淫羊藿苷对原代星形胶质细胞炎症反应的抑制作用
王皓田 李娜 陈文芳
[摘要] 目的 探討淫羊藿苷(ICA)对脂多糖(LPS)诱导的原代星形胶质细胞炎症反应的抑制作用。方法将细胞种于6孔板,分为对照组、LPS组和ICA给药组。对照组先用1 μL二甲基亚矾(DMSO)处理细胞1 h,再加入1 μL生理盐水;LPS组先用1 μL DMSO预处理细胞1 h,再加入LPS (终浓度1 mg/L)处理细胞6 h;ICA给药组先用不同浓度的ICA (0.1、1.0、10.0、20.0 μmol/L)预处理细胞1 h,然后与LPS共同孵育细胞6 h。采用荧光定量PCR(RT-PCR)法分别检测各组细胞胶质纤维酸性蛋白(GFAP)mRNA的表达。应用RT-PCR法检测ICA给药组(10.0 μmol/L)肿瘤坏死因子α(TNF-α)和白细胞介素1β(IL-1β) mRNA的表达,并与对照组、LPS组检测结果比较。结果 与对照组相比较,LPS组的GFAP mRNA表达明显升高(F=10.63,q=9.775,P<0.01);1.0、10.0、20.0 μmol/L的ICA均能够明显抑制LPS诱导的GFAP mRNA的表达(q=4.496~6.111,P<0.05),以10.0 μmol/L ICA的抑制效果最明显(q=6.111,P<0.01)。与对照组相比较,LPS组的TNF-α和IL-1β mRNA表达明显升高(F=243.50、378.60,q=30.040、36.000,P<0.01),10.0 μmol/L ICA组TNF-α和IL-1β mRNA表达较LPS组显著降低(q=7.689、5.199,P<0.05)。结论 ICA能够抑制LPS诱导的原代星形胶质细胞的活化和炎症反应。
[关键词] 淫羊藿甙;脂多糖;星形细胞;肿瘤坏死因子α;白细胞介素1β;神经胶质原纤维酸性蛋白质
[中图分类号] Q421 [文献标志码] A [文章编号] 2096-5532(2020)02-0181-04
doi:10.11712/jms.2096-5532.2020.56.094 [开放科学(资源服务)标识码(OSID)]
[网络出版] http://kns.cnki.net/kcms/detail/37.1517.R.20200519.1433.006.html;2020-05-19 17:25
[ABSTRACT] Objective To investigate the inhibitory effect of icariin (ICA) against lipopolysaccharide (LPS)-induced inflammatory response in primary astrocytes. Methods Cells were seeded in a 6-well plate and divided into control group, LPS group, and ICA groups. The control group was treated with 1 μL DMSO for 1 h and then 1 μL normal saline for 6 h. The LPS group was treated with 1 μL DMSO for 1 h and then LPS (final concentration, 1 mg/L) for 6 h. The ICA groups received 1 h pretreatment with ICA at 0.1, 1.0, 10.0, and 20.0 μmol/L, respectively, followed by co-incubation with LPS for 6 h. RT-PCR was used to determine the mRNA expression of glial fibrillary acidic protein (GFAP) in all the groups, and to determine the mRNA expression of TNF-α and IL-1β in the control group, the LPS group, and the 10.0 μmol/L ICA group. Results Compared with the control group, the LPS group showed a significantly higher mRNA expression level of GFAP (F=10.63,q=9.775,P<0.01); LPS-induced mRNA expression of GFAP was significantly inhibited by ICA at 1.0,10.0, and 20.0 μmol/L (q=4.496-6.111,P<0.05), with the strongest inhibitory effect at 10 μmol/L (q=6.111,P<0.01). The mRNA expression levels of TNF-α and IL-1β in the LPS group were significantly higher than those in the control group (F=243.50,378.60;q=30.040,36.000;P<0.01), and also significantly higher than those in the 10.0 μmol/L ICA group (q=7.689,5.199;P<0.05). Conclusion ICA inhibits LPS-induced activation of primary astrocytes and inflammatory response.
[KEY WORDS] icariin; lipopolysaccharides; astrocytes; tumor necrosis factor-alpha; interleukin-1beta; glial fibrillary acidic protein
星形胶质细胞是中枢神经系统数量最多、分布最为广泛的一类神经胶质细胞[1],在修复再生、免疫应答、能量代谢调节、神经递质代谢、营养、突触可塑性以及血-脑脊液屏障完整性的维持等多个方面发挥重要作用[2-5]。许多研究表明,星形胶质细胞的过度激活导致的炎症反应在阿尔茨海默症、帕金森病等神经退行性疾病的病理过程有重要作用[6-7]。因此,抑制星形胶质细胞的炎症反应,减轻星形胶质细胞的过度活化是神经退行性疾病的一种有效治疗策略。脂多糖(LPS)可通过与星形胶质细胞的Toll样受体4(TLR4)结合,导致星形胶质细胞的活化,引起胶质纤维酸性蛋白(GFAP)表达的增加并释放肿瘤坏死因子-α(TNF-α)和白细胞介素1β(IL-1β)等炎性因子,引发神经炎症损伤神经元[8-10]。淫羊藿苷(ICA)是淫羊藿总黄酮中的一种重要生物活性成分,具有调节内分泌、补肾壮阳、诱导成骨细胞分化、抗衰老和抗肿瘤等功效[11-13]。有研究发现,ICA通过抑制TNF-α、白细胞介素-6(IL-6)、环氧合酶-2(COX-2)、诱导型一氧化氮合酶(iNOS)的mRNA表达,抑制LPS诱导的小鼠急性肺部炎症反应[14]。那么ICA是否能通过抑制星形胶质细胞的炎症反应来保护神经元从而阻止神经退行性疾病的进展,目前尚未见报道。本研究采用分子生物学技术,研究ICA对LPS诱导的原代星形胶质细胞GFAP、TNF-α和IL-1β mRNA 表达的影响。
1 材料与方法
1.1 试剂及其来源
ICA购自上海同田生物技术有限公司,用二甲基亚砜(DMSO)溶液稀释为所需实验浓度。LPS购自Sigma公司。DMEM/F12培养基和胎牛血清购自BI公司;青霉素/链霉素储存液购于新华制药厂,开启前分装,-20 ℃保存备用。新生24 h的SD大鼠购于青岛大任富城畜牧有限公司。TRIzol购自Invitrogen 公司;逆转录试剂盒和SYBR green购于Takara公司;引物由Takara公司设计并合成。
1.2 细胞培养及分组
在超净工作台取新生24 h左右的SD大鼠(SPF级)大脑,然后分离出中脑,用灭菌后的PBS清洗3次后,轻轻剥离脑膜和血管。将脑组织剪碎,吹打使细胞分散,收集细胞悬液至大离心管中,以1 000 r/min 离心5 min。弃上清,加入DMEM/F12完全培养基(含胎牛血清、青霉素/链霉素混合液),将细胞吹打重悬,然后接种于铺有poly-D的150 cm2培养瓶中,放置在含体积分数0.05 CO2、37 ℃的细胞培养箱中培养,每隔2 d更换1次培养液。待细胞长满后,放到恒温摇床以37 ℃、210 r/min震荡16~18 h后,弃掉上清,用DMEM/F12基础培养基清洗细胞3次,加入2.5 g/L的胰酶消化1~3 min,用含血清的完全培养基终止消化,轻柔吹打将贴于培养瓶上的细胞吹下,收集细胞。然后,以1 000 r/min离心5 min后,将星形胶质细胞接种到6孔板中。当6孔板内的细胞融合达到80%左右时,将细胞分为对照组(A组)、LPS组(B组)、0.1 μmol/L ICA+LPS组(C组)、1.0 μmol/L ICA+LPS(D组)、10.0 μmol/L ICA+LPS(E组)、20.0 μmol/L ICA +LPS(F组)。A组给予1 μL的DMSO处理1 h,再加入1 μL生理盐水;B组用1 μL DMSO预处理1 h,再加入LPS(终浓度1 mg/L)共同作用6 h;C组、D组、E组、F组分别用0.1、1.0、10.0和20.0 μmol/L ICA 1 μL预处理1 h,再加入LPS(终浓度1 mg/L)共同作用6 h。
1.3 荧光定量PCR(RT-PCR)检测GFAP、TNF-α和IL-1β的mRNA表达
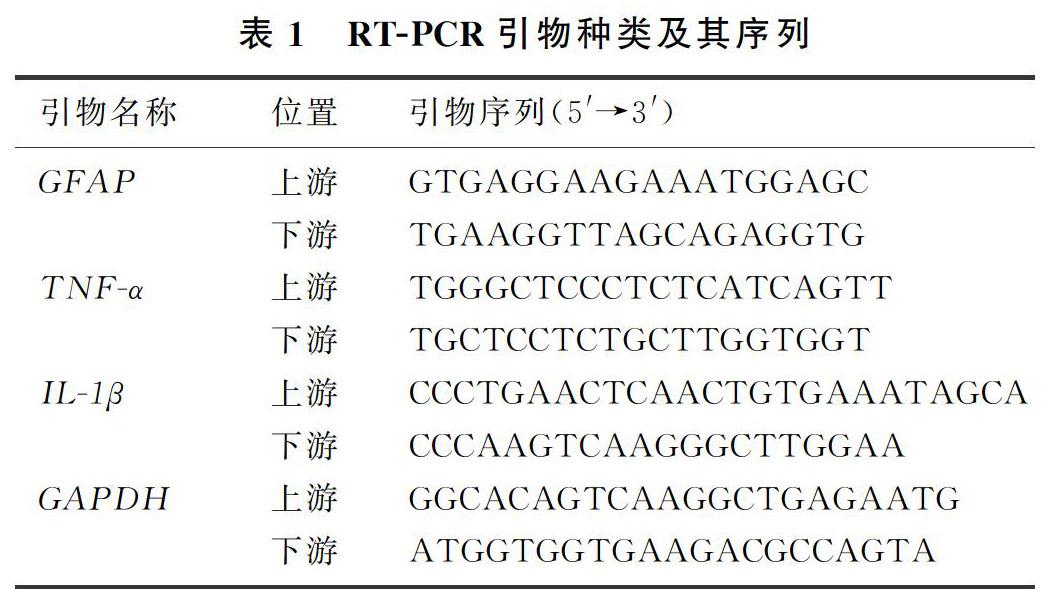
按照TRIzol reagent说明书提取总RNA,然后按照逆转录试剂盒说明书的要求将mRNA逆转录为cDNA。将cDNA放入实时荧光定量PCR仪中,采用SYBR green染料法分别检测相关基因的表达。PCR反应体系为20.0 μL,内含:SYBR green染料10.0 μL,RNA free water 8.2 μL,上下游引物各0.4 μL以及cDNA 1.0 μL。经过40个循环完成扩增,并在程序后添加溶解曲线,以检测扩增品质。以不添加cDNA的扩增溶液为空白对照组。使用2-ΔΔCT法计算目的基因(GFAP、TNF-α、IL-1β)和内参基因GAPDH的相对表达量,同时检查溶解曲线是否为单峰、引物扩增效率及扩增的相关系数等情况。RT-PCR引物及其序列见表1。
1.4 统计学处理
应用Graph Pad Prism 5.0统计软件进行数据处理,计量资料结果以±s形式表示,数据间比较采用单因素方差分析(One-Way ANOVA),并继以Tukey法进行两两比较。
2 结 果
2.1 各组GFAP mRNA表达比较
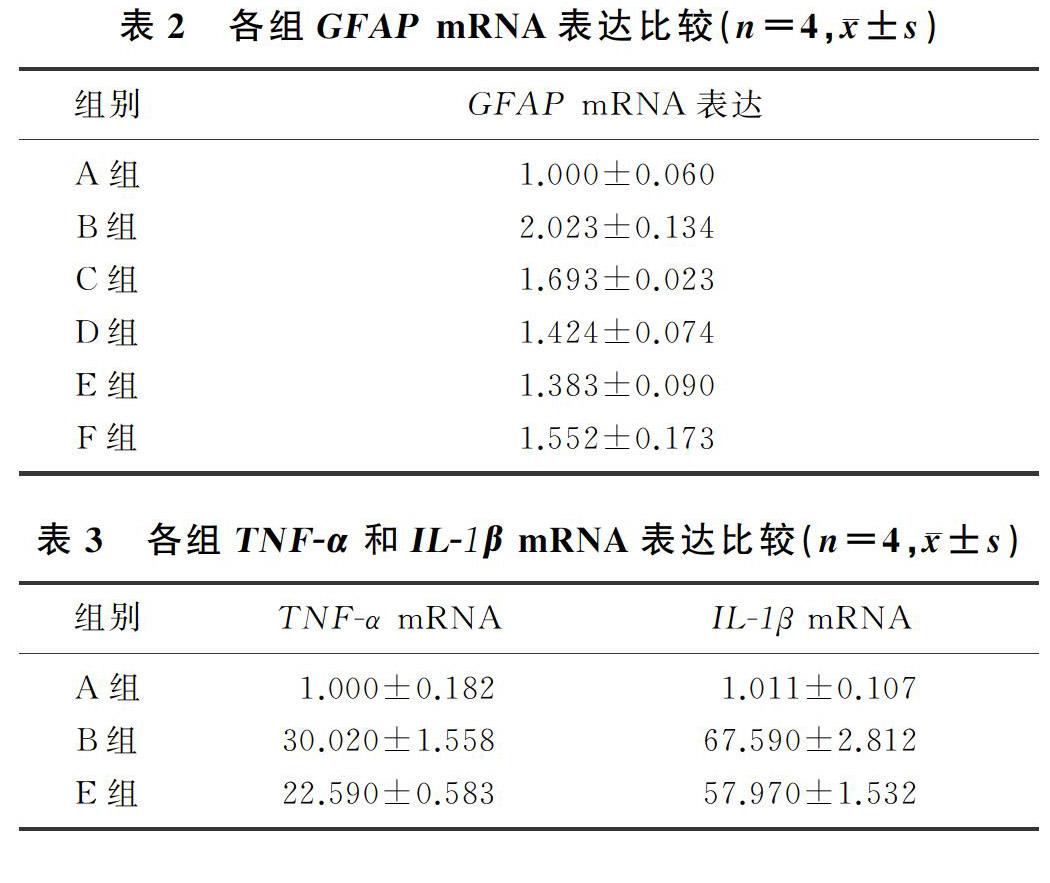
与对照组比较,LPS组的GFAP mRNA表达明显升高,差异有显著性(F=10.63,q=9.775,P<0.01);1.0、10.0、20.0 μmol/L的ICA均可明显抑制LPS诱导的原代星形胶质细胞GFAP mRNA的表达(q=4.496~6.111,P<0.05),其中10.0 μmol/L ICA的抑制作用最明显(q=6.111,P<0.01)。因此,后续实验我们将10.0 μmol/L的ICA作為最佳用药浓度。见表2。
2.2 各組TNF-α和IL-1β mRNA表达比较
与对照组相比较,LPS组原代星形胶质细胞TNF-α和IL-1β的mRNA表达均明显升高,差异有统计学意义(F=243.50、378.60,q=30.040、36.000,P<0.01);与LPS组相比,10 μmol/L ICA能够明显抑制原代星形胶质细胞TNF-α和IL-1β mRNA的表达,差异均有统计学意义(q=7.689、5.199,P<0.05)。见表3。
3 讨 论
体内和体外研究均表明,星形胶质细胞在神经退行性疾病的发病过程中起着至关重要的作用。与小胶质细胞相似,星形胶质细胞受到炎性刺激过度激活,会产生大量的炎性因子如IL-1β和TNF-α等,从而导致神经元的损伤[15-16]。已有研究显示,在不同的帕金森病动物模型中均发现星形胶质细胞反应性增生;且在啮齿类动物的中枢神经系统和周围神经系统实验性损伤模型中观察到,星形胶质细胞特异性标志物GFAP表达增加[17],提示星形胶质细胞可能参与了神经退行性疾病的发病与进展[18-19]。
ICA是从小檗科植物淫羊藿中提取的一种黄酮类化合物,具有骨保护、促生殖、抗肿瘤、改善心脑血管功能、神经保护、抗炎等多种功效[20-24]。本课题组前期研究证明,在离体细胞和整体动物水平,ICA均能够对抗神经毒素对多巴胺能神经元的损伤,发挥神经保护作用[25]。本研究通过LPS诱导原代星形胶质细胞的炎症反应以制备神经炎症模型,探讨ICA的抗炎作用。GFAP是主要分布于中枢神经系统星形胶质细胞的一种蛋白质,是星形胶质细胞活化的标志物[26-27]。在脑损伤和中枢系统发生病变的过程中,星形胶质细胞会被大量激活,GFAP的表达上调[28-30]。本实验结果显示,LPS能够使原代星形胶质细胞GFAP的表达明显上调,应用不同浓度的ICA预处理后,GFAP mRNA的表达被不同程度地抑制,提示ICA能够抑制星形胶质细胞的过度活化,其中10 μmol/L的ICA作用效果最明显,因此后续实验我们将10 μmol/L作为ICA的最佳用药浓度。此外,LPS能够明显诱导星形胶质细胞炎性因子TNF-α和IL-1β mRNA的表达,而应用浓度为10 μmol/L的ICA预处理后能够明显抑制LPS诱导的上述两种炎性因子的基因表达,因而有效地减少了星形胶质细胞的炎症反应,将有利于减低炎性因子对神经元的损伤。
综上所述,ICA能够抑制LPS诱导的原代星形胶质细胞的活化和炎症反应,为今后应用ICA对抗神经炎症提供了实验依据。
[参考文献]
[1] JI R R, DONNELLY C R, NEDERGAARD M. Astrocytes in chronic pain and itch[J]. Nature Reviews Neuroscience, 2019,20(11):667-685.
[2] SPAMPINATO S F, BORTOLOTTO V, CANONICO P L, et al. Astrocyte-derived paracrine signals: relevance for neurogenic niche regulation and blood-brain barrier integrity[J]. Frontiers in Pharmacology, 2019,10:1346.
[3] PAJARILLO E, RIZOR A, LEE J, et al. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics[J]. Neuropharmacology, 2019,161:107559.
[4] FALKOWSKA A, GUTOWSKA I, GOSCHORSKA M, et al. Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism[J]. International Journal of Molecular Sciences, 2015,16:25959-25981.
[5] BURDA J E, BERNSTEIN A M, SOFRONIEW M V. Astrocyte roles in traumatic brain injury[J]. Experimental Neurology, 2016,275(3, SI):305-315.
[6] JAYARAJ R L, BEIRAM R, AZIMULLAH S, et al. Lycopo-dium attenuates loss of dopaminergic neurons by suppressing oxidative stress and neuroinflammation in a rat model of Parkinsons disease[J]. Molecules, 2019,24:2182.
[7] ACOSTA C, ANDERSON H D, ANDERSON C M. Astrocyte dysfunction in alzheimer disease[J]. Journal of Neuroscience Research, 2017,95(12):2430-2447.
[8] LIU Tong, GAO Yongjing, JI Rurong. Emerging role of Toll-like receptors in the control of pain and itch[J]. Neuroscience Bulletin, 2012,28(2):131-144.
[9] GORINA R, FONT-NIEVES M, MARQUEZ-KISINOUSKY L, et al. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NF kappa B signaling, MAPK, and Jak1/Stat1 pathways[J]. Glia, 2011,59(2):242-255.
[10] BOWMAN C C, RASLEY A, TRANGUCH S L, et al. Cultured astrocytes Express toll-like receptors for bacterial pro-ducts[J]. Glia, 2003,43(3):281-291.
[11] ZHANG Lei, ZHANG Xuan, LI Kuifeng, et al. Icariin promotes extracellular matrix synthesis and gene expression of chondrocytes in vitro[J]. Phytotherapy Research, 2012,26(9):1385-1392.
[12] HUANG Xin, ZHU Danyan, LOU Yijia. A novel anticancer agent, icaritin, induced cell growth inhibition, G1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cells[J]. European Journal of Pharmacology, 2007,564(1/3):26-36.
[13] CHUNG B H, KIM J D, KIM C K, et al. Icariin stimulates angiogenesis by activating the MEK/ERK-and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells[J]. Biochemical and Biophysical Research Communications, 2008,376(2):404-408.
[14] XU Changqing, LIU Baojun, WU Jinfeng, et al. Icariin atte-nuates LPS-induced acute inflammatory responses: involvement of PI3K/Akt and NF-kappa B signaling pathway[J]. European Journal of Pharmacology, 2010,642(1/3):146-153.
[15] TANAKA T, KAI S, MATSUYAMA T, et al. General anesthetics inhibit LPS-induced IL-1beta expression in glial cells[J]. PLoS One, 2013,8(12): e82930.
[16] SAIJO K, WINNER B, CARSON C T, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death[J]. Cell, 2009,137(1):47-59.
[17] ZHANG Fangxue, XU Renshi. Juglanin ameliorates LPS-induced neuroinflammation in animal models of Parkinsons di-sease and cell culture via inactivating TLR4/NF-κB pathway[J]. Biomedicine& Pharmacotherapy, 2018,97:1011-1019.
[18] TRONCOSO-ESCUDERO P, PARRA A, NASSIF M, et al. Outside in: unraveling the role of neuroinflammation in the progression of Parkinsons disease[J]. Frontiers in Neurology, 2018,9:860.
[19] FELLNER L, IRSCHICK R, SCHANDA K, et al. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia[J]. Glia, 2013,61:349-360.
[20] 路宇仁,陈昳冰,崔元璐,等. 淫羊藿苷药理作用研究进展[J]. 中国实验方剂学杂志, 2018,24(17):209-220.
[21] ZHOU Heng, YUAN Yuan, LIU Yuan, et al. Icariin atte-nuates angiotensin Ⅱ-induced hypertrophy and apoptosis in H9c2 cardiomyocytes by inhibiting reactive oxygen species-dependent JNK and p38 pathways[J]. Experimental and Therapeutic Medicine, 2014,7:1116-1122.
[22] ZHENG Yaxin, ZHU Guofu, HE Jingyi, et al. Icariin targets Nrf2 signaling to inhibit microglia-mediated neuroinflammation[J]. International Immunopharmacology, 2019,73:304-311.
[23] ZHANG Shuncong, FENG Pengbo, MO Guoye, et al. Icariin influences adipogenic differentiation of stem cells affected by osteoblast-osteoclast co-culture and clinical research adipogenic[J]. Biomedicine & Pharmacotherapy, 2017,88:436-442.
[24] 陳茹,苏莹,柳江. 淫羊藿苷通过Wnt/β-catenin信号通路对卵巢癌细胞CAOV3增殖的影响[J]. 医学研究杂志, 2019,48(3):44-49.
[25] CHEN Wenfang, WU Lin, DU Zhongrui, et al. Neuroprotective properties of icariin in MPTP-induced mouse model of Parkinsons disease: involvement of PI3K/Akt and MEK/ERK signaling pathways[J]. Phytomedicine, 2017,25:93-99.
[26] 戴淑馨,王宇,林丽芳,等. PR-957对A1反应性星形胶质细胞形成的影响[J]. 中国当代儿科杂志, 2019,21(11):1110-1115.
[27] 苏晓梅,张丹参. 星形胶质细胞与神经退行性疾病的相关性[J]. 中国药理学与毒理学杂志, 2019,33(10):868-869.
[28] ULLAH F, ASGAROV R, VENIGALLA M, et al. Effects of a solid lipid curcumin particle formulation on chronic activation of microglia and astroglia in the GFAP-IL6 mouse model[J]. Scientific Reports, 2020,10(1):2365.
[29] PEKNY M, WILHELMSSON U, TATLISUMAK T, et al. Astrocyte activation and reactive gliosis-a new target in stroke[J]? Neuroscience Letters, 2019,689:45-55.
[30] KOSUGE Y, KANEKO E, NANGO H, et al. Bidens pilosa extract administered after symptom onset attenuates glial activation, improves motor performance, and prolongs survival in a mouse model of amyotrophic lateral sclerosis[J]. Oxidative Medicine and Cellular Longevity, 2020,2020:1020673.
(本文编辑 黄建乡)

