Effects of Growth Regulator MeJA on Nitrogen Metabolism in Rice under Cadmium Stress
ZENG Jing, RONG Xiang-min, ZHANG Zhen-hua, LIU De-ming, ZHOU Yong
Hunan Agricultural University, Changsha 410128, PRC
Abstract Taking high nitrogen efficiency and low nitrogen tolerance C156 and C111 rice varieties as materials, the aboveground and underground biomass, total nitrogen uptake, nitrate nitrogen content and other indicators, the effects of methyl jasmonate (MeJA) on the growth, development and NO3- distribution of rice under cadmium stress were studied. The results showed that under cadmium stress, more nitrate nitrogen of rice seedlings was distributed to roots, which enhanced the stress resistance of crops. The concentration of methyl jasmonate at 70 μmol/L is a boundary line, and the methyl jasmonate at 70 μmol/L is recommended as the later research material. Methyl jasmonate can increase the biomass and total nitrogen uptake of C156 under cadmium stress, but the effect is not significant. It can significantly improve the NUE of C111 based on biomass.
Key words Methyl jasmonate; Nitrate nitrogen; Cadmium; Nitrogen efficiency
1. Introduction
As a typical crop highly susceptible to cadmium pollution, rice is at high risk of growth retardation and declines in quality or yield under Cd stress. The situation is even worse if the cadmium content in rice grains exceeds the national limit, where frequent consumption of this “cadmium rice” may cause chronic cadmium poisoning[1]. Rice is the main grain crop in Asia and the second largest grain crop in the world. In China, large areas of the soil have been contaminated by Cd. The point-location over-limit rate of Cd in the soil has reached 7.0% according to theNational Soil Survey Bulletin(2014), making Cd head the list of the eight overlimit metal pollutants[2]. Therefore, to improve the quality and safety of rice grains, we need to enhance the resistance of rice to cadmium, which is also the main direction of research into rice quality safety.
Part of the nitrate nitrogen absorbed into the plant is assimilated by root system, while the rest is transported to the aboveground plant parts for various physiological processes[3]. The distribution and proportion of nitrate nitrogen content in different plant parts depend on a number of factors, such as the plant variety, external NO3-concentration, temperature, light intensity,etc[4]. Nitrogen metabolism is an important way of nutrition metabolism during the growth and development of rice. Heavy metal stress would affect the absorption and accumulation of nitrogen in plants and influence the activity of relevant enzymes[5-6].
Methyl jasmonate (MeJA), a common chemical substance in plant kingdom, is closely related to the regulation of stress resistance. As a signal transduction substance, MeJA can also induce the closing of stomas[7]. MeJA is applicable to various plant adversities, such as drought, low temperature, high temperature, plant diseases, insect pests, heavy metal contamination and so on. It is reported that exogenous MeJA could enhance the activity of various enzymes (i.e. AOX, LOX, and PAL) and increase the contents of soluble osmotic regulators (i.e. sugars and prolines), thus improving the cold resistance of fruits and reducing the damage caused by low temperature during storage[8-9]. According to the research findings of YANG H Get al.[10]on phalaenopsis seedlings under high temperature stress, MeJA enhanced the antioxidase activity and the contents of soluble proteins and prolines in plants as well as reducing the membrane relative conductivity and malondialdehyde content. It is therefore possible to use exogenous MeJA to improve the heat resistance of phalaenopsis seedlings by increasing their endogenesis antioxidase activity.
Studies have shown that MeJA under drought conditions could improve the photosynthesis capacity of wheat by increasing its water use efficiency and oxidation resistance[11]. Similar findings were reported by DONG T X in her research on rice seedlings[12], which proved that MeJA could help maintain the chlorophyll level, protect the photosystem, and consequently improve the photosynthesis efficiency of rice plants under drought conditions. Exogenous MeJA is also a very effective regulator for dealing with biotic stress like plant diseases and insect pests. According to the research of ZHANG Z Het al.[13], MeJA could produce good results when dealing with rice blast by enhancing the activity of POD, CAI, PAL and LOX of rice leaves. Studies on rice bacterial leaf blight showed similar results[14]. Exogenous MeJA could greatly enhance the oxidation resistance and photosynthetic pigment contents in maize seedling leaves, increase the accumulation of biomass, lower the levels of MDA and H2O2in maize leaves, and con- sequently reduce the damage caused by Cd stress[15]. As regards the effects of exogenous MeJA on maize plants under heavy metal stress, some have proposed that MeJA can significantly increase the contents of photosynthetic pigments (e.g. chlorophyll and carotene) in maize plants and remedy the impaired photosynthesis to achieve higher biomass[16]. But the effects of MeJA on nitrogen metabolism in rice plants are rarely seen in existing reports. In this research, high nitrogen efficiency and low nitrogen tolerance rice varieties C156 and C111 are chosen to evaluate the effects of MeJA on the growth anddistribution of rice seedlings under Cd stress. The findings reveal the relationship between the Cd resistance and nitrogen utilization efficiency of rice under MeJA regulation, providing theoretical support for the improvements of rice stress resistance.
2. Materials and Methods
2.1. Testing materials
Two rice varieties were screened for the test: the nitrogen-efficient and low-nitrogen-tolerant Qingshuidao C156; the nitrogen-sensitive Beizinuo C111. Both were provided by the Science of Plant Physiology and Ecology, SIBS, CAS.
2.2. Experimental design
The control group contained no Cd or MeJA, while the test group was composed of five treatments—Cd (40 µmol/L), 10 MeJA+Cd (MeJA 10 µmol/L+Cd 40 µmol/L), 40 MeJA+Cd (MeJA 40 µmol/L+Cd 40 µmol/L), 70 MeJA+Cd (MeJA 70 µmol/L+Cd 40 µmol/L), and 100 MeJA+Cd (MeJA 100 µmol/L+Cd 40 µmol/L). Experiments were conducted on C156 and C111 respectively, which made 12 treatments in total (4 replications for each treatment; random block arrangement).
The hydroponic experiment of rice plants at seedling stage was done in the phytotron of the Plant Nutrition Research Group (Hunan Agricultural University) during March to August 2017. Daytime and nighttime temperatures were 28℃ and 22℃ respec- tively; the relative humidity was 70%; natural sunlight for illumination; Yoshida nutrient solution was used for hydroponics. The hydroponic trays were 50 cm in length, 20 cm in width, and 8 cm in height; there were six 96-pore plates floating in each tray; each plate had 96 pores (12 pores/row and 8 pores/column); each plate was sown with 24 seeds.
First of all, the chosen seeds were placed on the moist absorbent paper in culture dishes and kept in an incubator for 3 d at 30℃ illumination to encourage ger- mination. The nutrient solution was changed every 3 d; 0.1 mol/L NaOH or 0.1 mol/L HCl was added to the solution every day to fix the pH at 5.5. These rice seedlings were cultivated in nutrient solution for about 30 d until they reached similar growth status. Then, we started the experiment of Cd stress (40 µmol/L) and Cd+MeJA treatments (Cd: 40 µmol/L; MeJA: 10, 40, 70, and 100 μmol/L). The nutrient solution was changed every 3 d; 0.1 mol/L NaOH or 0.1 mol/L HCl was added to the solution every day to fix the pH at 5.5. Phenotypes were collected 14 d after the treatment by random sampling; in each replication, 6 plants were sampled.
2.3. Determination items and methods
2.3.1. Determination items
The determination items were the biomass, total nitrogen uptake, and nitrate nitrogen content in the aboveground and underground parts of rice plants at the seedling stage.
2.3.2. Determination methods
Biomass of the aboveground and underground parts: randomly sample 3 plants from each block; dry the plants in the sun; take the seeds and dry at 40℃ until constant weight; store the dried seeds in bags; treat the remaining plant parts at 105℃ for green removing and dry to constant weight at 70℃; smash into pieces. The total nitrogen uptake was determined by a continuous flow analyzer (AA3). Nitrate nitrogen content: take some samples→cut into small pieces and mix thoroughly; weigh 1 g of the smashed sample and pour into a 10 mL centrifuge tube→add 8 mL of deionized water→put the tube in a boiling water bath for 30 min; cool the tube with tap water, adjust the volume to 10 mL and shake well→draw 0.1 mL of the sample liquid and release into a 10 mL centrifuge tube→add 0.4 mL of 5% salicylic-sulfuric acid solution→shake well and cool off at room temperature for 20 min→add 9.5 mL of 8% sodium hydroxide solution→cool off to room temperature and carry out colorimetric determination at a wavelength of 410 nm.
2.4. Data processing
Statistical analysis of the experimental data was done by Microsoft Excel 2007 and DPS software.
3. Results and Analysis
As for the underground parts of C156, the highestcontent was measured in the control group and the lowest appeared in the treatment of 100 MeJA+Cd (Fig. 1). There were significant differences between thecontents of the control and the test group of Cd treatment and 70 MeJA+Cd. Thecontent under Cd treatment was slightly higher than that under 70 MeJA+Cd, but the difference was not distinct. In contrast, the difference betweencontents under Cd treatment and 10 MeJA+Cd was remarkable. Thecontent under 10 MeJA+Cd was not significantly different from that under 70 MeJA+Cd or 40 MeJA+Cd, but it did vary considerably from the one under 100 MeJA+Cd. Thecontent under 40 MeJA+Cd was significantly different from those under 70 MeJA+Cd and 100 MeJA+Cd. For the underground parts of C111, the highestcontent was observed under Cd treatment and the lowest appeared in 100 MeJA+Cd. Thecontent of Cd treatment varied greatly from those of the control, 10 MeJA+Cd, 40 MeJA+Cd, 70 MeJA+Cd, and 100 MeJA+Cd. Thecontents under 40 MeJA+Cd and 100 MeJA+Cd were significantly different from those under 10 MeJA+Cd and 70 MeJA+Cd. Great difference incontents was also found between 40 MeJA+Cd and 100 MeJA+Cd.

Fig. 1 Nitrate nitrogen content in underground parts of rice seedlings
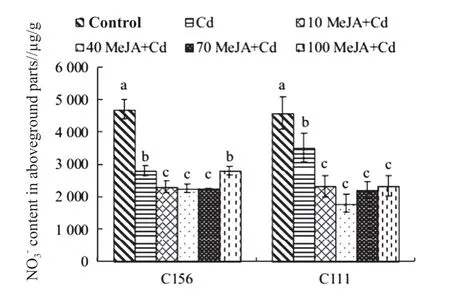
Fig. 2 Nitrate nitrogen content in aboveground parts of rice seedlings
As shown in Fig. 3, the contents ofin the aboveground/underground parts reached the peak under 70 MeJA+Cd treatment and hit rock bottom under 100 MeJA+Cd treatment for both C156 and C111 seedlings. In the aboveground/underground parts of C156, there were no significant differences among the NO3-contents under Cd, 10 MeJA+Cd, 40 MeJA+Cd, and 70 MeJA+Cd treatment. Thecontent under 70 MeJA+Cd was much higher than that of the control. There were no significant differences between the NO3-contents of the control and Cd treatment, 10 MeJA+Cd and 40 MeJA+Cd. Thelevels under normal treatment, Cd treatment, 10 MeJA+Cd, 40 MeJA+Cd, and 70 MeJA+Cd were considerably higher than that under 100 MeJA+Cd. For the aboveground/underground parts of C111, thecontents under 70 MeJA+Cd and 10 MeJA+Cd varied greatly from that under 40 MeJA+Cd, but they were not significantly different from that under Cd treatment. Thecontents of Cd treatment, 10 MeJA+Cd, 40 MeJA+Cd, and 70 MeJA+Cd were significantly higher than that of the control, whereas the control was significantly higher than 100 MeJA+Cd. This means more nitrate nitrogen was distributed to roots under Cd stress, which is conducive to stronger stress resistance.
“70 µmol/L” marked the boundary concentration of MeJA in relation tocontents. Thecontents in rice seedlings under treatments with MeJA less than 70 µmol/L were higher than the control, but when the concentration of MeJA went beyond 70 µmol/L, thecontents were lower than the control. For this reason, “70 µmol/L MeJA” was chosen as the treatment material for later studies.

Fig. 3 Nitrate nitrogen content in aboveground/underground parts of C156 and C111 at seedling stage
3.2. Nitrogen efficiency of rice with different genotypes
3.2.1. Biomass of different rice varieties
For rice variety C156, the biomass of the control was significantly different from that of Cd treatment (Fig. 4); the biomass of 70 MeJA+Cd was higher than that of Cd treatment, but there were no significant differences in biomass between 70 MeJA+Cd and the control or Cd treatment. For rice variety C111, there were no significant differences between the biomass of each treatment.

Fig. 4 Biomass of C156 and C111 at seedling stage
3.2.2. Total nitrogen uptake of rice with different genotypes
According to Fig. 5, the total nitrogen uptake of C156 under normal treatment was significantly different from the ones under other treatments; the total nitrogen uptake of 70 MeJA+Cd was higher than that of Cd treatment. In contrast, there were no significant differences between the total nitrogen uptake of C111 under each treatments.
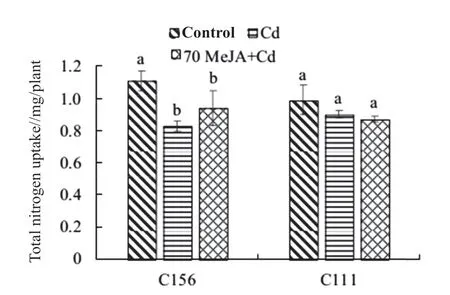
Fig. 5 Total nitrogen uptake of C156 and C111 at seedling stage
3.2.3. Biomass-based NUE of rice with different genotypes
For C156, there were no significant differences between the biomass-based NUE under each treatment (Fig. 6). But for C111, the biomass-based NUE under 70 MeJA+Cd was significantly higher than that under Cd treatment; there were no significant differences between normal treatment and other treatments.

Fig. 6 Biomass-based NUE of C156 and C111 at seedling stage
4. Conclusion and Discussion
In the aboveground/underground parts of both C156 and C111 seedlings, the contents ofreached the peak under 70 MeJA+Cd treatment and hit rock bottom under 100 MeJA+Cd treatment. For the aboveground/underground parts of C156, thecontent under 70 MeJA+Cd was significantly higher than the control; the NO3-contents of normal group, Cd, 10 MeJA+Cd, 40 MeJA+Cd, and 70 MeJA+Cd treatment were considerably higher than that under 100 MeJA+Cd treatment. In the aboveground/ underground parts of C111, the NO3-levels under 70 MeJA+Cd and 10 MeJA+Cd were significantly different from that under 40 MeJA+Cd; Cd, 10 MeJA+Cd, 40 MeJA+Cd, and 70 MeJA+Cd were much higher than the control, while the control varied greatly from 100 MeJA+Cd. This means more nitrate nitrogen was distributed to rice roots under Cd stress, which could enhance the stress resistance of rice at seedling stage. In this experiment, “70 µmol/L” was the boundary concentration of MeJA: if the concen- tration of MeJA in a treatment was less than 70 µmol/L, thecontent in this treatment would be higher than that in the control; when the concentration of MeJA exceeded 70 µmol/L, thelevel would be lower than the control. Therefore, it is feasible to use “70 µmol/L MeJA” as research material in future studies.
As regards the biomass of C156, significant differences were observed between the control and Cd treatment group. Specifically, the biomass of C156 under 70 MeJA+Cd was higher than that under Cd treatment. For the total nitrogen uptake of C156, there were significant differences between the control and other treatments; the total nitrogen uptake under 70 MeJA+Cd was higher than that under Cd treatment. The biomass-based NUE of C111 under 70 MeJA+Cd was considerably higher than that under Cd treatment. These results indicate that MeJA is capable of improving the biomass and total nitrogen uptake of C156 under Cd stress, but the performance is not outstanding. In contrast, MeJA can effectively enhance the biomass-based NUE of C111.
 Agricultural Science & Technology2020年1期
Agricultural Science & Technology2020年1期
- Agricultural Science & Technology的其它文章
- Purification of Starch from Cadmium-Contaminated Rice and Development of Functional Recombinant Rice
- Soil Heavy Metal Absorption Performance of the New Sorghum Combination Dunuo 201
- Optimization of Blueberry Wine Fermentation Process
- Ultrasound-assisted Enzymatic Extraction of SDF on Passion Fruit Seeds
- Study on the Effects of Reducing Blood Lipid by Flavonoids from Ampelopsis grossedentata on Hyperlipidemia Rats
- Isolation, Identification and Phosphate Solubilizing Capacity of Organophosphorus Solubilizing Bacteria in Rhizosphere Soil of Camellia oleifera
