Ascorbic acid and curcumin alleviate abnormal estrous cycle and morphological changes in cells induced by repeated ultraviolet B radiations in female Wistar rats
Gayatri Rai, Narendra Namdev, Payal Mahobiya
Department of Zoology, Dr Harisingh Gour (A Central University) Vishwavidhyalaya, Sagar (M.P.), India
ABSTRACT
KEYWORDS: Estrous cycle; Ultraviolet-B radiation; Vaginal smear; Ascorbic acid; Curcumin
1. Introduction
Ultraviolet (UV) rays include a band of electromagnetic radiations with a wavelength from 200 nm to 400 nm. Being present in the sunlight, UV radiations are an important source of energy and have sufficient power to penetrate the body cells; consequently,the chemical and biological effects generated by these radiations are much greater than those by simple heating effects. Radiations emitted and transmitted through different sources are absorbed by the animal body, which tends to be very high levels of environmental toxin. UV radiations bear the ability to induce both positive and negative effects, thereby altering the well-being of both animals. UV radiations are non-ionizing and are classified into three types UVC, UVB, and UVA. UVC (200-280 nm, shortwave length)is more lethal than UVB (280-320 nm, medium wavelength) and UVA (320-400 nm high wavelength)[1-3].
Mammalian germ cells are very sensitive to radiation, which can change the structure of the cell cytoplasm and nucleus[4] and affect the sensitivity of cells and tissues. The sensitivity of cells and tissues to the effects of radiation vary, with actively dividing cells (blood cells, embryonic cells and cells of gonads) being more sensitive. Cells exposed to radiation are more sensitive than the normal viable cells i.e. cells of the gonads (ovaries and testes),embryonic cells and blood cells. Many factors including age, stress,noise, light, temperature, nutrition, and social relationships impact the estrous cycle length[5-9]. Radiations also affect the reproductive behaviour by inducing imbalance of the production of the hormones by mammalian gonads[10].
In order to maintain healthy reproductive performance, breeding conditions (light cycle) and timing need to be properly controlled and regulated. The artificial light-dark cycle of an animal facility is critical to the synchronous development of eggs. Female rats should be allowed to stand around one week so as to acclimatize to the artificial conditions of light-dark cycle. The release of luteinizing hormone, a pituitary hormone that induces ovulation, is regulated by the light-dark cycle. The animal rooms are usually of 14 h light and 10 h dark cycles. The reproductive cycle of female rats called estrous cycle is categorized into four main stages i.e. proestrus,estrus, metestrus, and diestrus. The onset of sexual maturity is up to the age of 12 months and the mean cycle length is 4 days[11]. Due to the short cycle length, rats are ideal models to investigate various changes of the reproductive cycle[12,13].
Previous studies concerned the reproductive system as well as the influence of the estrous cycle on the non-reproductive functions[14-17] and vaginal smear cytology was used for the determination of the estrous cycle phases[1,18]. The characterization of each phase is based on the proportion among three types of cells observed in the vaginal smear: epithelial cells, cornified cells, and leukocytes. The collection of vaginal secretions and the use of stained material generally take 1-2 hours or more. Although having much lesser penetrating power, UVB radiations are potent enough to cause DNA damage[19]. UVB radiation is the main stimulator of the constitutive xanthine oxidase and nitric oxide synthase in human endothelial cells[20], as well as in human keratinocytes[21].
To the best of our knowledge, the present study is pioneering in its field, which for the first time demonstrates the effect of repeated UVB radiation exposure on the estrous cycle of female Wistar rats.In the present study, we also investigated the protective effect of antioxidants (ascorbic acid and curcumin) against the morphological changes in cells induced by UVB radiation.
2. Methods and materials
2.1. Chemicals
All the chemicals and reagents used were of analytical grade.Ascorbic acid was purchased from Sigma-Aldrich Co., USA.Curcumin was obtained from Himedia, India and crystal violet and rest of the chemicals used were obtained from Central Drug House(P) Ltd, New Delhi, India.
2.2. UV irradiation
UVB light (TL 20W/01 UVB Narrowband made in Germany),which emitted UVB in the range of 280nm (UVB), was used as the source to irradiate the Wistar rats. The irradiance was two hours daily for a period of 15 days[22].
2.3. Experimental animals and treatment
Female adult Wistar rats weighing 130-150 g and aged 12-16 weeks were purchased from the College of Veterinary Sciences and Animal Husbandry Mhow (22.55° N, 75.75° E, M.P), India.All animals (n=16) were housed in plastic cages and were fed on standard laboratory diet daily food and water ad libitum. Rats were kept on laboratory conditions, with standard temperature (20±2) ℃,relative humidity of (40%-60%) and 12h/12h light and dark cycle.The experimental rats were randomly divided into four groups, with 4 rats in each group. The control group received normal food and water ad libitum. The UVB group received a dose of 280 nm of UVB radiation for 2 h daily for a period of 15 days. The UVB+curcumin group received a dose of 280 nm of UVB radiation for 2 h daily and also an oral dose of curcumin (25 mg/kg body weight) daily for a period of 15 days. The UVB+ascorbic acid group received a dose of 280 nm of UVB radiation for 2 h daily and also an oral dose of ascorbic acid (250 mg/kg body weight) daily for a period of 15 days[22-24].
2.4. Sample collection
At the end of the experiment, animals were anesthetized and sacrificed by cervical dislocation; ovaries were dissected out, washed in ice-cold phosphate buffer saline and stored at -20 ℃ for further analysis.
2.5. Measurement of body and ovary weight
Measurement of body and ovary weight was done with an electronic balance (Sartorius, BP210 S). Body weight measurement was performed before and after the experiment. Ovary weight measurement was performed after the experiment.
2.6. Calculationof gonadosomatic index
Calculation of gonadosomatic index between body weight and gonad weight individually was done by the following formula.
Gonadosomatic index = Gonad weight×100/Body weight
2.7. Estrous cycle
Estrous cycle, the main reproductive cycle of non-primate vertebrates such as mice, rats, horses, etc represented the cyclic pattern of the ovarian activity that allowed females to go from a period of reproductive receptivity to non receptivity, eventually leading to pregnancy after successful mating. In rodents such as rat the estrous occurred after every 4-5 days, with sequential stages of proestrus, estrus, metaestrus and diestrus which lasted for 1, 1, 1 and 2 days, respectively. The stages of the estrous cycle were estrus,metaestrus, diestrus and proestrus[25]. These stages occurred in each cycle and in a sequential manner. The day of estrus was usually designated as day 1 of the cycle. The stages of the estrous cycle were best determined by the cell types observed in the vaginal smear.Normally, vaginal smears should be examined in the morning (08:00 to 09:00 a.m.).
Sexually mature female animals were selected and vaginal smears were collected by using a sterilized micropipette. The micropipette was filled with a small amount of double-distilled water and was then inserted into the vagina of the female rat. The vagina was flushed two to three times with the double-distilled water and then the fluid was placed onto a glass slide. The fluid was equally distributed onto the glass slides and the smear was stained with crystal violet (1%) and observed under a light microscope with 10×and 40× magnification (Carl Zeiss, Germany)[26].
2.8. Statistical analysis
All statistical analysis was performed by using one-way analysis of variance. The data were expressed as mean±standard deviation(mean±SD). Dunnett test was applied for the comparison between the control group and each treated group individually. P<0.05 was considered statistically different.
2.9. Ethics statement
This study was approved by the Department of Pharmaceutical Sciences Dr. Harisingh Gour Vishwavidyalaya (A Central University)Sagar (M.P.), India (Ethical registration No. 379/CPCSEA/IAEC-2018/2017). Also, international guidelines were followed for the care and use of laboratory animals.
3. Results
3.1. Body weight, ovary weight, and gonadosomatic index
Female Wistar rats in the UVB group showed a significant reduction in body weight as compared with the control group(P<0.05). It was observed that curcumin and ascorbic acid treatment increased the body weight significantly (P<0.05) as compared with the UVB group and no significant difference was found when compared with the control group. A significant decrease in ovary weight was observed in the female Wistar rats of the UVB group as compared with the control group (P<0.01), while a significant increase was noted in the UVB+curcumin and UVB+ascorbic acid groups as compared with the UVB group. No significant difference was observed in UVB+curcumin and UVB+ ascorbic acid groups as compared with the control group. Gonadosomatic index decreased in the UVB group while increased in UVB+curcumin and UVB+ascorbic acid groups and no significant difference was found when compared with the control group (Table 1).
3.2. Estrous cycle
In our experiment, it was found that the UVB exposure irradiated on whole body with a dose of 280 nm wavelength for 2 h expressively influenced the phases of estrous cycles (Figures 1 to 4). Exposure of UVB radiation on the animal showed significant differences and irregular alterations in the estrous cycle and their cells.
During the proestrus phase, the appearance of epithelial cells was shown in different groups (Figure 1). Nucleated epithelial cells were observed in the control group (Figure 1 A1 and A2). Nucleated epithelial cells in cohesive clusters were seen in the UVB group(Figure 1 B1 and B2). Separation of cohesive clusters of epithelial cells was shown in the UVB+curcumin group (Figure 1 C1 and C2).Epithelial cells appeared in the UVB+ascorbic acid group (Figure 1 D1 and D2)
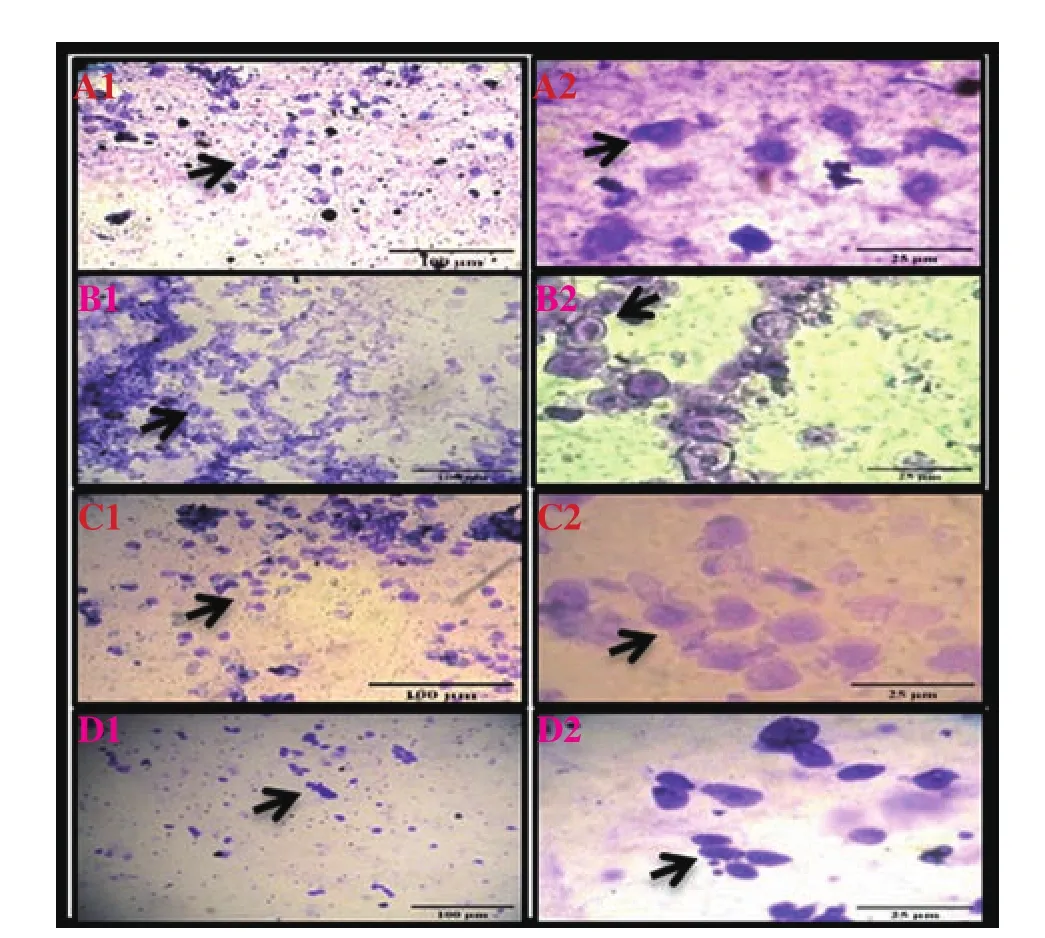
Figure 1. Vaginal smears of proestrus stain with crystals violet in UVB-irradiated Wistar rats treated with curcumin and ascorbic acid (Images show 10× and 40× magnification). Nucleated epithelial cells are observed in the control group (A1 and A2); nucleated epithelial cells in cohesive clusters are seen in the UVB group (B1 and B2); separation of cohesive clusters of epithelial cells is shown in the UVB+curcumin group (C1 and C2); epithelial cells appear in the UVB+ascorbic acid group (D1 and D2).

Table 1. Body weight, ovary weight and gonado-somatic index in ovaries of female Wistar rats in all groups.
During the estrus phase, the control group showed normal cornified squamous anucleated cells and the predominance of nucleated and cornified epithelium cells was shown (Figure 2 A1 and A2). The UVB treated group presented the degraded cornified squamous fluid and structure and showed less nucleated and cornified epithelial cells (Figure 2 B1 and B2). In the UVB+curcumin group, curcumin prevented cornified squamous anucleated cells (Figure 2 C1 and C2).In the UVB+ascorbic acid group, ascorbic acid showed a curative effect on the degrading anucleated cells (Figure 2 D1 and D2).Compared with ascorbic acid, curcumin prevented the nucleated and cornified epithelial cells.
During the metestrus phase, the UVB group presented the emergence of neutrophils interspersed or clumped among the nucleated epithelial cells as compared with the conrtol group. UVB changed the cornified epithelial cell. Rats had fewer numbers of nucleated epithelial cells (Figure 3 B1 and B2). As metestrus progressed, an increase of neutrophil number resulted in smears of less cellularity. In the UVB+curcumin group, curcumin repaired the cell cornified structure(Figure 3 C1 and C2). In the UVB+ascorbic acid group, ascorbic acid prevented cell cornified structure (Figure 3 D1 and D2).
During diestrus phase, the predominance of nucleated epithelium cells was shown in the control group (Figure 4 A1 and A2).However, in the UVB group, UVB changed the cornified epithelial cell; mucous entrapped and distorted the epithelial cells, neutrophils and leukocytes were observed (Figure 4 B1 and B2). In the UVB+curcumin group, curcumin repaired the cell cornified structure(Figure 4 C1 and C2). In the UVB+ascorbic acid group, ascorbic acid prevented cell ornified structure (Figure 4 D1 and D2).
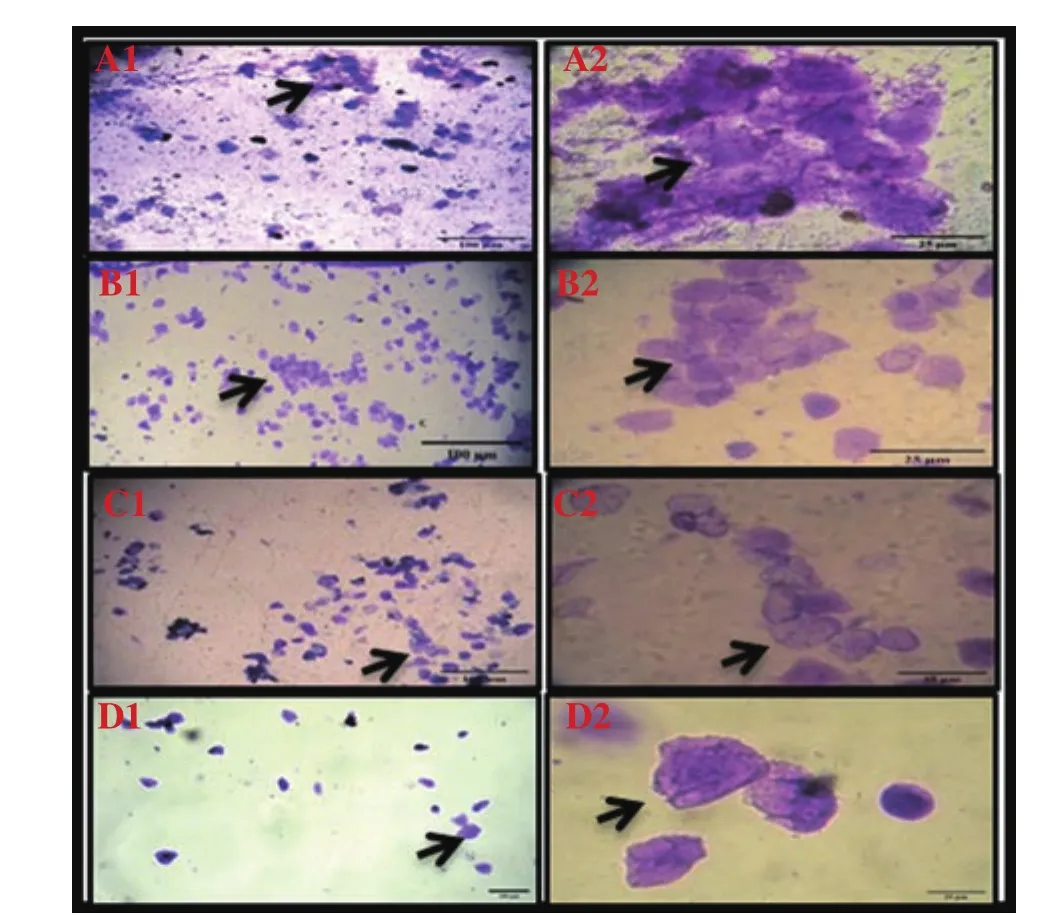
Figure 2. Vaginal smears of estrus stain with crystals violet in UVB-irradiated Wistar rats treated with curcumin and ascorbic acid (Images show 10× and 40× magnification). Normal cornified squamous anucleated cells are shown in the control group (A1 and A2). Degraded cornified squamous fluid and structure are present in the UVB group (B1 and B2). Curcumin prevents the cornified squamous anucleated cells in the UVB+curcumin group (C1 and C2). Ascorbic acid has a curative effect on the degrading anucleated cells in the UVB+ascorbic acid group (D1 and D2).
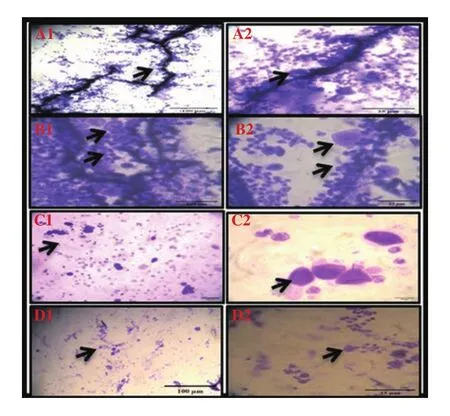
Figure 3. Vaginal smears of metestrus stain with crystals violet in UVB-irradiated Wistar rats treated with curcumin and ascorbic acid (Images show 10× and 40× magnification). A1 and A2 show the control group. In B1 and B2 (the UVB group), UVB changes the cornified epithelial cell. In C1 and C2 (the UVB+curcumin group), curcumin repairs the cell cornified structure.In D1 and D2 (the UVB+ascorbic acid group), ascorbic acid prevents cell cornified structure.
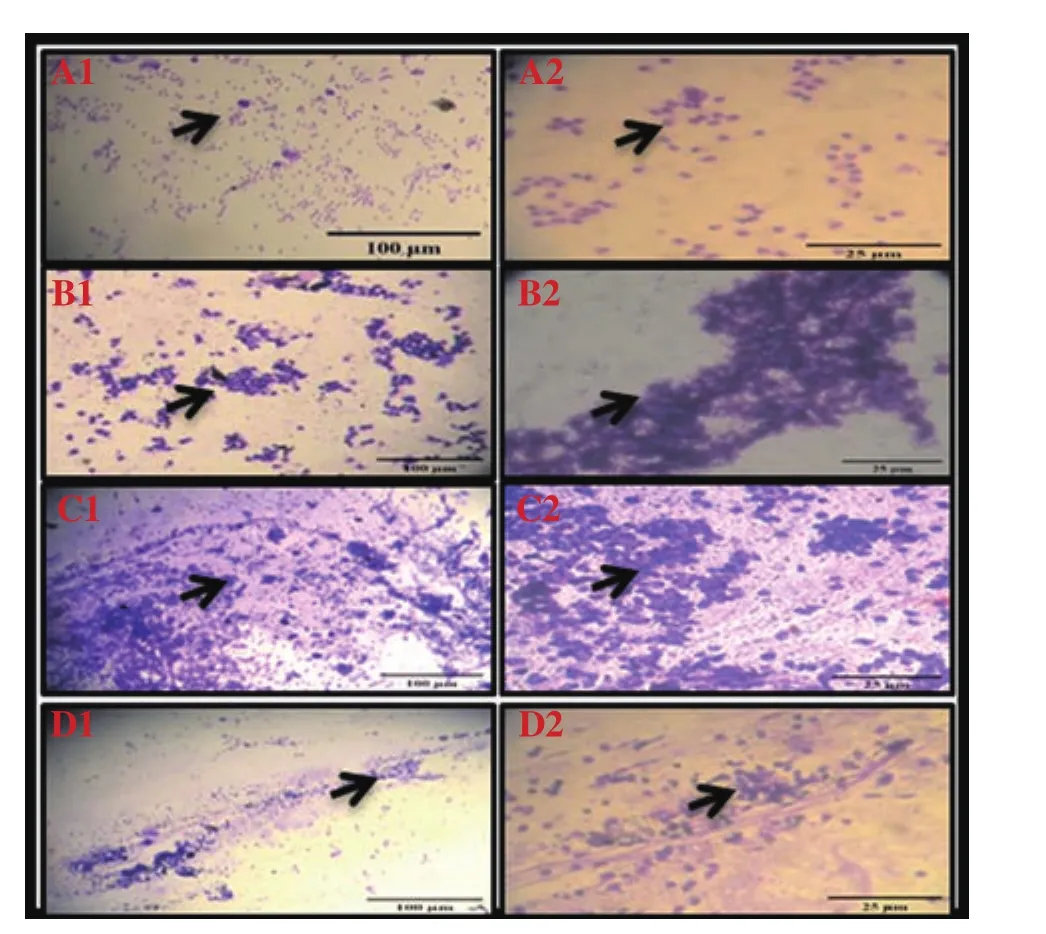
Figure 4. Vaginal smears of diestrus stain with crystals violet in UVB-irradiated Wistar rats treated with curcumin and ascorbic acid (Images show 10× and 40× magnification). A1 and A2 show the control group. B1 and B2(the UBV group) show aggregated white blood cells due to UVB irradiation.In C1 and C2 (the UVB+curcumin group), curcumin prevents aggregation of the white blood cells and cures mucus. In D1 and D2 (the UVB+ascorbic acid group), ascorbic acid prevents aggregation of white blood cells and less mucus is seen.
4. Discussion
The present study reveals that UVB exposure does influence the oestrus cycle in female rats. A whole estrous cycle in rats takes seven to fifteen days among UVB exposure days. This cycle involves four stages: proestrus, estrus, metestrus, and diestrus.
UVB radiation was non-ionizing radiation, which meant that this radiation was able to non-ionise the material in its path. In this case, the cells of the ovaries were actively divided into blood cells and ovarian gametes. These cells were very sensitive to the effect of radiation and as a result, damaged ovarian cells. The results of different phases of the estrous cycle were irradiated with UVB radiation[27]. The rodent estrous cycle was very sensitive to alterations in the radiation. Our result showed the alteration of nucleated epithelium cells, cornified epithelium cells, leukocyte cells.
Morphological changes in vaginal smear were detected with conventional stains and crystals violet also showed significant changes. The striking change in the nucleated epithelial cells of proestrus-estrus is especially evident by this method[28]. UV radiation affected the blood hormonal level significantly[29]. Repeated UVB exposure hasan effect on the estrous cycle, presents a cluster of cells in the estrus cycle phases and reduces the ovary weight. In contrast,the estrous cycle becomes longer when mice are exposed to a repetition of gamma radiation[4]. Previous studies showed thatwholebody exposure to radiation could result in a slight decrease in weight gain of rats[30-32]. In our experiment, UVB exposure showed less significant alterations in the estrous cycle. Moreover, the UVB group showed tight clusters of cells and increased length of estrous phases.It was also evident from the results that the repeated UVB exposure significantly decreased body weight, ovary weight, and sexual maturity.
Both antioxidants (curcumin and ascorbic acid) showed the protective effect of the reproductive system, increased body and ovary weight as well as brought an increase in gonadosomatic index.Lower doses of antioxidants (curcumin and ascorbic acid) used in the present study (25 mg/kg and 250 mg/kg respectively) showed a preventive effect on different phases of the estrous cycle in female Wistar rats. However, higher dose concentration of these antioxidants(curcumin and ascorbic acid) induced the blockage of estrous phases and showed significant antifertility activity and elevated estrogenic activity, with inhibition of ovulation and impairment of fertility[33]. Additionally, a higher dose of another medicinal plant such as Calotropis procera (25, 50 and 100 mg/kg of dry roots)[34],Rivea hypocrateriformis (200 and 400 mg/kg body weight)[35],Momordica chrantia (25, 100 mg/g body weight)[36], Momordica cymbalaria (250 and 500 mg/kg body weight)[37], Gacinia kola(200 mg/kg body weight)[38] were also reported to affect the estrous cycle phases and showed reduced fertility in mammals[39]. Moreover,the estrus cycle in the rats treated with a high dose of Momordica cymbalaria extracts also showed a decrease in the duration of estrus and metestrus phases and prolongation of the proestrus phase[40]. A high dose of alcoholic extract of Azadirachts indica flower disrupts the estrous cycle in Sprague-Dawley rats and causes a partial block in ovulation and hence has the potential to develop female contraception[41].
In conclusion, UVB exposure causes significant disturbance in the estrous cycle and leads to irregularity in the estrous cycle and their cells. UV radiations have effects on the reproductive cycles and result in abnormalities. Both curcumin and ascorbic acid protect estrous cycle and increase body and ovary weight besides maintaining the gonadosomatic index.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors’ contributions
Payal Mahobiya designed the experiment plan and supported Gayatri Rai. Gayatri Rai managed the experimental animals,performed the treatment and completed data analysis and wrote the manuscript. Narendra Namdev assisted in collecting the veginal smear and the treatment. Payal Mahobiya and Gayatri Rai contributed to the editing and completion of the manuscript. All authors read and approved the final manuscript.
 Asian Pacific Journal of Reproduction2020年3期
Asian Pacific Journal of Reproduction2020年3期
- Asian Pacific Journal of Reproduction的其它文章
- COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery
- Effects of extender and packaging method on morphological and functional characteristics of cryopreserved Ossimi ram semen
- Antifertility effects of Azadirachta indica methanol seed extract on canine spermatozoa in vitro
- Effects of vascular endothelial growth factor supplementation and alginate embedding on human oocyte maturation in vitro
- Effect of double cleavage stage versus sequential cleavage and blastocyst stage embryo transfer on clinical pregnancy rates
- Blastocyst elective single embryo transfer improves perinatal outcomes among women undergoing assisted reproductive technology in Indonesia
