Antifertility effects of Azadirachta indica methanol seed extract on canine spermatozoa in vitro
Chike Fidelis Oguejiofor, Ifeanyi Gabriel Eke, Kenneth Orji Anya
1Department of Veterinary Obstetrics and Reproductive Diseases, Faculty of Veterinary Medicine, University of Nigeria, Nsukka 410001, Nigeria
2Department of Veterinary Physiology and Pharmacology, Faculty of Veterinary Medicine, University of Nigeria, Nsukka 410001, Nigeria
ABSTRACT
KEYWORDS: Antifertility; Azadirachta indica; Canine;Spermatozoa; Contraceptive
1. Introduction
Suppression of fertility or contraception is often desirable in the canine species for several reasons including the avoidance of pregnancy from indiscriminate or accidental mating, and the management of animal overpopulation in cities, zoos, etc.Suppression of fertility may be performed via surgical and nonsurgical means.
Surgical methods of contraception such as oviductal ligation(sterilization), ovariohysterectomy (spaying) and orchidectomy(castration) have been traditionally and intensively used in canines in many parts of the world, but these have been reported to cause some significant complications and side effects[1,2]. In male dogs,other contraceptive techniques including chemical castration,hormonal treatment and immunological contraception have also been associated with significant limitations and untoward side effects[1,3].
There has been increasing interest in ethno-veterinary medicine and the application of plant-derived pharmacologic agents in veterinary science. This is also underscored by the need to develop inexpensive, reliable and reversible contraceptives with minimal adverse effects. Azadirachta (A.) indica (neem) is a tropical evergreen tree of the family Meliaceae that grows in many parts of the world. Its products have been used extensively in ancient medicine and traditionally as treatment for various human and animal ailments[4,5]. Extracts from A. indica leaves and the seed oil(oil of margosa) are believed to have antifertility or contraceptive effects[6]. There are previous reports of antifertility effects of A.indica aqueous leaf extract in vivo when administered to male rats or in vitro when incubated with human spermatozoa[7-9]. When administered orally, A. indica seed oil extract was reported to have in vivo antispermatogenic, antiandrogenic or antifertility effects in male mice and rats[10,11]. A. indica seed oil has also been reported to have antispermatogenic effect when injected into the vas deferens[12]and have contraceptive effect when administered intravaginally in rats, monkeys and humans[13]. To the best of our knowledge,information is not available on the antifertility effect of A. indica seed oil in the canine species. Therefore, the aim of this study was to investigate the effects of A. indica methanol seed extract on canine male fertility in vitro. The specific objectives were to evaluate the effect of A. indica methanol seed extract treatment on canine spermatozoal total motility, progressive motility, vitality and total abnormalities as a prelude to development of an intra-vaginal precoital contraceptive cream for bitches.
2. Materials and methods
2.1. Preparation of A. indica seed and extraction
Fresh samples of the fruit of A. indica were collected from the botanical garden of University of Nigeria Nsukka Enugu State Nigeria. The plant was identified by a taxonomist at the University’s Department of Plant Science and Biotechnology. Voucher specimen(PSB/2018/4506/22) was deposited at the institution’s herbarium.The seeds were removed from the fruits and dried in the shade for several days. Dried seeds weighing 300 g were pulverized by using hammer mill. Cold extractions were performed with analytical grades of solvents (JHD, Guangdong Guanghua Sci-Tech Co.,Ltd., Guangdong, China) sequentially, starting with n-hexane (min 97%) followed by chloroform (min 99.7%) and finally methanol(min 99.5%). The methanol extract was dried in vacuo by using rotary evaporator, and then stored at 4 ℃ until use. The extract was subsequently referred to as A. indica methanol seed extract.
2.2. Brine shrimp lethality bioassay
The lethal concentration 50 (LC50) of A. indica methanol seed extract was evaluated by using the brine shrimp (Artemia salina; brine shrimp eggs; Sanders Great Salt Lake, Brine Shrimp Company L.C., U.S.A.)lethality bioassay as previously described[14]. Brine shrimp eggs were hatched in artificial seawater (Sigma, St. Louis, MO, USA). Toxicity of A. indica methanol seed extract in 1% dimethyl sulfoxide (DMSO)(Sigma, St. Louis, MO, USA) was tested at 0.1, 1.0, 10.0, 100.0 and 1 000.0 ppm in triplicate after 48 h incubation. The LC50of A. indica methanol seed extract was derived by probit analysis using SPSS,version 20 (IBM Corp, Armonk, NY, USA).
2.3. Animal preparation and semen collection
For the purpose of this study, semen was routinely collected from three sexually mature male Basenji dogs (9-11 months old)weighing 8.1-9.5 kg. The dogs were acclimatized for 4 weeks, and were clinically examined and determined to be apparently healthy with no obvious signs of systemic or reproductive pathologies. They were kept in standard kennels, fed with commercial pelletized dry dog feed twice daily with water provided ad libitum. During this period, the dogs were trained for semen collection twice a week using the artificial vagina as previously described[15]. Following semen collection using the artificial vagina, the time of ejaculation was recorded. The semen was maintained between 32 ℃- 37 ℃ and quickly taken to the laboratory for further studies.
2.4. Semen and sperm evaluation
Grossly, semen was evaluated to determine the color, volume and pH. Semen color was assessed by using a color chart. Semen pH was measured by using a digital pH meter (Hanna; Woonsocket, RI,USA) while semen volume was determined by using a graduated tube. Microscopic semen evaluation was performed as previously described[16,17]. Sperm total motility (%) and progressive motility(%) were determined by examining diluted semen wet mount at×400 using a phase-contrast microscope (Motic B3; Motic, Carlsbad,CA, USA) equipped with a stage slide warmer (TCS-100; Amscope,Ivrine, CA, USA) set at 37 ℃. Sperm vitality (%) was evaluated by using eosin-nigrosin vital staining method. Sperm were identified as live (unstained head) or dead (red/pink-stained head) by using light microscopy at ×1 000 magnification. Sperm morphology and sperm abnormalities were evaluated at ×400 and ×1 000 magnifications by using phase-contrast microscopy, light microscopy (eosinnigrosin staining) and Papanicolaou staining methods. Fixed smears of semen samples were also stained with Papanicolaou method(Harris’s hematoxylin, G-6 orange and EA-50 green stains) and examined for acrosomal morphology using light microscopy at×1 000 magnification. All values in percentage were determined by examining 200 sperm cells in duplicates. Sperm concentration per mL of semen was determined by using a hemocytometer (Weber,England). Total sperm count in each ejaculate was determined by multiplying the volume of the ejaculate by its sperm concentration per mL. All micrographs were captured by using Moticam 2.0 image system (Motic, Carlsbad, CA, USA).
2.5. In vitro testing of A. indica methanol seed extract
The effect of the A. indica methanol seed extract on canine semen was investigated in vitro. The experiment was carried out three times(n=3) with semen collected at three different times, and all tests were performed in duplicates for all the following six groups. The treatment concentrations of A. indica methanol seed extract was selected based on the minimum concentration required to immobilize 100% of sperm within 1 min in vitro. Various concentrations of A. indica methanol seed extract was tested for 1 min with sperm samples containing 1 million sperm. Measured quantities of A.indica methanol seed extract was dissolved in DMSO and then made up with phosphate-buffered saline (PBS) (pH 7.4). Dilutions of these suspensions were then made using 100 μL of sperm sample(10 million sperm/mL) to a final volume of 200 μL in each well to yield four treatment groups with 2.5, 5.0, 10.0 and 20.0 mg/mL of A.indica methanol seed extract in 1% DMSO, respectively. Also, two control groups were included in the evaluation. Group 1 (the control A group) comprised 200 μL of sperm sample while group 2 (the control B group) comprised 1:2 dilution of sperm sample in PBS and 1% DMSO to a final volume of 200 μL. The other experimental groups were groups 3 to 6 comprising treatments with 2.5, 5.0, 10.0 and 20.0 mg/mL A. indica methanol seed extract in 1% DMSO,respectively. All the groups were incubated at 37 ℃ and samples were evaluated for sperm total motility, progressive motility, vitality and total abnormalities at 1.0, 2.5, 5.0 and 10.0 min post-treatment.Sperm revival test was carried out to investigate the extent of immobilizing capacity of A. indica methanol seed extract treatment and any reversal of sperm incapacitation. At the end of 10 min of treatment with A. indica methanol seed extract, the samples were washed twice. Briefly, samples were diluted to 10 mL with PBS,mixed gently and centrifuged at 800×g for 10 min. The supernatant was removed and the sperm pellet re-suspended in fresh PBS and the procedure was repeated. Washed sperm samples were then incubated at 37 ℃ for 1 h in PBS (pH 7.4) supplemented with 4 mg/mL bovine serum albumin, 0.36 mM sodium pyruvate, 23.8 mM sodium lactate and 5.5 mM glucose. After incubation, sperm total motility was assessed as a test for sperm revival.
2.6. Statistical analysis
Data were analyzed by using mixed analysis of variance with repeated measures via a general linear model built in SPSS, version 20. The effect of treatment concentration (mg/mL) and duration of treatment (min) and their interaction were determined for each of the studied sperm parameters. The Greenhouse-Geisser correction was used where Mauchley’s test of sphericity was violated. Results were reported as main effects and simple main effects depending on the significance of any interactions. Where analysis of variance showed significant difference, differences between means were confirmed by using Tukey’s honestly significant difference post hoc multiple comparison.α= 0.05 was considered as statistically significant level. Charts were prepared by using GraphPad Prism version 6.01(GraphPad Software Inc.).
2.7. Ethics statement
The welfare of the semen-donor dogs was observed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals[18] and the experiment was approved by the Research Ethics Committee of the Faculty of Veterinary Medicine,University of Nigeria, Nigeria (approval No. 2016/10-173537).
3. Results
3.1. Brine shrimp lethality test
The calculated LC50of A. indica methanol seed extract was 119 μg/mL (95% CI: 21-2454).
3.2. Preliminary evaluation of dog semen
Grossly, semen collected from the donor dogs had normal milky color, volume of (3.6±0.3) mL [reference range: (1-30) mL] and pH of (6.8±0.1) (reference range: 6.3-7.0). Further microscopic analysis of the semen samples revealed the following: sperm total motility=(88.0±2.6)% (reference range: 70%-90%), sperm progressive motility=(79.3±2.3)% (reference range: ≥70%), sperm vitality=(96.7±0.6)% (reference range: >50%), sperm total abnormalities=(8.3±2.1)% (reference range: <10%-20%), sperm concentration (98.0±4.0)×106(reference range: 10×106- 2×109)spermatozoa per mL and total sperm count=(353.5±40.1)×106[reference range: (300-2 000)×106] spermatozoa per ejaculate.
3.3. Effect of A. indica methanol seed extract treatment on sperm total motility
The effects of treatment with different concentrations of A.indica methanol seed extract on sperm total motility were shown in Figure 1 A. There was a statistically significant interaction between the effects of treatment concentration and duration of treatment on sperm total motility [F(15, 36)=169.62, P<0.001,partial η2=0.986]. There was a significant main effect for treatment with A. indica methanol seed extract on sperm total motility [F(5, 12)=741.84, P<0.001, partial η2=0.997] with a concentration-dependent decrease in total motility in all the A. indica methanol seed extract-treated groups. Pairwise comparison showed that all the treatment groups were significantly different (P<0.001)at the four time points. As the concentration of A. indica methanol seed extract increased from 2.5 mg/mL to 10.0 mg/mL (from Group 3 to Group 5), there was a corresponding significant decrease in total motility from 76% to 37% at 1.0 min, 71% to 25% at 2.5 min, 58%to 8% at 5.0 min and 42% to 0% at 10.0 min respectively compared to the control groups. Sperm treated with the highest concentration of 20.0 mg/mL A. indica methanol seed extract (Group 6) had absolute loss of motility (0%) for all the duration of treatment observed(P<0.001). There was also a significant main effect for duration of treatment with A. indica methanol seed extract on sperm total motility [F(3, 36)=843.23, P<0.001, partial η2=0.986]with a decrease in total motility following longer duration of treatment. From 1.0 min to 10.0 min post-treatment, total motility decreased from 76% to 42% (Group 3), 52% to 29% (Group 4) and 37% to 0% (Group 5), respectively.
3.4. Effect of A. indica methanol seed extract treatment on sperm progressive motility
Treatment with A. indica methanol seed extract also affected sperm progressive motility as shown in Figure 1 B. Analysis of variance with Greenhouse-Geisser correction showed significant interaction between the effects of treatment concentration and duration of treatment on sperm progressive motility [F(8.61, 20.66)=130.77,P<0.001, partial η2=0.982]. Main effects analysis showed a significant effect for treatment with A. indica methanol seed extract on sperm progressive motility [F(5, 12)=670.11, P<0.001, partial η2=0.996] with a concentration-dependent decrease in progressive motility in all the A. indica methanol seed extract-treated groups.All the treatment groups were also different (P<0.001) at the four time points following post hoc comparison. An increase in A. indica methanol seed extract concentration from 2.5 mg/mL (Group 3) to 5.0 mg/mL (Group 4) caused a significant decrease in progressive motility from 61% to 35% at 1.0 min, 55% to 27% at 2.5 min, 42%to 19% at 5.0 min and 23% to 7% at 10.0 min respectively, compared to the control groups. At 10 mg/mL (Group 5), progressive motility declined from 77% to 11% at 1 min and then to 0% subsequently.At the highest dose of 20 mg/mL A. indica methanol seed extract(Group 6), there was absolute loss of motility (0%) for all the time points (P<0.001). A significant main effect was also observed for duration of treatment with A. indica methanol seed extract on sperm progressive motility [F(1.72, 20.66)=488.18, P<0.001, partial η2=0.976] with a decrease in progressive motility following longer duration of treatment. From 1.0 min to 10.0 min post-treatment,progressive motility decreased from 61% to 23% (Group 3), 35% to 7% (Group 4) and 11% to 0% (Group 5), respectively.
3.5. Effect of A. indica methanol seed extract treatment on sperm vitality
Treatment with A. indica methanol seed extract also affected sperm vitality as shown in Figures 2 and 3. There was a statistically significant interaction between the effects of treatment concentration and duration of treatment on sperm vitality [F(15, 36)=532.41,P<0.001, partial η2=0.996]. There was a significant main effect for treatment with A. indica methanol seed extract on sperm vitality[F(5, 12)=149.10, P<0.001, partial η2=0.984) with a concentrationdependent decrease in sperm vitality in all the A. indica methanol seed extract-treated groups. Pairwise comparison also showed that all the treatment groups were significantly different at the four time points. As the concentration of A. indica methanol seed extract increased from 2.5 mg/mL to 20.0 mg/mL (from Group 3 to Group 6), there was a decrease in sperm vitality from 91% to 89%at 1.0 min (P<0.05), 83% to 52% at 2.5 min (P<0.01), 62% to 32% at 5.0 min (P<0.001) and 54% to 11% at 10.0 min (P<0.001)respectively, compared to the control groups. A significant main effect was also observed for duration of treatment with A. indica methanol seed extract on sperm vitality [F(3, 36)=4457.22, P<0.001,partial η2=0.997] with a decrease in sperm vitality following longer duration of treatment. From 1.0 min to 10.0 min post-treatment,sperm vitality decreased from 91% to 54% (Group 3), 90% to 38%(Group 4), 88% to 17% (Group 5) and 88% to 11% (Group 6),respectively.

Figure 2. Evaluation of sperm vitality using eosin-nigrosin staining (scale bar: 10 μm). Live sperm (LS) remain unstained whereas dead sperm (DS)are stained red. SM: swollen mid-piece.
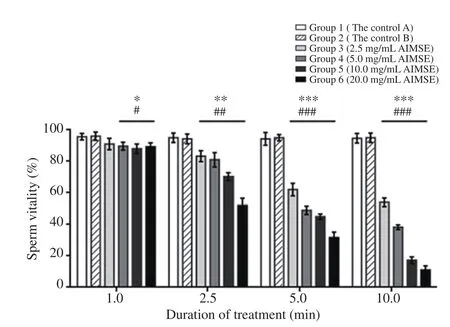
Figure 3. Effects of different concentrations of A. indica methanol seed extract (AIMSE) on sperm vitality in vitro. There is a significant interaction between the effects of the extract concentration and duration of treatment on sperm vitality (P<0.001). There are also significant main effects(P<0.001) for treatment with A. indica methanol seed extract and duration of treatment. *P<0.05, **P<0.01, ***P<0.001: statistically significant differences between each extract-treated group and the controls within each time point; #P<0.05, ##P<0.01, ###P<0.001: statistically significant differences between the extract-treated groups within each time point.
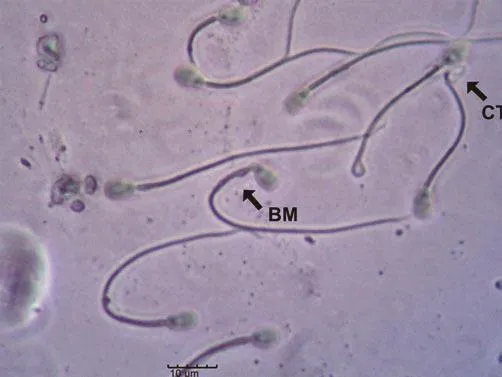
Figure 4. Evaluation of sperm morphology using phase-contrast microscopy (scale bar: 10 μm). Note the sperm with bent midpiece (BM)and coiled tail (CT).
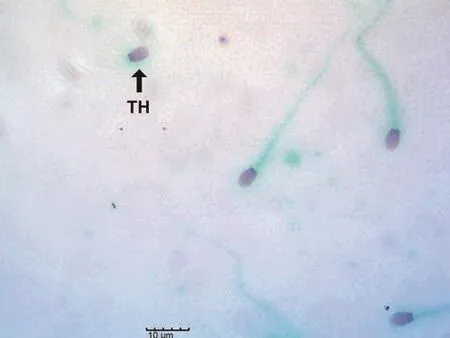
Figure 5. Evaluation of sperm morphology using Papanicolaou staining(scale bar: 10 μm). Note the sperm with tailless head (TH).
3.6. Effect of A. indica methanol seed extract treatment on sperm total abnormalities
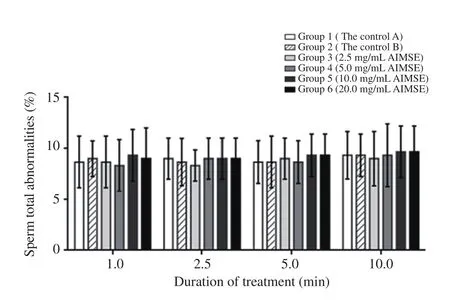
Sperm abnormalities observed following morphological evaluation included headless sperm, tailless sperm, and sperm with coiled tail, bent midpiece, swollen midpiece and cytoplasmic droplets.The effects of treatment with different concentrations of A. indica methanol seed extract on sperm total abnormalities were shown in Figures 4 to 6. Analysis showed no significant interaction between the effects of treatment concentration and duration of treatment on sperm total abnormalities [F(15, 36)=0.274, P=0.995, partial η2=0.102]. Main effects analysis showed no significant effect for treatment with A. indica methanol seed extract on sperm total abnormalities [F(5, 12)=0.034, P=0.999, partial η2=0.014] as the mean sperm total abnormalities did not differ with increased concentrations of A. indica methanol seed extract at the four time points. There was also no significant main effect for duration of treatment with A. indica methanol seed extract on sperm total abnormalities [F(3, 36)=2.325, P=0.091, partial η2=0.162] as the mean sperm total abnormalities did not differ within the groups for all the duration of treatment.
3.7. Effect of A. indica methanol seed extract treatment on sperm revival
Following treatment, sperm revival test showed that total sperm motility did not differ between the control A group [(58.30±3.21)%]and the control B group [(55.70±3.06)%], while groups 3 to 6 recorded zero values for total sperm motility. Therefore, there was complete absence of sperm motility in all the A. indica methanol seed extract-treated groups when compared to the control groups.
4. Discussion
A. indica methanol seed extract caused a concentration and timedependent decrease in sperm total motility, progressive motility and vitality but had no effect on sperm total abnormalities. The complete absence of sperm motility in all the A. indica methanol seed extracttreated sperm following the sperm revival test provided evidence of a permanent or irreversible incapacitation of sperm by A. indica methanol seed extract in vitro. This suggests that A. indica methanol seed extract has the potential to produce effective or reliable contraceptive effect on canine spermatozoa. The brine shrimp lethality test showed that the LC50of A. indica methanol seed extract was 119 μg/mL, indicating that A. indica methanol seed extract contains potent bioactive compounds. According to Meyer et al and Para et al[19,20], LC50values < 1 000 μg/mL is considered bioactive and possibly cytotoxic in toxicological assessment of medicinal plants. Therefore, at LC50of 119 μg/mL, A. indica methanol seed extract is strongly bioactive and may explain its spermicidal effect on canine spermatozoa. The initial evaluation of the donor semen used in the in vitro experiment revealed semen characteristics that were within the normal range for dogs as previously reported[21,22].Therefore, the semen samples were validated for further in vitro studies. Due to its oily constituent, A. indica methanol seed extract was dissolved in DMSO to allow for its suspension in PBS and mixture with sperm samples. The control B group comprising of sperm in 1% DMSO was included in the experiment to observe for any confounding effect due to DMSO. However, the presence of DMSO at a concentration of 1% did not have detectable effect on the spermatozoa as there were no significant differences between the control A and control B groups with respect to all the sperm parameters assessed. This study investigated the in vitro effects of treatment with 2.5, 5.0, 10.0 and 20.0 mg/mL of A. indica methanol seed extract on canine sperm total motility, progressive motility,vitality and total abnormalities at 1.0, 2.5, 5.0 and 10.0 min posttreatment.
Treatment with A. indica methanol seed extract caused a concentration-dependent decrease in sperm total motility with a complete loss of sperm motility within 1.0 min at the highest concentration of 20.0 mg/mL. A previous study on aqueous neem leaf extract, in contrast with neem oil used herein, also reported similar effect on sperm motility. About 3 mg of neem leaf extract was required to immobilize and kill 100% of 1 million human sperm within 20 s[9]. Interestingly, a fraction isolated from neem oil (NIM-76) was also reported to inhibit sperm motility in human spermatozoa in a dose-dependent manner[23].
Treatment with A. indica methanol seed extract also disrupted sperm motility by causing a concentration-dependent increase in abnormal sperm movement such as circular, backward, rolling and wobbling motions. Normal sperm motility is defined as a rapid, progressive, forward motion[23]. This disruption of sperm movement was not likely an endpoint effect but may be related to the functional incapacitation in the sequence towards the eventual complete inhibition of motility. The mechanism through which A.indica methanol seed extract inhibits sperm motility is not known but may be related to disruption of the sperm plasma membrane and the spermicidal effect of the extract. Motility is an indication of the structural and functional competence of spermatozoa. Therefore,the proportion of progressively motile sperm is usually positively correlated with sperm plasma membrane integrity and normal morphology[24]. Fertility may be compromised in dogs with total and progressive sperm motility of less than 70%[23]. Therefore,the inhibition of sperm total and progressive motility even at lower concentrations of A. indica methanol seed extract is likely to have significant impact on the fertility potential of treated canine spermatozoa.
There was also a significant decrease in sperm vitality, in a concentration-dependent manner, following treatment with A. indica methanol seed extract. However, this effect was relatively delayed in comparison to the observed inhibition of sperm motility. By 10.0 min post-treatment with A. indica methanol seed extract, a small proportion (11%) of sperm still showed evidence of vitality in the group treated with 20.0 mg/mL A. indica methanol seed extract.However, the loss of motility in the affected sperm means that their fertility potential is poor. Treatment at lower concentrations is also likely to have increased spermicidal effect with longer exposure of sperm to A. indica methanol seed extract. An increase in the proportion of dead spermatozoa in semen has adverse effect on the fertility of affected dogs[22]. The spermicidal effect of A. indica methanol seed extract therefore suggests a potent antifertility effect.An increase in the proportion of sperm with abnormal morphology is known to adversely affect canine fertility[21,22]. There was no effect of A. indica methanol seed extract treatment on the proportion of sperm with abnormal morphology in vitro. Treatment with aqueous neem leaf extract also did not alter sperm morphology in human sperm[9]. The antifertility potential of A. indica methanol seed extract may be related to its sperm immobilization and spermicidal effects rather than by altering sperm morphology. However, a previous ultra-structural study using electron microscopy revealed the formation of pores and vesicles over the sperm head following treatment with the fraction NIM76[25].
A few limitations in the study may include possible differences in effects due to differences in dog breed or other animal species.Also, the evaluation of morphologic changes in spermatozoa was limited to the observations possible with light and phase-contrast microscopy.
Products from A. indica seed offer potential as sources of new contraceptive principles for application in some areas of veterinary practice such as canine reproduction. Neem oil when applied intra-vaginally before sexual intercourse was reported to be 100%effective in preventing pregnancy in treated rats, monkeys and humans[13]. Although a few products have been formulated from A.indica seed oil, they have limited commercial availability and usage.Most applications of A. indica seed extracts are within traditional settings.
In conclusion, the total inhibition of canine sperm motility and the significant spermicidal effect suggests that A. indica methanol seed extract, particularly at the concentration of 20.0 mg/mL,may produce a potent antifertility or contraceptive effect in dogs.Further studies would investigate possible contraceptive effects of A. indica methanol seed extract in dogs in vivo, followed by further characterization of active principles.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors’ contributions
All the authors were involved in the conception and design of the study. Kenneth Orji Anya supervised all aspects of the study.Ifeanyi Gabriel Eke performed extract preparation and brine shrimp lethality bioassay. Chike Fidelis Oguejiofor and Kenneth Orji Anya were involved in semen collection and evaluation. All authors were involved in the in vitro analyses. Chike Fidelis Oguejiofor and Ifeanyi Gabriel Eke were involved in literature search, data analysis and manuscript preparation. All authors contributed to data interpretation and discussion, and were involved in the review and final approval of the manuscript.
 Asian Pacific Journal of Reproduction2020年3期
Asian Pacific Journal of Reproduction2020年3期
- Asian Pacific Journal of Reproduction的其它文章
- COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery
- Effects of extender and packaging method on morphological and functional characteristics of cryopreserved Ossimi ram semen
- Ascorbic acid and curcumin alleviate abnormal estrous cycle and morphological changes in cells induced by repeated ultraviolet B radiations in female Wistar rats
- Effects of vascular endothelial growth factor supplementation and alginate embedding on human oocyte maturation in vitro
- Effect of double cleavage stage versus sequential cleavage and blastocyst stage embryo transfer on clinical pregnancy rates
- Blastocyst elective single embryo transfer improves perinatal outcomes among women undergoing assisted reproductive technology in Indonesia
