Sensitivity of two drug-resistant bacteria to low-temperature air plasma in catheterassociated urinary tract infections under different environments
Yuanyuan HE (何媛媛), Chengbiao DING (丁呈彪), Tao JIN (金涛),Yueyao FAN(范玥瑶),Zhengwei WU(吴征威),8,Mengwen SUN(孙梦雯),Kun WANG (王琨) and Tuo JI (纪拓)
1 Department of Geriatrics,The First Affiliated Hospital of USTC,Division of Life Sciences and Medicine,University of Science and Technology of China, Hefei 230001, People’s Republic of China
2 Department of Rehabilitation Medicine,The Second Hospital of Anhui Medical University,Hefei 230001,People’s Republic of China
3 Department of Engineering and Applied Physics, University of Science and Technology of China, Hefei 230026, People’s Republic of China
4 School of Food and Biological Engineering, Hefei University of Technology, Hefei 230009, People’s Republic of China
5 Polar Research Institute of China, Shanghai 200136, People’s Republic of China
6 Science Island Branch,University of Science and Technology of China,Hefei 230026,People’s Republic of China
Abstract The high incidence of catheter-associated urinary tract infections,which are dominated by drugresistant bacteria, has attracted an increasing number of researchers interested in solving this public health problem. The purpose of this study was to explore the killing effect of lowtemperature air plasma (LTAP) on extended-spectrum beta-lactamase-producing Escherichia coli and high level gentamycin resistance enterococci under two simulated environments in vitro.The results showed that the survival rate of these two kinds of bacteria decreased to less than 20% after being treated by LTAP in different environments for 5 min. A comparison of the LTAP treatments showed that the killing efficacy of the two kinds of bacteria in the early stage(0–1 min) was up to 50%. Moreover, the results of transmission electron microscopy, reactive nitrogen species measurement,and a temperature test indicated that the bactericidal effect of the LTAP treatment on the two kinds of bacteria worked through the destruction of the ribosome and other organelles inside the bacteria, rather than the thermal effect, to achieve sterilization.
Keywords:CAUTI,low-temperature air plasma,antibacterial,transmission electron microscopy(Some figures may appear in colour only in the online journal)
1. Introduction
Hospital-acquired infections (HAIs) are a public health issue that has drawn increased attention worldwide, among which catheter-associated urinary tract infections (CAUTIs) are the most common, accounting for about 40% of HAIs [1].According to health surveys, patients who use catheters for a long time are at a high risk of getting a urinary tract-induced fever[2].In addition,patients who use indwelt catheters for a long time are at greater risk of being infected with bacteremia and other urinary complications than patients who have not used them. The continual and repeated application of highefficiency and broad-spectrum antibiotics in clinical practice can make the bacteria become more strongly drug resistance.Pathogenic bacteria with a high drug-resistant property results in CAUTIs mainly including Escherichia coli (E. coli),Pseudomonas spp.,and rare gram-negative bacilli [3,4].The inner and outer surfaces of the catheter can form a biofilm to protect the pathogen from antimicrobial agents and host an immune response [5]. The latest research has found that silver-ion and furacillin coated catheters can reduce the occurrence of bacteriuria in patients with indwelt catheters.However, if the indwelling time exceeds seven days, the occurrence of a CAUTI could not be reduced compared with silicone catheters. Besides, furacillin coated catheters can produce discomfort in clinical use [6]. The current use of antibacterial catheters,either over the short term or long term,has not been highly effective in the laboratory or clinical studies. As the urine pH value increases due to the pathogen of the CAUTI, the crystals of calcium and magnesium phosphate in the urine blocks the eyelet and lumen of the urinary catheter,which can further result in a scabby catheter,catheter obstruction, bladder distension, nephropyelitis, sepsis, and additional pain to the patients [7]. Traditional sterilization methods,such as ultraviolet(UV)radiation and hightemperature sterilization, are difficult to apply to the internal environment of the human body.Therefore,it is urgent to find a new method as a substitute for traditional chemical antibacterials, which could greatly shorten hospitalization and treatment times, and even improve the quality of life of patients.
Plasma is the fourth state of matter besides solid,gas,and liquid, and exists widely in the Universe. It is produced by ionizing argon, helium, nitrogen, oxygen, or air between two high-pressure electrodes.Plasma can be divided into low-and high-temperature plasma, which is mainly a mixed state composed of a large number of electrons,ions,and unionized neutral particles. Plasma is electrically neutral at the macro level [8]. Low-temperature plasma (LTP) is mainly produced under atmospheric pressure, so it is also called atmospheric LTP. The prepared neutral particles and ions are low temperature and widely used in many applications such as material surface treatment, ozone production, and pollution control[9–11]. The literature has shown that plasma can generate large amounts of free radicals and chemicals in all-air and airliquid environments such as charged particles, UV radiation,free radicals, reactive oxygen species (ROS), and reactive nitrogen species (RNS). Some of these active substances can enter adjacent liquid phases and affect cells [12, 13]. The working conditions of LTP are mild at a low temperature,which makes it possible to be applied in living tissue such as the human body.At present,LTP technology has been widely used in biomedicine such as in the disinfection and sterilization of thermal materials[14,15],surface modification of medical devices [16, 17], tumor treatment [18, 19], oral disease treatment [20, 21], wound healing [22, 23], and skin disease treatment[24,25],etc.Though the bactericidal effect of plasma on medical devices has been proven, there are few studies on multi-drug-resistant bacteria on implanted and semi-implanted biomedical materials due to its complex reactive species and biological reactions. In this work,according to the pathogenic characteristics of urinary tract infection bacteria found in the First Affiliated Hospital of USTC, two kinds of drug-resistant bacteria were isolated,identified, and cultured from urine samples of clinically diagnosed CAUTI patients: gram-negative bacillus extendedspectrum beta-lactamase (ESBL)-producing E.coli (ESBLE. coli) and high level gentamycin resistance enterococci(HLGRE).Two different environments that the bacteria might live in the catheter were stimulated: first, when the bladder was completely empty, the bacteria on the surface of the catheter were in an all-air environment, and second, bacteria on the surface of the catheter were in an air–liquid interface before the bladder was completely empty. Then the sensitivities of the two bacteria treated with low-temperature air plasma (LTAP) under different environments were observed.
2. Materials and methods
2.1. LTAP device
As shown in figure 1, an LTAP device was designed and manufactured. The device directly used air as the reactant without adding other special gases. A built-in resistor was used to control the voltage and current. For operational purposes, a movable electrode was fixed through a wire to place the processed samples.When the power was switched on,the copper electrodes were connected to a high-voltage DC power supply, and plasma was generated between the copper electrodes and the external electrodes by ionizing the surrounding air. The input voltage was 10 kV with 5 mA of current peak,and the temperature of the air plasma gas was about 27°C.
2.2. Antibacterial experiment
GN13 gram-negative and GP67 gram-positive bacilli drugsensitive cards on a 64-well plate were used to identify and select two drug-resistant bacteria from the urine specimens of 18 clinical patients in our hospital by a VITEK2 system analyzer. Then, the two bacteria were cultured on Columbia blood agar medium at 37°C overnight, separately. Single colonies were selected to prepare the bacterial suspension,and the concentration was adjusted to 106CFU/ml. 25 μl of diluted bacterial suspension was uniformly coated on the Columbia blood agar medium (set as an all-air environment model) and treated with LTAP for 0, 1, and 5 min, respectively. Another prepared 500 μl of diluted bacterial suspension was added into the empty culture dishes(regard as an airliquid environment model), and treated with LTAP for 0, 1,and 5 min,respectively.Then 25 μl of treated diluted bacterial suspension was evenly coated on the Columbia blood agar medium. After being cultured for 12 h at 37°C, the active bacteria were counted by the flat colony counting method.Finally, the bactericidal effect and sterilization rate of the LTAP treatment on two kinds of bacteria were studied. The experiment was repeated three times.

Figure 1.Schematic of LTAP device (a) and photo of the plasma device treating bacteria (b).
2.3. Transmission electron microscopy (TEM)
The treated bacteria samples were fixed with an electron microscope fixative for 4 h at 4°C.Mung-bean sized bacteria masses were obtained by low-speed centrifugation. Then bacteria were wrapped with 1% agarose and rinsed with 0.1 M of phosphate acid buffer (pH 7.4) three times for 15 min each. The bacteria were fixed with a composition solution of 1% osmic acid and 0.1 M of phosphoric acid buffer(1:1,v:v)for 2 h at room temperature(20°C),followed by rinsing with 0.1 M of phosphoric acid buffer(pH 7.4)three times for 15 min each and dehydrated with ethanol water and acetone of different volume fractions for 15 min each. In the next step, the samples were permeated with an acetone/812 embedding agent mixture, then inserted into the embedding plates and dried for 12 h at 37°C.The samples were put in an oven for 48 h at 60°C to polymerize. The 60 nm slices were obtained by ultra-thin slices, and then stained with uranium lead (2% uranium acetate alcohol solution and lead citrate solution,each for 15 min). The slices were dried overnight at room temperature. Finally, the images were observed and analyzed under TEM (Hitachi HT7700, Japan).
2.4. Measurement of intracellular ROS and RNS production
The ROS and RNS contents were detected by DCFH-DA(C24H16Cl2O7, 487.29) and BBoxiProbe O52 (C2H6O6,78.13), respectively. The LTAP treated bacteria on a petri dish were washed and collected using PBS (10 mM,pH=7.4). Then, 2 ml of treated bacterial solution was prepared by centrifugation and mixing with PBS.Finally,100 μl of 5 μM DCFH-DA or O52 was added into a 96-well enzyme-linked immunosorbent assay (ELISA) plate containing a 100 μl 1×106CFU/ml bacterial solution.The mixture was incubated for 25 min at 37°C.The fluorescence intensity of each reaction solution was measured by fluorescence ELISA at an excitation wavelength of 488 nm, and emission wavelengths of 525 nm (ROS) and 516 nm (RNS). Besides,the fluorescence intensity of 100 μl of PBS was recorded as zero.
2.5. Temperature measurements
To explore whether a thermal effect occurred in the process of the LTAP treatment on bacteria,infrared digital thermometers(Omron Mc-872)were used to measure the temperature of the surface of agar(all-air environment)and bacterial suspension(air-liquid environment) after treatment on bacteria. The experiments were repeated three times as well.
2.6. Statistical analysis
IBM SPSS Statistics 21 was used to compare the different processing groups. An Excel 2013 tool was used to record and calculate the experimental data. In addition, the experimental data were recorded as a mean±standard deviation(SD): the significance of the difference was set at P<0.05,and a highly significant difference was set at P<0.01.
3. Results and discussion
3.1. Bactericidal effect of LTAP treatment of two kinds of bacteria in different environments
Figure 2 shows the growth of the two kinds of bacteria which were treated by LTAP in different environments on Columbian blood agar medium. It can be seen that a few bacteria grew on the Columbian blood agar medium with 5 min of LTAP treatment compared with 0 min. In addition, as shown in figure 3, the survival rate of the bacteria dropped rapidly with the increase of the LTAP treatment time and there were less than 20%bacteria alive at 5 min of LTAP treatment.The results indicate that LTAP showed a significant lethal effect on ESBL-E.coli and HLGRE in vitro which were isolated from the catheter of CAUTI patients.

Figure 2. After LTAP treatments of 0, 1, and 5 min (lines 1–3 in each picture) in different environments, the growth of two kinds of drug-resistant bacteria: (a) growth of ESBL-E.coli in an all-air environment; (b) growth of ESBL-E.coli in an air-liquid environment;(c)growth of HLGRE in an all-air environment;(d)growth of HLGRE in an air-liquid environment.

Figure 3.Effects of LTAP treatment on the survival rate of two kinds of drug-resistant bacteria in different environments. E-G: growth of ESBL-E.coli in an all-air environment; E-GL: growth of ESBLE.coli in an air-liquid environment. H-G: growth of HLGRE in an all-air environment; H-GL: growth of HLGRE in an air-liquid environment.
3.2. Effects of environmental media on the sterilization rate of the LTAP treatment
To further explore the rule of LTAP sterilization, the LTAP treatment was divided into two parts: 0–1 and 1–5 min. The 0–1 min part was defined as the early sterilization stage, and the 1–5 min part was defined as the late sterilization stage.The lethal rates on bacteria in the early and late sterilization stages were calculated according to the following formula:
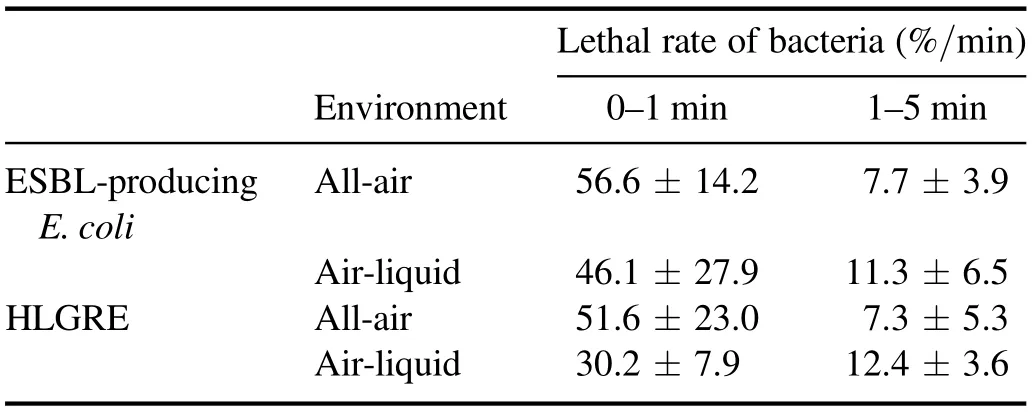
Table 1. Lethal rates of two kinds of bacteria treated by LTAP in different environments.
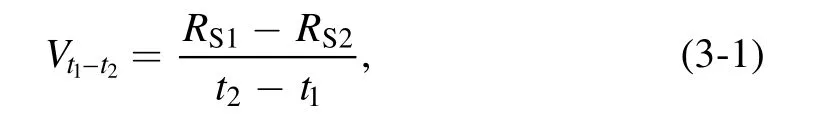
where t1and t2were the processing times, respectively, and t2>t1; RS1and RS2were the survival rates of resistant bacteria at t1and t2of the treatment, respectively.Vt1–t2was recorded asVt2for convenience. The results are shown in table 1.
According to table 1, during the early sterilization stage(0–1 min), the lethal rate of LTAP on bacteria in the all-air environment was significantly higher than that in the airliquid environment, which was consistent with the report of Mai-Prochnow et al [26]. However, in the late sterilization stage(1–5 min),the lethal rate of LTAP on bacteria in the airliquid environment was higher than that in the all-air environment. This indicates that LTAP had an obvious cumulative effect on killing bacteria in different environments [27].
3.3. TEM images of bacteria
As shown in figures 4–7,TEM was used to study the internal cellular structures of two kinds of bacteria treated by LTAP.As can be seen from figures 4 and 5, the lethal effects on the two kinds of bacteria were different at different lengths of LTAP treatment times:
(1) After 1 min of LTAP treatment on ESBL-E.coli in the all-air environment,the cell morphology of the bacteria was basically complete. The outer membranes of some bacteria were torn, but the cell walls were intact. In a few bacteria,the cytoplasm was uneven with intact cell nucleus (figures 4(a) and 5(a)).(2) After 5 min of LTAP treatment on ESBL-E.coli in the all-air environment, the extracellular membranes of most of the bacteria were broken. The cell walls were still intact, but the cytoplasm was uneven and even oozed (figures 4(b) and 5(b)).
(3) After 1 min of LTAP treatment on ESBL-E.coli in the air-liquid environment, the outer membranes of some bacteria were deformed.The cell walls were intact,and the cytoplasm was uniform (figures 4(c) and 5(c)).
(4) After 5 min of LTAP treatment on ESBL-E.coli in the air-liquid environment, the extracellular membranes of most of the bacteria were broken and opened.Besides,a nonuniform cytoplasm, unrecognizable nucleus, and intact cell walls could also be observed (figures 4(d)and 5(d)).
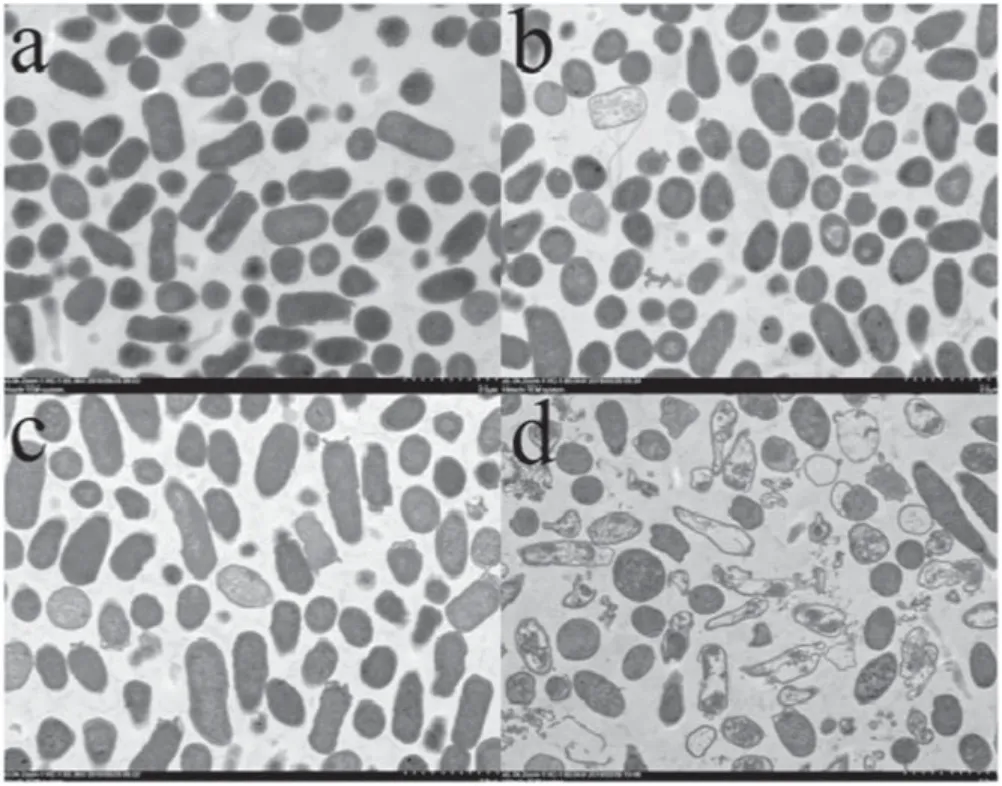
Figure 4.TEM images of the cell structures of ESBL-E.coli treated by LTAP (magnification times: 5k). (a) Bacterial cell structure treated by LTAP for 1 min under the all-air environment, (b)bacterial cell structure treated by LTAP for 5 min under the all-air environment, (c) bacterial cell structure treated by LTAP for 1 min under the air-liquid environment, (d) bacterial cell structure treated by LTAP for 5 min under the air-liquid environment.
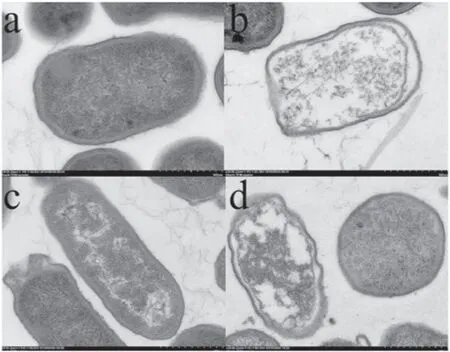
Figure 5.TEM images of the cell structures of ESBL-E.coli treated by LTAP (magnification times: 30k). (a) Bacterial cell structure treated by LTAP for 1 min under the all-air environment, (b)bacterial cell structure treated by LTAP for 5 min under the all-air environment, (c) bacterial cell structure treated by LTAP for 1 min under the air-liquid environment, (d) bacterial cell structure treated by LTAP for 5 min under the air-liquid environment.
Figures 6 and 7 show the morphological changes of HLGRE treated by LTAP under different environments.Comparing figures 6 and 7 with figures 4 and 5 showed that the lethal effect on HLGRE was the same as that on ESBLE.coli in that the LTAP treatment oxidized the double bonds in the lipid bi-layer of the bacteria and destroyed the ribosome and other organelles at an early stage (figures 6(a),(c) and 7(a),(c)). Thus it damaged the transport of macromolecules inside and outside the bacteria and inhibited the replication of bacteria, and consequently killed the bacteria [28]. Finally,the cytoplasm was nonuniform and even oozed (figures 6(b),(d) and 7(b), (d)).
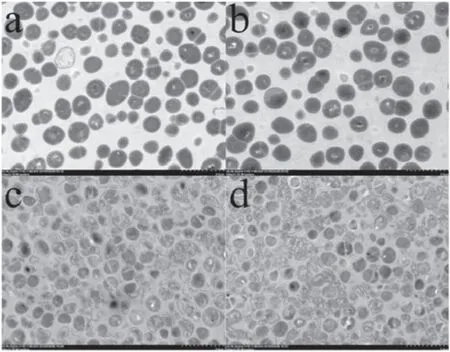
Figure 6.TEM images of the cell structures of HLGRE treated by LTAP (magnification times: 5k). (a) Bacterial cell structure treated by LTAP for 1 min under the all-air environment, (b) bacterial cell structure treated by LTAP for 5 min under the all-air environment,(c) bacterial cell structure treated by LTAP for 1 min under the airliquid environment, (d) bacterial cell structure treated by LTAP for 5 min under the air-liquid environment.

Figure 7.TEM images of the cell structures of HLGRE treated by LTAP (magnification times: 30k). (a) Bacterial cell structure treated by LTAP for 1 min under the all-air environment, (b) bacterial cell structure treated by LTAP for 5 min under the all-air environment,(c) bacterial cell structure treated by LTAP for 1 min under the airliquid environment, (d) bacterial cell structure treated by LTAP for 5 min under the air-liquid environment.

Figure 8.The intracellular RONS content of ESBL-E.coli and HLGRE treated by LTAP under different environments.The ROS content of ESBL-E.coli under all-air and air-liquid environments(a),the ROS content of HLGRE under all-air and air-liquid environments(b),the RNS content of ESBL-E.coli under all-air and air-liquid environments (c), the RNS content of HLGRE under all-air and air-liquid environments (d).

Table 2.The temperature of the treated bacteria for different lengths of time under each environment.
On the other hand,comparing figure 4(a)with figure 4(c)and figure 6(a)with figure 6(c),respectively,showed that the lethal effect of the LTAP treatment on bacteria for 1 min in the all-air environment was better than that in the air-liquid environment.It was consistent with the conclusions of Zheng et al [29], and illustrated that during the early sterilization stage and under the all-air environment, the UV rays, ozone,and other reactive species generated by the plasma could directly contact the bacteria and kill the closest bacteria at the early stage.Otherwise,it should take a specific time to arrive at the air–liquid interface under the air-liquid environment.Therefore, the sterilization rate of the LTAP treatment in the air-liquid environment was lower during the early sterilization period.In addition,comparing figure 4(b)with figure 4(d)and figure 6(b) with figure 6(d), respectively, the lethal effect of the LTAP treatment on bacteria for 5 min under the air-liquid environment was significantly higher than that in the all-air environment. The results were verified by Mai-Prochnow et al [26] and illustrated that ROS and RNS produced by plasma could react with water molecules to generate more stable active substances (H2O2and ONOO−, etc) and accumulate continuously. These new and stable substances could have a more significant bactericidal effect on bacteria [30].Nevertheless, when the bacteria died on the surface of the media in the all-air environment, the dead bacteria covered and protected the below bacteria from the LTAP making it impossible for the LTAP to further kill the bacteria. Therefore,the LTAP treatment has a higher bactericidal effect in an air-liquid environment than in an all-air atmosphere in the late sterilization stage.
3.4. ROS and RNS analyses
As shown in figure 8,the intracellular ROS and RNS content increased after being treated by LTAP for 1 min. Moreover,the ROS and RNS content in each bacterium decreased or continued to increase under different environments. This was consistent with the results in references [31, 32], which indicated that the antibacterial performances of LTAP were mainly due to the interactions between the ROS and the RNS.In this work, the fluorescence intensity of each bacteria decreased at the late stage (1–5 min) of LTAP treatment.These results are the same as those mentioned in references[33, 34], which indicates that the redox reaction products formed inside bacteria could be washed away by PBS when they died. According to the results of a TEM analysis(figures 4–7) and reactive nitrogen species (RONS) measurement, it was found that the ESBL-E.coli and HLGRE were almost all killed by LTAP under either an all-air or airliquid environment.
3.5. Temperature change after LTAP treatment
As shown in table 2, the temperatures (surface temperature of blood agar media) of ESBL-E.coli after 1and 5 min of LTAP treatment under the all-air environment were 20.4°C±0.3°C,and 20.6°C±0.1°C, respectively. The temperatures (surface temperature of bacterial suspension) of ESBL-E.coli at 1 and 5 min of LTAP treatments in the air-liquid environment were 24.4°C±0.2°C and 24.7°C±0.3°C, respectively, which indicated that there were no obvious temperature changes.Moreover, the same results could be obtained from HLGRE treated by LTAP under an identical environment.Overall, our results prove that the bactericidal effect of LTAP on the two kinds of bacteria in vitro was independent of the thermal effect with a short treatment [35].
4. Conclusion and future outlook
The purpose of our work was mainly to explore the sensitivity of two kinds of drug-resistant bacteria to LTAP in CAUTI patients under two different simulated environments in vitro.The results show that the survival rate of the two kinds of bacteria decreased to less than 20% and the lethal rate of the two kinds of bacteria in the early stage (0–1 min) was up to 50%. Moreover, the results of TEM, RONS measurement,and a temperature test indicated that the bactericidal effect of the LTAP treatment on the two kinds of bacteria worked through the destruction of the ribosome and other organelles inside the bacteria, rather than the thermal effect, to achieve sterilization. In this work, only the bactericidal effect of LTAP on the two representative drug-resistant bacteria in urethral infection of CAUTI patients was studied. But the optimal time and the effect of LTAP treatment on the normal cells in the urethra were not further explored. Overall, LTAP was found to be a mild,safe,and effective method,making it possible to solve clinical medical problems in the future.
 Plasma Science and Technology2020年6期
Plasma Science and Technology2020年6期
- Plasma Science and Technology的其它文章
- First experimental results of intrinsic torque on EAST
- Gas pressure effect on plasma transport in a magnetic-filtered radio-frequency plasma source
- Design of a variable frequency comb reflectometer system for the ASDEX Upgrade tokamak
- Optimization of plasma-processed air (PPA)inactivation of Escherichia coli in button mushrooms for extending the shelf life by response surface methodology
- Reconstruction of hollow areas in density profiles from frequency swept reflectometry
- Fullwave Doppler reflectometry simulations for density turbulence spectra in ASDEX Upgrade using GENE and IPF-FD3D
