogenesis in the Brazilian population.In addition,we also evaluate the influence of both polymorphisms and H.pylori infection on TLR2 mRNA expression.The results may highlight important polymorphisms that act on gastric carcinogenesis.
Research methods
A case-control study was con ducted to evaluate two TLR2 SNPs (TLR2 19216T/C-rs3804099 and TLR2-196 to-174 ins/del-rs111200466) in CG and GC patients.A total of 854 DNA samples of peripheral blood [269 CG,202 GC,and 383 samples from healthy individuals (C)] were genotyped by allele-specific PCR or restriction fragment length polymorphism (RFLP)-PCR.Quantitative polymerase chain reaction by TaqMan® assay was used to quantify TLR2 mRNA from fresh gastric tissues (48 GC,26 CG,and 14 C).
Research results
The data showed that for the TLR2-196 to-174 polymorphism,the ins/del and del/del genotypes were associated with a higher risk of GC compared with the C and CG groups.In contrast,TLR2 19216T/C was associated with a protective effect in the GC group compared to the C group.Regarding the association of polymorphisms with H.pylori infection,for the TLR2-196 to-174 ins/del polymorphism,an association was observed with H.pylori-positive,while TLR2 19216T/C was associated with protection against H.pylori infection.TLR2 mRNA levels were significantly higher in the GC group compared to the CG group and normal mucosa.In addition,when the samples were grouped according to polymorphic genotypes and the presence of H.pylori,the two SNPs (TLR2-196 to-174del and TLR2 19216 C alleles) and H.pylori infection influenced TLR2 mRNA expression.
Research conclusions
Our findings highlight that the polymorphisms of the TLR2-196 to-174 ins/del and TLR2 19216 T/C receptors are associated with gastric cancer (an increased risk and a protective effect,respectively) and H.pylori infection,and therefore may act as a potential factor in the progression of gastric carcinogenesis.TLR2 mRNA expression levels are upregulated in gastric cancer tissues and are influenced by the TLR2-196 to-174 del variant allele,the TLR2 19216 T wild-type allele and H.pylori infection.Considering that most cases of GC have a good prognosis and are treatable when diagnosed at an early stage,it is of the utmost importance to establish molecular markers capable of identifying risk groups and providing early diagnosis in individuals with increased risk of developing this neoplasm.Thus,polymorphisms in genes that affect its expression,such as TLR2,could have an effect on the development and clinical manifestation of disease.
Research perspectives
The pattern of the host's immune response associated with genetic and environmental factors are essential for understanding the pathology of gastric cancer.Overall,our results indicate that theTLR2gene plays an important role in gastric carcinogenesis,highlighting the importance of theTLR2-196 to-174delandTLR2 19216 Tpolymorphisms in increasing gene expression andH.pyloriinfection,possibly triggering a stronger inflammatory response,which in turn enhances the risk of tumor progression.In the future,it would be important to increase the biopsies collected during the endoscopic evaluation to quantifyTLR2mRNA levels,and investigate another polymorphism in theTLR2gene (rs3804100,rs7696323,and rs10116253),described in the literature as associated with cancer,but not yet analyzed in our Brazilian population.
ACKNOWLEDGEMENTS
The authors are grateful to Lilian Castiglione for her support with the statistical analysis.
 World Journal of Gastrointestinal Oncology2020年5期
World Journal of Gastrointestinal Oncology2020年5期
- World Journal of Gastrointestinal Oncology的其它文章
- Espresso coffee,caffeine and colon cancer
- Perineural invasion as a prognostic factor in patients with stage l-lll rectal cancer-5-year follow up
- Association of Helicobacter pylori infection with colorectal polyps and malignancy in China
- Gastric cancer with peritoneal metastases:Efficiency of standard treatment methods
- Perioperative chemotherapy for advanced gastric cancer-results from a tertiary-care hospital in Germany
- Neutropenia in colorectal cancer treated with oxaliplatin-based hyperthermic intraperitoneal chemotherapy:An observational cohort study
