Effects of extender and packaging method on morphological and functional characteristics of cryopreserved Ossimi ram semen
Wael A. Khalil, Abdel-Khalek E. Abdel-Khalek, Laura Falchi, Bedir E. El-Saidy, Ahmed I. Yousif
1Department of Animal Production, Faculty of Agriculture, Mansoura University, Mansoura, 35516, Egypt
2Department of Veterinary Medicine, Section of Obstetrics and Gynecology, University of Sassari, Sassari, 07100, Italy
3Animal Production Research Institute, Agriculture Research Centre, Ministry of Agriculture, Egypt
ABSTRACT
KEYWORDS: Cryopreservation; Egg-yolk; Lecithin; Sperm;Ossimi ram
1. Introduction
The optimization of techniques for semen cryopreservation is one of the key steps for enhancing fertility rates following artificial insemination. Besides its importance for genetic improvement,it also plays a role in the control of diseases and in facilitating management of herd fertility in farm animals[1].
Spermatozoa of small ruminants are more sensitive to cryopreservation than those of other species[2]. Changes in temperature and processing stress can cause massive ultrastructural, biochemical and functional damages on these cells[3] with decrease in motility and viability and consequently in fertility[4].Spermatozoa stored at low temperatures undergo oxidative stress,with accumulation of reactive oxygen species and perturbation of the redox status of the cell.
The composition of the extender, type of cryoprotectants used and freezing-thawing rates[5] are among the most influencing factors on semen cryopreservation. Therefore, the efficiency of extender types and freezing protocols have been investigated to compare the detrimental effects of cryopreservation on sperm morphologic and functional parameters in ram[6]. Lysolecithin and low-density lipoproteins of egg yolk have been proved to be effective in protecting spermatozoa from cold shock during freezing procedures[7].
Soybean lecithin is the alternative lipid/lipoprotein source of nonanimal origin employed in extenders for semen cryopreservation for its ability in protecting the phospholipids in mammalian biological membranes, providing stability to cell structure[8].
Another approach for assessing the deleterious effects of cryopreservation on sperm quality has been the use of antioxidants.Among others, butylatedhydroxytoluene[9], a phenolic synthetic analogue of vitamin E with anti-oxidant properties, acts as a membrane lipid perturbant, reducing the changes in permeability of cold shocked sperm membranes and inactivating lipid-containing viruses[10].
Among factors affecting the fertilizing ability of semen, storage packaging (straws or pellets) during freezing has been demonstrated to have variable effects on post-thaw sperm motility in ram[11].Although semen stored in pellets can achieve low temperatures faster,it has several post-freezing issues, especially the identification of the sample at thawing and the potential risk of cross-contamination with heterologous sperm cells when the carbon dioxide block or metal plate are reused[12].
Therefore, we aimed to study the effects of soybean lecithin or butylatedhydroxytoluene as alternative components to egg yolk,and packaging methods (straws or pellets) on morphologic and functional characteristics of frozen thawed semen of Ossimi rams.
2. Materials and methods
2.1. Animal management
The experiment was carried out at the Animal Production Research Station, Sakha, Kafrelsheikh Governorate (in the northern part of Nile Delta, latitude 31o15’ N and longitude 31o45’ E), Animal Production Research Institute, Agricultural Research Center,Ministry of Agriculture, Egypt, in cooperation with the Physiology and Biotechnology Laboratory, Animal Production Department,Faculty of Agriculture, Mansoura University, Egypt.
A total of 5 sexually mature Ossimi rams of proven fertility(weighting 60-80 kg; aged 2-4 years), trained to serve an artificial vagina were selected for semen collection. Only clinically healthy rams were used, and all rams were raised under the same environmental conditions in the experimental farm and kept under the semi-open shaded yard. Feeding requirements were calculated according to the recommendations of Animal Production Research Institute, Ministry of Agriculture, Egypt. Each ram was fed concentrate feed mixture at a level of 1.250 kg (14% calorific power)plus 5 kg Egyptian fresh berseem (Trifolium alexandrinum) during December to February or 1.250 kg berseem hay during August to November, with free access to trace mineralized salt lick blocks and drinking water ad libitum.
2.2. Collection of semen
Ejaculates were collected once weekly from each ram for nine weeks before feeding at 7-8 a.m. by using the conventional artificial vaginal method (a total of 45 ejaculates). In detail, the artificial vagina (in vitro maturation technologies) consisted in an open hard rubber hose fitted with an inner sleeve of soft latex and a rubber cone. The space between the hose and the inner sleeve was filled with water at 45 ℃ and air through a valve, and a 5-mL tube for semen collection was mounted on the rubber cone.
2.3. Experimental plan
The design of the experimental work was represented in Figure 1.Briefly, the 45 ejaculates of 5 rams were collected, kept in a water bath at 37 ℃, and submitted to an initial assessment. Only those ejaculates that scored ≥80% for mass motility, progressive motility and viability, ≥70% membrane integrity, ≤10% abnormality and sperm concentration of 2.4×109sperm cells/mL were selected for the experiment. The ejaculates were then pooled and subsequently divided into 3 aliquots diluted at 37 ℃ in 3 different extenders (post dilution stage): Tris-citric-egg yolk extender (TEY); Tris-soybean lecithin extender (TSBL); Tris-butylatedhydroxytoluene extender(TBHT). Samples were gradually cooled to 5 ℃ for 4 h (equilibration stage). In post dilution and equilibration stages, progressive motility,viability, abnormalities and membrane integrity of spermatozoa were assessed for each extender used. Samples were then frozen by pellets or straws and stored in liquid nitrogen until further evaluation.Frozen semen was thawed in a water bath at 37 ℃ for 30 s. In detail,3 straws and 3 pellets for each extender were thawed and assessed for progressive motility, viability, abnormalities and membrane integrity of sperm cells as well as oxidative stress, integrity of DNA and apoptosis. The experiment was carried out in 9 replicates.
2.4. Preparation of semen extenders
Tris egg yolk extender used in this study as a control contained 3.025 g Tris (Sigma Chemical Co., St. Louis, MO, USA), 1.660 g citric acid monohydrate (Sigma), 1.250 g glucose (Sigma, Aldrich),15% fresh egg yolk, 5% glycerol, and antibiotics (100 IU/mL penicillin and 100 μg/mL streptomycin). Egg yolk (15%) was replaced by 1% soybean lecithin (L-a-phosphatidyl choline, LAB:product number MC041) in the 2nd extender (TSBL) or 2 mM butylatedhydroxytoluenein the 3rd extender (TBHT).
Extenders were gently shaken and warmed up to 37 ℃ in a water bath before use. Osmolarity and pH were assessed and adjusted respectively to 300 mosm/kg H2O and 7.3 before addition of cryoprotectants.
2.5. Semen freezing and thawing
Pooled semen was diluted at 37 ℃ with the three extender types at a ratio of 1:5 (semen/extender) for a final concentration of 480×106sperm/mL (dilution stage). Extended semen was then gradually cooled at 5 ℃ for 4 h (equilibration stage).
Equilibrated semen was frozen by two methods of packaging:0.25 mL French straws (IMV, L’Agile France) or 0.25 mL pellets.The straws were loaded with extended semen, exposed to liquid nitrogen vapours for 5 min and then plunged into liquid nitrogen at -196 ℃. Pellet freezing procedure was performed according to Awad[11]. Briefly, 0.25 mL of extended semen were placed on small depressions of the surface of a block of dry ice for 2 min and then stored in small aluminium goblets in liquid nitrogen.
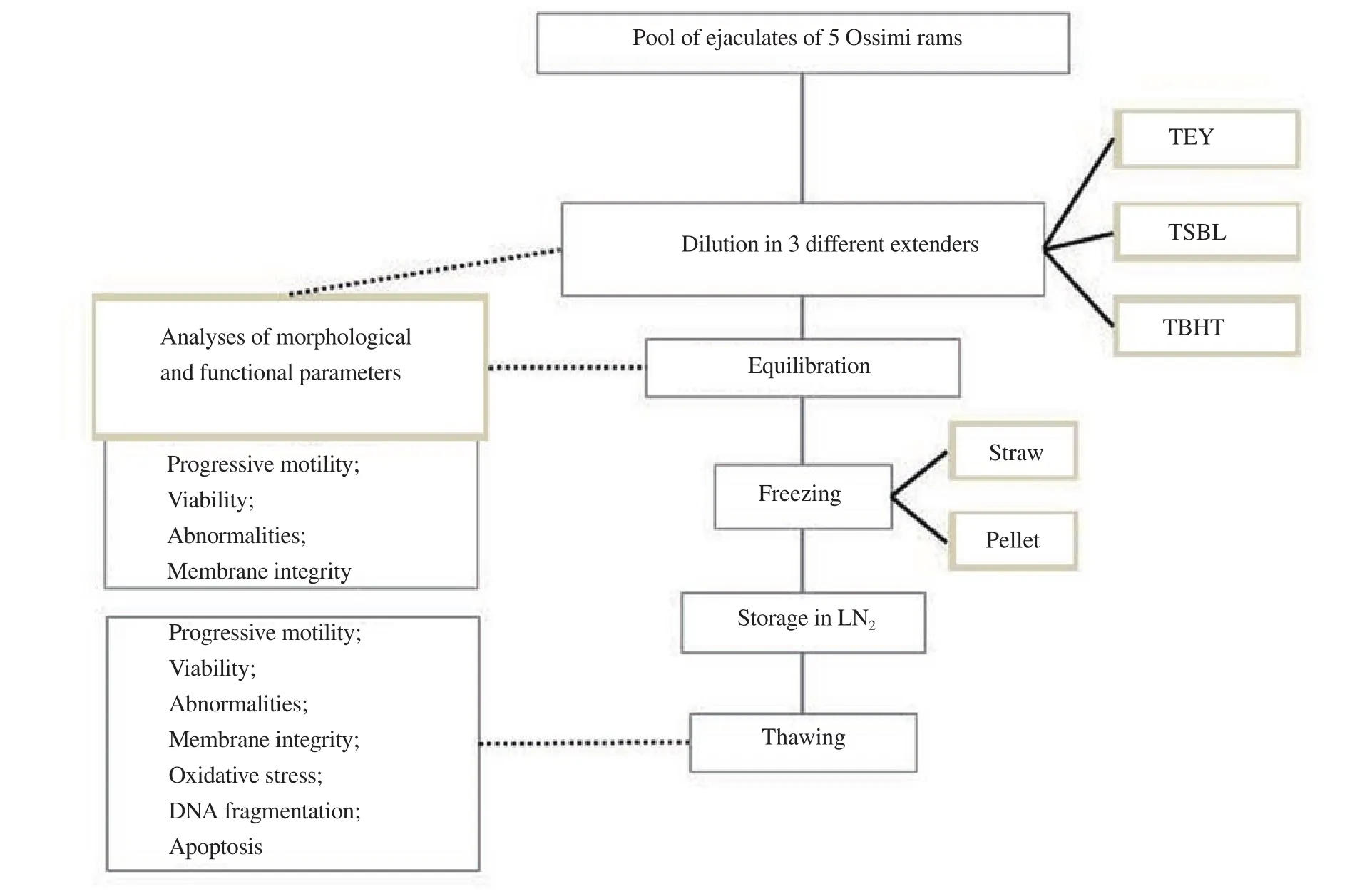
Figure 1. Experimental design of the study. TEY: Tris-egg yolk; TSBL: Tris-soybean lecithin; TBHT: Tris-butylatedhydroxytoluene; LN2: liquid nitrogen.
2.6. Semen evaluation
Semen was evaluated for sperm progressive motility, viability,abnormality, and plasma membrane integrity in 9 replicates for each treatment (extender type * packaging method) post dilution and at equilibration and thawing. Total antioxidant activity,malondialdehyde, and lactic dehydrogenase in thawed seminal plasma as well as comet assay analysis and annexin-Ⅴassay of thawed semen were determined in 3 replicates for each treatment.
2.6.1. Sperm progressive motility
A phase-contrast microscope with magnification (100×) and supplied with a hot stage at 37 ℃ (DM 500, Leica, Switzerland) was used to determine the percentage of progressive sperm motility which was defined as the ability of sperm to move forward in a long semiarc pattern. A 10 μL aliquot of diluted semen was placed on a warm slide and covered with a coverslip. The same professional investigator performed the blind analysis that was conducted in 3 replicates.
2.6.2. Sperm viability
Live spermatozoa were determined in a smear of semen stained by a mixture of 5% eosin (vital stain) and 10% nigrosin (background stain). Percentage of the live spermatozoa (unstained ones) per sample was calculated for 300 sperm cells examined at high magnification (400×) by using a light microscope (DM 500, Leica,Switzerland).
2.6.3. Sperm abnormalities
Abnormalities were assessed during the examination of 300 live/dead spermatozoa by using a light microscope (DM 500, Leica,Switzerland) with high magnification (400×). The following criteria were considered: i) tail defects (abnormal tails), ii) abnormal heads,iii) cytoplasmic droplets.
2.6.4. Plasma membrane integrity
In order to assess the functionality and integrity of plasma membranes of spermatozoa, the hypo-osmotic swelling test was used according to a previously described protocol by Neild et al[13]. Semen (10 μL) was incubated at 37 ℃ for 30 min with hypo-osmotic solution (100 μL at osmolarity level of 75 mOsmol/L), containing fructose (6.75 g/L) and sodium citrate (3.67 g/L).A sample of the mixture was placed on a slide and covered with a coverslip. Spermatozoa with coiled or swollen tails (with functional intact membranes) were counted in each sample at 400× under phase-contrast microscopy on a total count of 300 sperm cells/slide.
2.6.5. Oxidative stress
Concentration of total antioxidants[14], MDA[15] and lactic dehydrogenase activity[16] were determined in post-thawed seminal plasma by using commercial kits (Biodiagnostic, Egypt)and spectrophotometer (SPECTRO UV-VIS AUTO, UV-2602,Labomed, USA). All procedures were carried out according to the manufacturers’ instructions.
2.6.6. Sperm DNA damage (Comet assay analysis)
The integrity of sperm DNA was assessed by comet assay analysis according to Boutet et al[17]. Briefly, the comet assay detected DNA damage in individual sperm cells based on micro-electrophoresis of DNA content. Fluorescence stained comet images were captured with a couple charged device camera (Optica version pro, Italy)attached to a fluorescence microscope and linked to a comet assay software program (Comet score, Version 5.0). Sperm cells were classified as: i) negative = intact sperm cells (with non-fragmented DNA) that did not form a comet; ii) positive = sperm cells with fragmented DNA exhibiting increase in DNA migration outside the nucleus and brightly fluorescent head and tail. For quantifying sperm DNA damage, tail moment was calculated as the product of % tail DNA and tail length.
2.6.7. Sperm apoptosis
According to Chaveiro et al[18], semen samples were stained by annexin-Ⅴ(AⅤ) (calcium-dependent probe) that tracks phosphatidylserine externalization in the membrane of sperm cells.A commercial Phosphatidylserine Detection Kit (IQP, Groningen,Netherlands) was used according to the manufacturer’s instructions.Briefly, semen samples were thawed and washed twice by centrifugation (300×g for 10 min at 4 ℃) with phosphate buffer solution. The supernatant was removed and the sperm pellet was resuspended in binding buffer at a concentration of 1×106sperm cells/mL. Afterwards, an aliquot of sample (100 μL) was transferred to culture tube (5 mL) containing 5 μL AⅤ (fluorescein isothiocyanate, FITC label, BD Biosciences, US) and 5 μL propidium iodide (PI) (BD Biosciences, US), and then incubated in dark at room temperature (25 ℃) for 15 min and 400 μL of additional binding buffer was added to each tube. Within 5 min,evaluation was conducted by flow cytometry.
Flow cytometry analysis was carried out on Accuri C6 Cytometer(BD Biosciences, San Jose, CA) by using Accuri C6 software(Becton Dickinson) for acquisition and analysis[19]. The percentages of negative or positive for AⅤ (A-or A+), and PI (PI-or PI+), as well as double positive cells were evaluated. Following flow cytometry,sperm subpopulations were classified as percentage of viable sperm cells, negative for both AⅤ and PI staining (A-/PI-); early apoptotic sperm cells, positive for AⅤ and negative for PI (A+/PI-); apoptotic sperm cells, positive for both AⅤ and PI (A+/PI+); dead sperm cells,negative for AⅤ and positive for PI (A-/PI+).
2.7. Statistical analysis
The general linear model analysis of variance (ANOVA) was used for statistical analysis of data[20]. One-way ANOVA was used for testing the effect of extender type on different sperm characteristics in diluted and equilibrated semen prior to packaging method.The effect of each extender type with each packaging method on sperm characteristics in thawed semen, total antioxidants capacity,malondialdehyde concentration and lactic dehydrogenase activity in seminal plasma of thawed semen, and parameters of annexinⅤ/propidium iodide assay were tested by one-way ANOVA.
The significant differences among treatments for all considered parameters were tested according to Duncan multiple range tests at a level of P<0.05. Arcsine transformation was performed before the analysis of variance, then pre-arcsine values were expressed as mean±standard deviation (mean±SD).
2.8. Ethics statement
This study was done under proper research ethics and care that was approved by Mansoura University Research Committee, Egypt,which was in agreement with the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes.
3. Results
3.1. Sperm characteristics in diluted and equilibrated semen
Among all sperm characteristics analysed, only sperm membrane integrity percentage in equilibrated semen significantly improved(P<0.05) in TEY and TSBL compared with TBHT extender.However, percentages of motility, viability and abnormality in diluted and equilibrated semen were nearly similar for all extender types (P>0.05) ( Table 1).
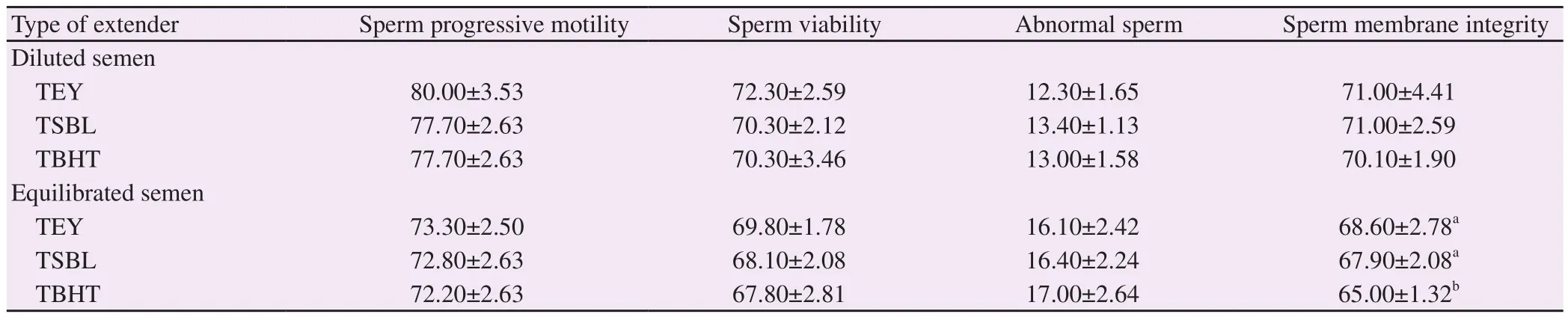
Table 1. Effect of extender type on sperm characteristics in diluted and equilibrated ram semen (%).
3.2. Sperm characteristics in thawed semen
Both progressive motility and viability percentages of spermatozoa were higher (P all <0.05) in straws than in pelleted semen for each extender. For pelleted semen, the progressive motility and vitality of sperm cells were the highest in TEY extender, and the lowest for TBHT. However, no significant differences were shown in the percentages of abnormal sperm and sperm membrane integrity (P both >0.05) (Table 2).
3.3. Antioxidants capacity and lactic dehydrogenase activity in seminal plasma of thawed semen
In thawed seminal plasma, there was no significant difference in total antioxidant capacity among three extenders (P>0.05). But,TBHT or TSBL in straws and TBHT in pellets significantly reduced malondialdehyde (P<0.05). Lactic dehydrogenase activity was affected by both extender and packaging method. Activity of lactic dehydrogenase was the highest in TBHT, moderate in TEY and the lowest in TSBL extenders, regardless of packaging methods. TBHT in pellets significantly increased lactic dehydrogenase comparing with all other groups (P all <0.05) (Table 3).
3.4. Analysis of apoptosis by flow cytometer
Levels of apoptosis analysed by AV/PI assay revealed that viable sperm percentage was significantly higher in TEY semen stored either in straws or pellets compared with the corresponding packing methods of TSBL and TBHT (P<0.05). Within the extender used,apoptotic sperm cells were lower in semen stored in straws than in pellets for all the three extenders. Percentages of early apoptotic sperm cells showed the same trend, being lower in straws compared with pellets within the extender used (P all <0.05). Moreover,between extenders significant differences were also found in TEY and TSBL. Early apoptotic sperm cells were higher in TEY and lower in TSBL stored semen in both straw and pellet (P both<0.05). Percentage of apoptotic spermatozoa was lower for semen in straws compared with pellets independently from the extender used (P<0.05). Apoptotic sperm cells were the lowest in straws with TEY and the highest in pellet with TSBL. The percentage of necrotic sperm cells was not significantly affected by extender type or packaging method (P>0.05) (Table 4).
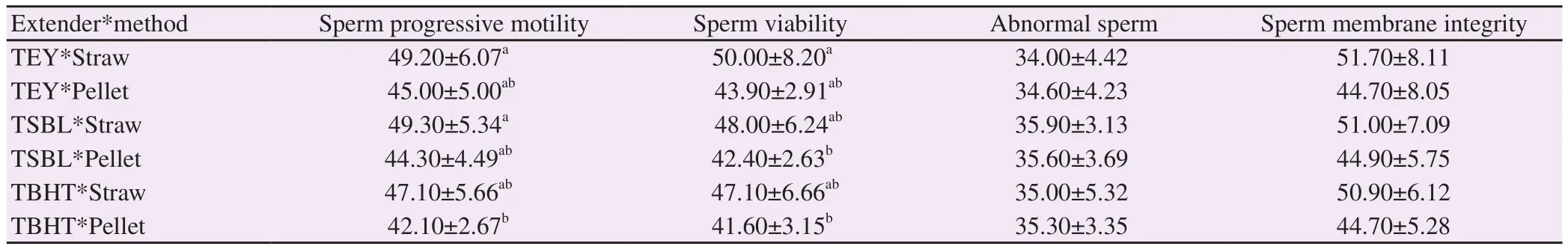
Table 2. Effect of extender type and packaging method on sperm characteristics in thawed ram semen (%).
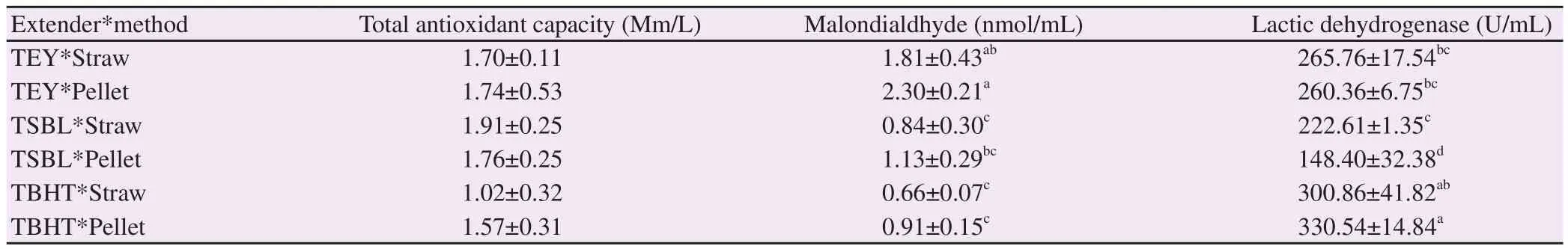
Table 3. Effect of extender type and packaging method on total antioxidants capacity, malondialdehyde concentration and lactic dehydrogenase activity in seminal plasma of thawed ram semen.
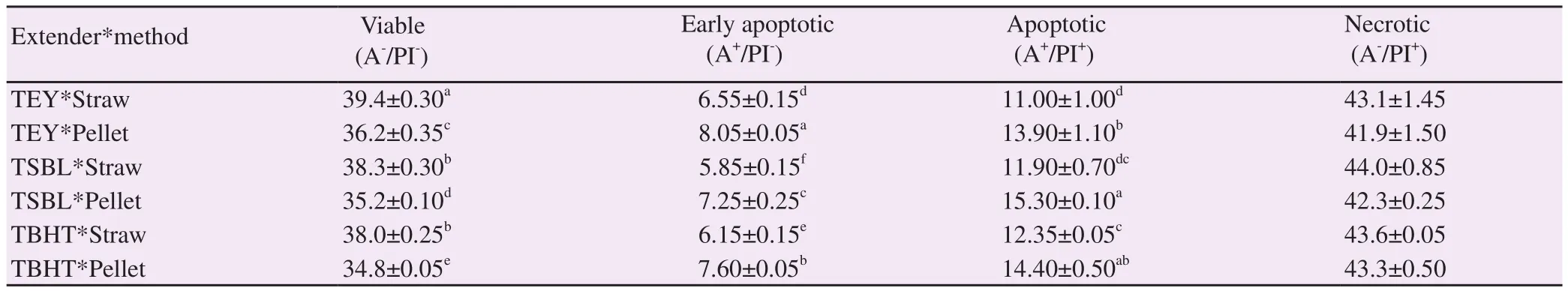
Table 4. Effect of extender type and packaging method on percentage of viable, early apoptotic, apoptotic and necrotic spermatozoa in thawed ram semen using annexinⅤ/propidium iodide assay (%).
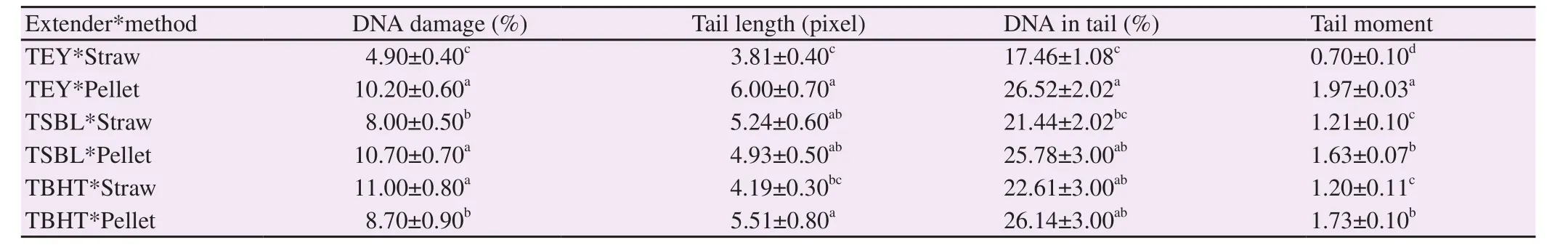
Table 5. Effect of extender type and packaging method on comet assay parameters in thawed ram semen.
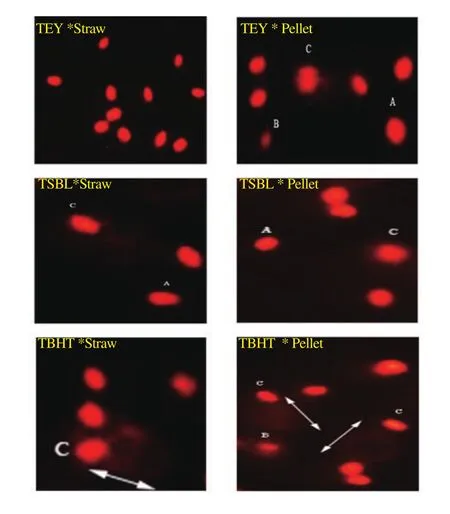
Figure 2. Typical images of spermatozoa observed from comet assay in thawed Ossimi ram semen (ethidium bromide stain;magnification: ×400).A: Spermatozoa with intact nuclei, undamaged comet (without DNA fragmentation). B: Spermatozoa with low damaged comet (low DNA fragmentation). C: Spermatozoa with high damaged comet (high DNA fragmentation). Arrow points to a degree of tail length.
3.5. DNA integrity
Packaging semen extended with TEY in straws showed the lowest percentage of DNA damage, tail length, DNA in tail, and tail moment in thawed semen. Contrarily, semen extended with TEY or TSBL in pellets and TBHT in straws showed the poorest results of sperm DNA damage (P all <0.05) (Table 5; Figure 2).
4. Discussion
Using egg yolk in semen extenders could potentially be a cause of allergic reactions and responsible for bacterial contamination in preserved semen[21]. The current study showed that semen diluted in TEY extender maintained functional parameters compared with those diluted in animal protein-free extenders, such as TSBL and TBHT. Similarly, no differences were reported in percentages of sperm viability, motility[22], and membranes integrity[23] in ram and goat semen extended either in TEY or TSBL or regarding the progressive motility percentages in goat semen extended in TBHT as compared with TEY[24].
The choice of a suitable concentration of extender was based on what found in literature. Extenders with 15% egg yolk had positive effects on the viability of ram cryopreserved spermatozoa[25].Soybean lecithin at 1% in the extenders has been proved to protect sperm membranes and to increase cryo-tolerance[26].Butylatedhydroxytoluene supplementation has been rarely used in the cryopreservation of ram semen. Therefore, the choice of the best concentration (2 mM) of butylatedhydroxytoluenewas made according to what previously reported in literature. In the ram, a dated experiment carried out by Farshad et al[27] showed that concentrations of butylatedhydroxytoluene from 2 to 3 mM provided the best protection to spermatozoa submitted to cold shock. In buffalo[9] concentrations of butylatedhydroxytoluene(2.0 mM) were considered to be effective in improving the quality of cryopreserved semen. In bull, high concentrations of butylatedhydroxytoluene increased plasma membrane fluidity and the amount of malondialdehyde in thawed semen with increase in spermatozoa acrosome damage[28]. Thawed canine semen,diluted in extender supplemented with increasing concentrations of butylatedhydroxytoluene (0.5−2.5mM) showed increased quality parameters such as sperm motility, acrosomal integrity,viability and plasma membrane integrity[29]. Equilibration time,during which spermatozoa are exposed to cryoprotectant prior to freezing, causes marked physic-chemical changes leading to various levels of damage in structure of sperm cells and deteriorating its characteristics after thawing[30]. According to our results, there is a noticeable reduction in the quality parameters of equilibrated semen. In buck semen diluted in TEY, equilibrated spermatozoa showed a reduction in motility, viability and integrity of plasma membrane of spermatozoa. This decrease in sperm quality may be in association with changes in pH value, osmolality level and bacterial contamination in the extended semen[31]. In ram semen extended in butylatedhydroxytoluene, the observed reduction of plasma membrane integrity post-equilibration compared with post-dilution can be attributed to the prolonged cooling during equilibration (4 h at 5 ℃). This factor seems to change sperm permeability by decreasing plasma membrane integrity after equilibration[32].
In the present study, the observations on morphologic functiona characteristics of thawed semen are different from those previously reported in the literature. The obtained results in our study indicate positive effects of storage in straws, regardless of the extender used,on sperm characteristics of thawed ram semen. Similarly, Dai et al[33] reported that boar semen diluted in TEY and packaged in 0.25 mL straws had higher post-thaw motility rates and plasma membrane integrity than semen stored in pellets.
In our study, the obtained results showed no significant effects of extender type or semen packaging method on the total antioxidant capacity, while malondialdehyde concentration pronouncedly decreased for TSBL in straw and for TBHT with both packaging methods. There was a tendency of increasing total antioxidant capacity to the highest values in semen extended with TSBL and reducing malondialdehyde to the lowest values in TBHT, being(numerically) better in straws than in pellets. In agreement with the present results, total antioxidant capacity was higher, while malondialdehyde concentration was lower in semen diluted with TSBL supplemented with Camellia sinensis extract and packaged in 0.25 mL French straws[34]. Also, packaging the ram semen in 0.25 mL straws significantly decreased malondialdehyde concentration in the egg yolk extender with 1% rainbow trout seminal as compared with the lecithin extender[35].
The present results showed that lactic dehydrogenase activity in seminal plasma of thawed semen was higher in TBHT extender than in TEY and TSBL extenders, respectively. Also, semen packaging method significantly increased activity of lactic dehydrogenase in seminal plasma of straws compared with pellets only in semen extended with TSBL. It could be responsible for driving glycolysis when oxygen is limited by carrying nicotinamide adenine dinucleotide-mediated reduction of pyruvate to lactate[36].The reduction in lactic dehydrogenase activity in seminal plasma is an indicator of disturbance in function and metabolism of spermatozoa[37].
Freezing and thawing caused sperm apoptosis and decreased the percentage of viable spermatozoa. The obtained results using AV/PI assay indicated beneficial effect of straw as a packaging method of ram semen cryopreservation as compared with pellets, in terms of higher viability and lower apoptosis percentage in each extender type, being the best in TEY as compared with other extenders.
The role that sperm DNA damage plays in reproductive outcome has become increasingly debated and investigated[38].Cryopreservation induced sub-lethal damage to the spermatozoa of Merino ram, which resulted in loss of motility, viability, in vivo fertilizing capacity, deterioration of acrosomal and plasma membrane integrity, and damage of DNA[39]. A relationship between sperm DNA integrity and fertility has been reported in goat[40].
According to the results obtained in the present study, extender type affects DNA integrity of cryopreserved spermatozoa as reported by many authors. Storage in straws and TEY preserved DNA integrity parameters of thawed semen compared with the other two extenders.Similarly, El-Badry et al[41] found that the addition of 15% egg yolk to extender resulted in increased DNA integrity in thawed spermatozoa. Poor fertilization, impaired implantation rates and increased incidences of abortion and disease in the offspring are positively correlated with DNA damage of spermatozoa[42].
Results obtained from the current study showed that the comet assay can be used as an additional parameter for the quality assessment of ram semen after cryopreservation.
In conclusion, despite the disadvantages of dilution of cryopreserved semen with egg yolk, ram semen cryopreserved with TEY gives the best physical, morphological and functional characteristics in straws compared with pellets, followed by semen diluted with TBSL. However, semen diluted with TBHT or TSBL,regardless of packaging method, showed the highest impact on antioxidant status of cyopreserved ram semen. Further strategies are required to improve quality parameters of thawed ram semen.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors’ contributions
Wael A. Khalil and Abdel-Khalek E. Abdel-Khalek made substantial contributions to conception and design; Ahmed I. Yousif and Bedir E. El-Saidyconducted acquisition of data; Laura Falchi and Ahmed I. Yousif made analysis and interpretation of data; Laura Falchi and Wael A. Khalil performed statistical analyses; Ahmed I. Yousif, Laura Falchi and Wael A. Khalil drafted the manuscript;Abdel-Khalek E. Abdel-Khalek, Laura Falchi, Wael A. Khalil and Ahmed I. Yousif critically revised the manuscript for important intellectual content; and all authors had final approval of the manuscript for publication.
 Asian Pacific Journal of Reproduction2020年3期
Asian Pacific Journal of Reproduction2020年3期
- Asian Pacific Journal of Reproduction的其它文章
- COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery
- Ascorbic acid and curcumin alleviate abnormal estrous cycle and morphological changes in cells induced by repeated ultraviolet B radiations in female Wistar rats
- Antifertility effects of Azadirachta indica methanol seed extract on canine spermatozoa in vitro
- Effects of vascular endothelial growth factor supplementation and alginate embedding on human oocyte maturation in vitro
- Effect of double cleavage stage versus sequential cleavage and blastocyst stage embryo transfer on clinical pregnancy rates
- Blastocyst elective single embryo transfer improves perinatal outcomes among women undergoing assisted reproductive technology in Indonesia
