Effect of double cleavage stage versus sequential cleavage and blastocyst stage embryo transfer on clinical pregnancy rates
Gözde Kaya, Begüm Alyürük, Ozge Senem Yucel Cicek, Sule Yildirim Köpük, Ahmet Yiğit Çakiroğlu,3,Emek Doğer, Serdar Filiz
1IVF Center, Faculty of Medicine, Kocaeli University, 41380, Izmit, Kocaeli, Turkey
2IVF Center, Acı badem Maslak Hospital, 34457, Sariyer, Istanbul, Turkey
3Medical Faculty, Acı badem Mehmet Ali Aydı nlar University, Istanbul, Turkey
ABSTRACT
KEYWORDS: Sequential transfer; Blastocyst; Cleavage stage embryo; In vitro fertilization
1. Introduction
Despite advancements in culture conditions and embryo transfer methods, pregnancy and live birth rates following assisted reproductive technologies (ART) treatment have not dramatically increased. Sequential embryo transfer, defined as consecutive or two-step transfer of embryos on different days within the same fresh embryo transfer cycle, has been proposed as an alternative approach to improve ART success rates[1].
Embryo implantation is only possible for a limited period called the window of implantation when the endometrium transforms into a suitable environment for implantation[2]. Endometrium acquires receptivity 4-5 days after endogenous or exogenous progesterone exposure and window of implantation has been suggested to be open for 2 to 4 days[3]. Displacement of the window of implantation has been shown in some patients especially with repeated implantation failures[4]. The sequential transfer may increase the chance of hitting the window of implantation which is only open for a short time.
In vivo, embryos do not reach the uterine cavity before the morula stage which corresponds to day 4 for in vitro cultured embryos[5].Thus, day-2 and day-3 embryos are physiologically premature for the uterine environment which has a different nutritional milieu from the oviduct. Therefore, the transfer of cleavage-stage embryos might result in metabolic stress leading to a reduced implantation potential[6,7]. Compared with the cleavage stage, the blastocyst stage enables the selection of embryos with higher implantation potential and increases the likelihood of synchronized endometrium and embryonic development[8]. However, an increased failure to transfer any embryos at the blastocyst stage was also observed[9].The sequential transfer of cleavage- and blastocyst-stage embryos may benefit from possible advantages from blastocyst transfer while not increasing transfer cancellation risk.
The sequential transfer has been extensively studied in patients with recurrent implantation failure. However, it is not clear whether it can be used as a routine embryo transfer technique to improve ART success. Hence, we aimed to investigate clinical pregnancy rates following sequential day-3 and day-5 embryo transfer compared to double or sequential cleavage-stage transfers. We further aimed to compare whether the double or sequential cleavage-stage transfer has higher clinical pregnancy rates.
2. Materials and methods
2.1. Study design and participants
This retrospective cohort study was undertaken at the ART center of Kocaeli University Faculty of Medicine, Kocaeli, Turkey between January 2011 and January 2014. The study consisted of. patients with≤40 years of age, having ≥3 good quality embryos on day 2 and ≥2 good quality embryos on day 3 were included in this study. Patients with any type of uterine anomaly were excluded from our study. A total of 242 patients undergoing gonadotropin-releasing hormone(GnRH) antagonist protocol and fresh embryo transfer were enrolled in this retrospective study. Of 242 women, 135 underwent double embryo transfer on day 2 or day 3 (the double group), 54 women underwent sequential transfer on day 2 and day 3 (the D2/D3 group)and 53 underwent sequential transfer on day 3 and day 5 (the D3/D5 group).
The data including female age, male age, body mass index(BMI), duration and type of infertility, ART indication, clinical and laboratory findings were collected from medical records.
2.2. GnRH antagonist protocol
GnRH antagonist protocol was used as the pituitary suppression protocol for all patients. The ultrasound examination was performed on the second or third day of the menstrual cycle and patients were started on daily subcutaneous injections of recombinant follicle stimulating hormone (FSH) preparation (Gonal F; Merck Serono,Switzerland). The initial doses recombinant FSH were 150-225 IU based on the antral follicle count, day 3 serum FSH level and anti-Müllerian hormone (AMH) level.
The patients were monitored by their serum estradiol (E2),luteinizing hormone (LH) and progesterone levels and serial transvaginal ultrasonographic examinations. The gonadotrophin doses were adjusted with respect to the follicular response assessed on ultrasonography. When the leading follicle reached ≥12 mm, the GnRH antagonist was administered at 0.25 mg/day. The duration of antagonist administration varied depending on the follicular development (Cetrotide 0.25 mg; Merck Serono, Switzerland). When at least 2 follicles reached a mean diameter of 18 mm or 3 or more follicles reached a mean diameter of 17 mm, 250 μg of recombinant choriogonadotropin alfa (Ovitrelle; Merck Serono, Switzerland)was administered. Transvaginal ultrasound-guided oocyte retrieval was performed 35-37 h after recombinant choriogonadotropin alfa administration and follicles with a mean diameter of ≥11 mm were aspirated. Semen parameters were evaluated according to the World Health Organization 2010 guidelines[10]. If the number of motile sperm in the ejaculate was high and the number of non-sperm cells was low, the “swim-up” sperm washing technique was used. If the number of non-sperm cells was high and the number of sperm was low, the “gradient” sperm washing method was used.
Intracytoplasmic sperm injection (ICSI) was performed 3-5 h after oocyte retrieval. Successful fertilization, defined as the presence of two pronuclei, was assessed 16-18 h post-ICSI. The embryos were initially cultured in G-1 PLUS™ embryo culture media (Vitrolife Sweden AB). On day 3, embryos were transferred into blastocyst media G-2 PLUS™ (Vitrolife Sweden AB) at 37 ℃ in 6% CO2.
2.3. Embryo quality
Embryo quality was determined according to the number and regularity of blastomeres, degree of fragmentation and presence of multinucleation. Grade 1 (uniform blastomers, <10% fragmentation,a single nucleus per blastomere) and grade 2 (slightly uneven blastomeres, 10%-25% fragmentation, a single nucleus per blastomere) embryos containing ≥4 cells on day 2 and ≥6 cells on day 3 were defined as good-quality embryos and transferred. Grade 3 (even or uneven blastomeres, ≥25% fragmentation, no nuclei visible or multinucleation) embryos were excluded from our study.
2.4. Embryo transfer
In the double group, two good-quality embryos on day 2 or day 3 were transferred. In the D2/D3 group, one embryo was transferred on day 2 and a consecutive transfer of one cleavage-stage embryo was performed on day 3. In the D3/D5 group, one day-3 embryo was transferred, then remaining good quality embryos were placed in blastocyst culture medium and cultured until day 5. On day 5, one blastocyst-stage embryo transfer was performed.
All embryo transfers were performed with a standard protocol under transabdominal ultrasound guidance. The patient was placed in the lithotomy position and the cervix was visualized using a speculum. The cervix and cervical mucus were wiped with a sterile gauze and saline solution. A mock embryo transfer procedure was performed by using a soft catheter (Full Echo®, Laboratoire CCD,Paris, France). Only the inner sheath of the catheter passed through internal os and the outer sheath was stopped before the internal os.After dummy embryo transfer, the embryologist loaded the embryos in a new soft catheter (Full Echo®, Laboratoire CCD, Paris, France).The loaded catheter was inserted through the external cervical os.Only the inner sheath was advanced through the internal cervical os. The tip of the catheter was positioned approximately 1.0-1.5 cm from the uterine fundus and embryos were discharged. The catheter was slowly withdrawn. The embryologist checked the transfer catheter for retained embryos. If any retained embryos were detected, they were immediately re-transferred.
2.5. Calculation of clinical pregnancy rates
The main measure of outcome was clinical pregnancy rates per fresh embryo transfer cycle. Clinical pregnancy was defined as the presence of a gestational sac with fetal heart activity.
2.6. Statistical analysis
The statistical analysis of the data was performed by using the Statistical Package for Social Sciences for Windows 13.0 (SPSS,Chicago, IL, USA). The Kolmogorov-Smirnov test was used to test the normality of the data distribution. The continuous variables with normal distribution were expressed as mean±standard deviation(mean±SD); the continuous variables without normal distribution were expressed as median (interquartile). Depending on the distribution of variables, one-way analysis of variance for normally distributed data or Kruskal Wallis tests for non-normally distributed data were used to compare the continuous variables. Dunn’s test was used for post-hoc analyses. Fisher’s exact test was used to compare clinical pregnancy rates between groups. A P-value less than 0.05 was considered statistically significant.
2.7. Ethics statement
The study was approved by the Kocaeli University Ethical Committee of Clinical Research, Kocaeli, Turkey (protocol number:KOU/KAEK 2014/80; approval date: 18.02.2014).
3. Results
3.1. Baseline characteristics and clinical findings
The indications for ART cycles were tuboperitoneal factor, male factor, unexplained infertility and others. The male factor constituted the highest percentage of our diagnosis in each group (Double group 43.7%, D2/D3 group 44.4% and D3/D5 group 47.1%). This was followed by tubal factor, unexplained infertility and others (Table 1).
The baseline characteristics and clinical findings of these women were presented in Table 2. Female age, BMI and male age were similar among all groups (P=0.049, P=0.550, and P=0.792,respectively). Although statistical significance was found between groups regarding female age (P=0.049), no significant difference was detected after post-hoc analysis. In addition, there was no significant difference between groups in regard to basal FSH, LH and E2levels (P=0.471, P=0.478, and P=0.906, respectively). The double group had significantly lower AMH levels compared with the D2/D3 and D3/D5 groups (P=0.006 and P=0.009, respectively). The D3/D5 group had a longer duration of infertility compared with the double group (P=0.023). The number of prior in vitro fertilization(IVF) attempts was similar between groups (P=0.292).
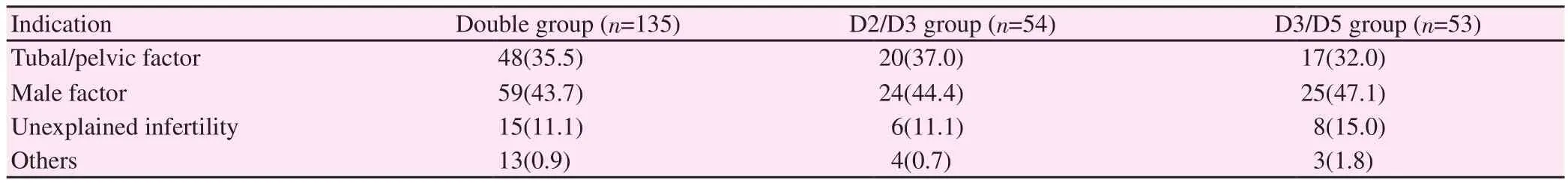
Table 1. Comparison of infertility diagnosis between groups [n(%)].
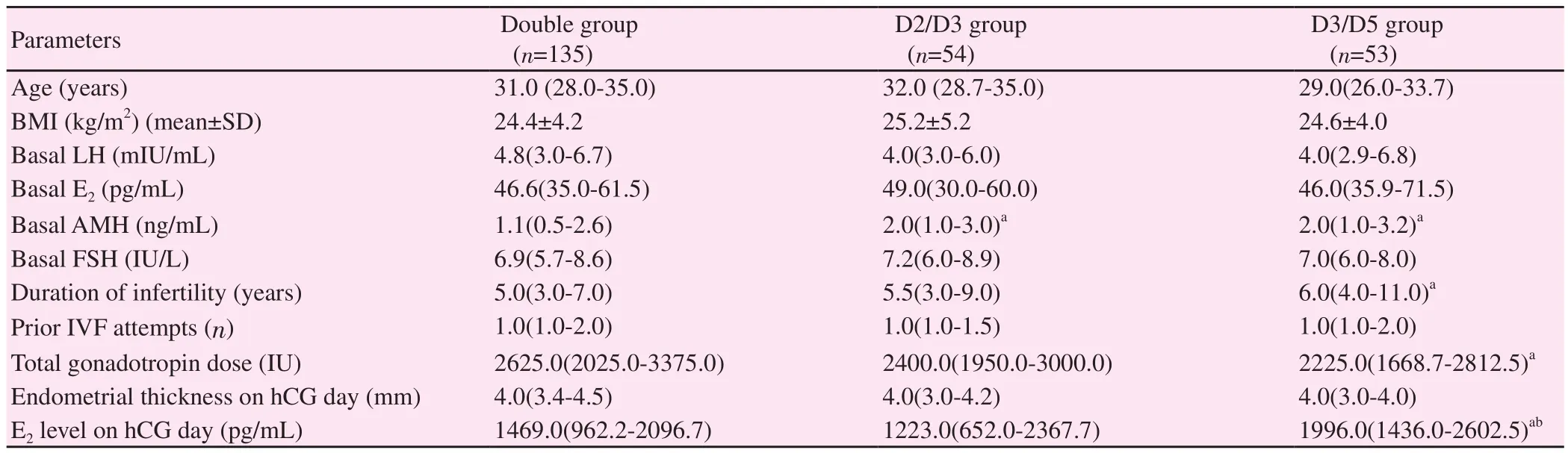
Table 2. Comparison of basal clinical and laboratory characteristics of patients between groups.
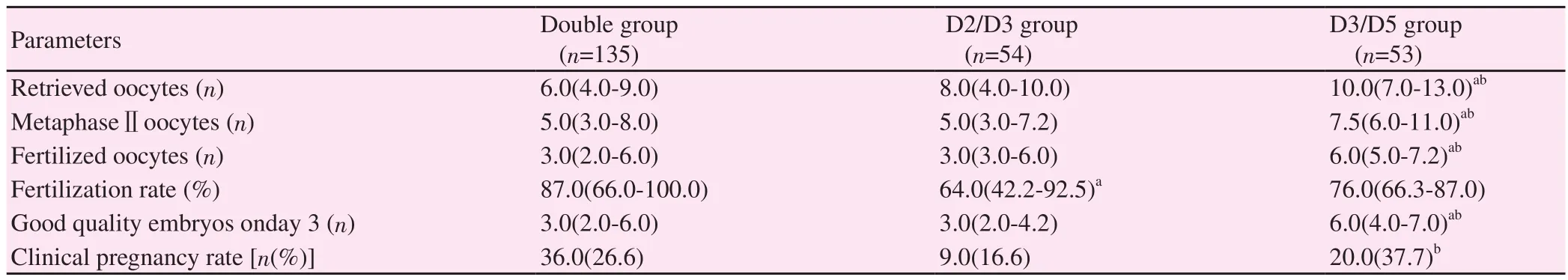
Table 3. Comparison of controlled ovarian stimulation outcomes and clinical pregnancy rates between groups.
The total dose of gonadotropins used during ovarian stimulation was significantly higher in the double group compared with the D3/D5 group (P=0.018). The endometrial thickness on the day of human chorionic gonadotrophin (hCG) was similar between groups(P=0.081). E2level on the day of hCG was significantly higher in the D3/D5 group as compared with the double and D2/D3 groups(P=0.016 and P=0.041, respectively).
3.2. Oocyte numbers and ICSI outcomes
The number of retrieved oocytes, metaphaseⅡoocytes, and fertilized oocytes were significantly higher in the D3/D5 group compared with the double and D2/D3 groups (P<0.001 and P=0.005,P<0.001 and P<0.001, P<0.001 and P<0.001, respectively). The fertilization rate in the D2/D3 group was significantly lower than that in the double group (P=0.002). In addition, a significantly higher number of good quality day-3 embryos were present in the D3/5 group compared with the double group (P=0.002) and the D2/3 group (P<0.001) (Table 3).
3.3. Clinical pregnancy rates
Clinical pregnancy rates in the double, D2/D3 and D3/D5 groups were 26.6% (36/135), 16.6% (9/54) and 37.7% (20/53), respectively(Table 3). The D3/5 group had a significantly higher clinical pregnancy rate compared with the D2/D3 group (P=0.025). Despite no statistical significance, there was a trend towards higher clinical pregnancy rates in the D3/D5 group compared with the double group(P=0.188). There was no significant difference in clinical pregnancy rates between the double and D2/D3 groups (P=0.204).
4. Discussion
A receptive endometrium, a viable embryo and embryoendometrium crosstalk are the necessities for successful implantation. Because embryo-endometrium asynchrony is a potential cause of implantation failure, the sequential transfer has been offered for improving implantation rates[1]. However, the present study demonstrated that there was no significant difference in clinical pregnancy rates between the transfer of two good quality cleavage-stage embryos and day-2 and day-3, or day-3 and day-5 sequential embryo transfer.
The sequential transfer has been shown to be beneficial under certain conditions. A previous study comparing day-2 and day-3 sequential transfer with day-3 transfer only found that day-2 and day-3 sequential transfer improved clinical pregnancy rates in patients with repeated IVF failures[11]. In contrast to this study,we found similar clinical pregnancy rates in the day-2 and day-3 sequential transfer group and double cleavage-stage embryo transfer group. Indeed, the higher clinical pregnancy rate was observed in the double cleavage-stage embryo transfer group in our study although it was not statistically significant. Our study was performed in an unselected ART population while the previous study was performed in a specific population of patients with recurrent IVF failures. Therefore, although performing day-2 and day-3 sequential transfer may improve clinical outcomes for patients with recurrent IVF failures. Nevertheless, catheter-related problems or multiple interferences into the endometrial cavity might be possible causes as previously suggested by Ashkenazi et al[12]. In our study, we did not consider only one diagnosis group. So, there may be a difference between the previous data.
Extensive research documented that fresh blastocyst stage transfer is associated with higher clinical pregnancy rates than cleavagestage transfer[8,9]. In line with these studies, our findings showed that day-3 and day-5 sequential transfer had higher clinical pregnancy rates compared to day-2 and day-3 sequential transfer. Because blastocyst stage transfer results in the transfer of embryos into a more synchronized uterine environment, the higher pregnancy rates observed in the D3/D5 group are more likely to be a consequence of higher implantation potential of blastocysts[13] rather than a benefit of sequential cleavage stage and blastocyst embryo transfer. However, one disadvantage of extended blastocyst culture is an increased failure to transfer any embryos[9]. A prospective randomized study showed that embryo transfer cancellation rate was higher in blastocyst stage transfer but the presence of two or more 8-cell embryos on day 3 in culture carried a high probability of obtaining blastocysts for transfer[14]. The D3/D5 group in our study had a higher number of good quality embryos on day 3 than the D2/D3 group, enabling extended culture to the blastocyst stage in this group. Thus, it is conceivable to assume that when there is a sufficient number of embryos, planning extended culture and sequential day 3 and 5 transfer provides better clinical pregnancy rates than performing sequential day 2 and 3 transfer.
The effect of cleavage and blastocyst stage sequential transfer on ART outcomes is controversial. Machtinger et al[15] compared sequential transfer of day-3 embryos and blastocysts to transfer on day 3 only in patients with repeated IVF failures and found higher pregnancy rates in the sequential transfer group. Several studies also demonstrated clinical pregnancy and implantation rates were improved when the sequential transfer of cleavage stage embryos and blastocysts was performed[1,11]. In contrast, we found no significant difference in clinical pregnancy rates between double cleavage-stage embryo transfer and day-3 and day-5 sequential transfer although there was a trend towards higher clinical pregnancy rates in the day-3 and day-5 sequential transfer group. In line with our results, a recent randomized controlled trial comparing sequential cleavage stage and blastocyst transfer with day 5 transfer alone in patients with three repeated IVF failures, found no benefit of sequential transfer[16].Furthermore, Ashkenazi et al[12] reported consecutive transfer of early embryos and blastocysts was ineffective and concluded that this may be due to the adverse effect of the second transfer on the implantation process. The authors suggested the second insertion of the catheter may cause trauma to the endometrium and stimulate the secretion of prostaglandins. In addition, more mucus or additional contamination to the uterine cavity damages the implantation process and decreases the pregnancy rate. Other authors also reported no improvement in pregnancy rates after applying this technique[17].
The main limitation of this study was its retrospective nature. In addition, Some level of heterogeneity was present between groups regarding basal AMH, duration of infertility, total gonadotropin dose, and E2level on hCG day. These factors may also affact the clinical pregnancy rate in our study. Another limitation was that the clinical pregnancy rate rather than live birth rate was used because of the difficulty in long-term follow-up of patients. Carefully designed randomized controlled trials are required to confirm its value in the future.
In conclusion, our study which consisted of an unselected population of patients undergoing ART treatment showed that sequential cleavage stage transfer (D2/D3) or cleavage-stage and blastocyst transfer (D3/D5) does not improve clinical pregnancy rates. Although sequential transfer might be an effective option in certain patient populations, the routine application of this technique does not seem to be a suitable approach in an unselected population to improve ART outcomes.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
We would like to thank Prof. Dr. Canan Baydemır for her help in the statistical analysis of the data.
Authors’ contributions
Gözde Kaya and Begüm Alyürük conducted the conception of the study, drafted the article and made the interpretation of data. Ozge Senem Yucel Cicek was responsible for the acquisition of data and analysis. Sule Yıldırım Köpük carried out the ethical procedure.Ahmet Yiğit, Çakıroğlu, Emek Doğer and Serdar Filiz made practical procedure.
 Asian Pacific Journal of Reproduction2020年3期
Asian Pacific Journal of Reproduction2020年3期
- Asian Pacific Journal of Reproduction的其它文章
- COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery
- Effects of extender and packaging method on morphological and functional characteristics of cryopreserved Ossimi ram semen
- Ascorbic acid and curcumin alleviate abnormal estrous cycle and morphological changes in cells induced by repeated ultraviolet B radiations in female Wistar rats
- Antifertility effects of Azadirachta indica methanol seed extract on canine spermatozoa in vitro
- Effects of vascular endothelial growth factor supplementation and alginate embedding on human oocyte maturation in vitro
- Blastocyst elective single embryo transfer improves perinatal outcomes among women undergoing assisted reproductive technology in Indonesia
