Efference ects of dietary cottonseed meal protein hydrolysate on growth, antioxidants and immunity of Chinese mitten crab Eriocheir Sinensis*
CHENG Huihui, LIU Wenbin, YUAN Xiangyang, JIA Erteng, ZHANG Dingdong, JIANG Guangzhen
Key Laboratory of Aquatic Nutrition and Feed Science of Jiangsu Province, National Experimental Teaching Center for Animal Science, College of Animal Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
Received Jun. 8, 2019; accepted in principle Aug. 14, 2019; accepted for publication Sep. 19, 2019 © Chinese Society for Oceanology and Limnology, Science Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract One hundred and sixty crabs (average initial weight: 51.32±0.08 g) were fed with four experimental diets containing cottonseed meal protein hydrolysate (CPH) at 0% (CPH0, control), 0.3% (CPH0.3), 0.6% (CPH0.6), and 1.2% CPH (CPH1.2). The experiment results show that no difference erence was observed in specifi c growth rate and survival rate of crabs fed with CPH diet. Moisture content of crabs fed with CPH0.6 diet was signifi cantly reduced than that of the CPH0 group. Superoxide dismutase and catalase activities of crabs fed with CPH0.6 diet were signifi cantly increased and the difference erence was not signifi cant between the CPH0.3 and CPH0.6 groups. Malondialdehyde content of CPH0.3 group was signifi cantly lower than that of the CPH0 group. Lysozyme, alkaline phosphatase, and acid phosphatase activities of CPH0.3 diet crabs were signifi cantly higher than that of the CPH0 group. Glutamic-pyruvic transaminase activity of crabs fed with CPH0.3 diet was signifi cantly decreased compared to the CPH0 group. The relative expression levels of Toll1, Toll2, MyD88, LITAF, and ILF- 2 of crabs fed with CPH0.3 diet were signifi cantly higher than that of the CPH0 group. The expression level of SOCS2 showed an opposite pattern. After CPH perfusion, the expression levels of SOCS2 and Toll1 in intestine at time 3 h and SOCS2 in hepatopancreas at time 18 h increased signifi cantly to the highest value. The expression level of Toll2, MyD88, LITAF, decreased at times 6 h, 6 h, 12 h, respectively, then increased gradually. Therefore, supplementation of dietary CPH could improve antioxidant capacity and immune function; the appropriate supplement dosage of CPH for crab could be 0.3%-0.6%. Furthermore, the short-term CPH stimulation could signifi cantly increase or decline the expression levels of immune-related genes at difference erent times after CPH perfusion.
Keyword: Eriocheir sinensis; cottonseed meal protein hydrolysate; growth; body composition; immunity; antioxidant
1 INTRODUCTION
Chinese mitten crab Eriocheir sinensis is one of the most economically valuable freshwater crabs in Asia (Rudnick et al., 2003). It was reported that world aquaculture output of Chinese mitten crab reached 796 622 tons in 2014 (Pauly and Zeller, 2017). However, with the development of aquaculture practice, a variety of diseases caused by viruses (Zhang and Bonami, 2012) and bacteria (Wang and Gu, 2002) may emerge and afference ect the production of Chinese mitten crab. When bacteria or viruses infect crabs, it would afference ect the health of crabs and consequently their growth, thus result in a burst of death (Fu et al., 2017). Therefore, it is important to strengthen the resistance of crabs. In the past few years, to reduce the risk of bacterial and viral infections in crabs, many researches carried out studies to enhance the antioxidant capacity and immune response of crabs, for example, supplying nutrient suitably of feed (Sun et al., 2013b; Wei et al., 2016), and adding small bioactive peptides (Guo et al., 2017) and immune-enhancers moderately (Sun et al., 2013a; Hong et al., 2017; Jia et al., 2017). However, most of the researchers concentrated on juvenile Eriocheir sinensis, and research on adult ones remains poor. As crustaceans, it is necessary to work more to enhance or regulate the immunological response of adult crab in order to avoid alteration of the immune system (Sánchez et al., 2001).
Plant protein as an ingredient of aquaculture feed is less cost compared with animal protein, especially fi sh meal. However, the utilization of plant proteins (e.g., cottonseed meal, rapeseed meal) is limited due to their antinutritional factors such as gossypol (Jiang et al., 2018). These antinutritional factors can cause negative efference ect on growth and health of aquatic animals (Figueiredo-Silva et al., 2015; Jiang et al., 2018). Plant protein hydrolysate is produced using processes such as hydrolytic enzymes (Colla et al., 2014; Muranova et al., 2017). Studies has proven that these hydrolysates are ideal nutrient sources, they could increase weight gain, improve immunity and antioxidant capability of aquatic animals when added within a suitable range (Gibbs et al., 2004; Kotzamanis et al., 2007; Möller et al., 2008; Gui et al., 2010; Yimit et al., 2012; Song et al., 2014).
A previous study by Liu (2005) suggested that cottonseed meal protein hydrolysate (CPH) had the best efference ect on the growth and immunity of fi sh compared with those hydrolysates of rapeseed meal, soybean meal, and peanut meal. Thus, we chose cottonseed meal (CM) to produce CPH. The proportions of small molecule peptide and free amino acid consisting in CPH were signifi cantly improved compared with CM. The peptide content of CPH was 13.4% (180-1 983 Da), whereas CM was only 1.9% (180-1 983 Da) (Gui et al., 2010). CPH can improve feed ingredient palatability as an attractant of Chinese mitten crab (Cheng et al., 2019), and boost immunity of Alogynogenetic crucian carp (Liu, 2005); it can also improve the activities of serum lysozyme and alkaline phosphatase in the intestines of Cyprinus carpio var. jian (Xia et al., 2012). However, presently, there is no available information on the efference ects of dietary CPH supplementation on immunity of Chinese mitten crab. Thus, the main aim of our study is to explore the efference ects of dietary CPH at difference erent levels on growth, antioxidant capability and immune response as well as the mRNA expression levels of immune-related genes of Chinese mitten crab.
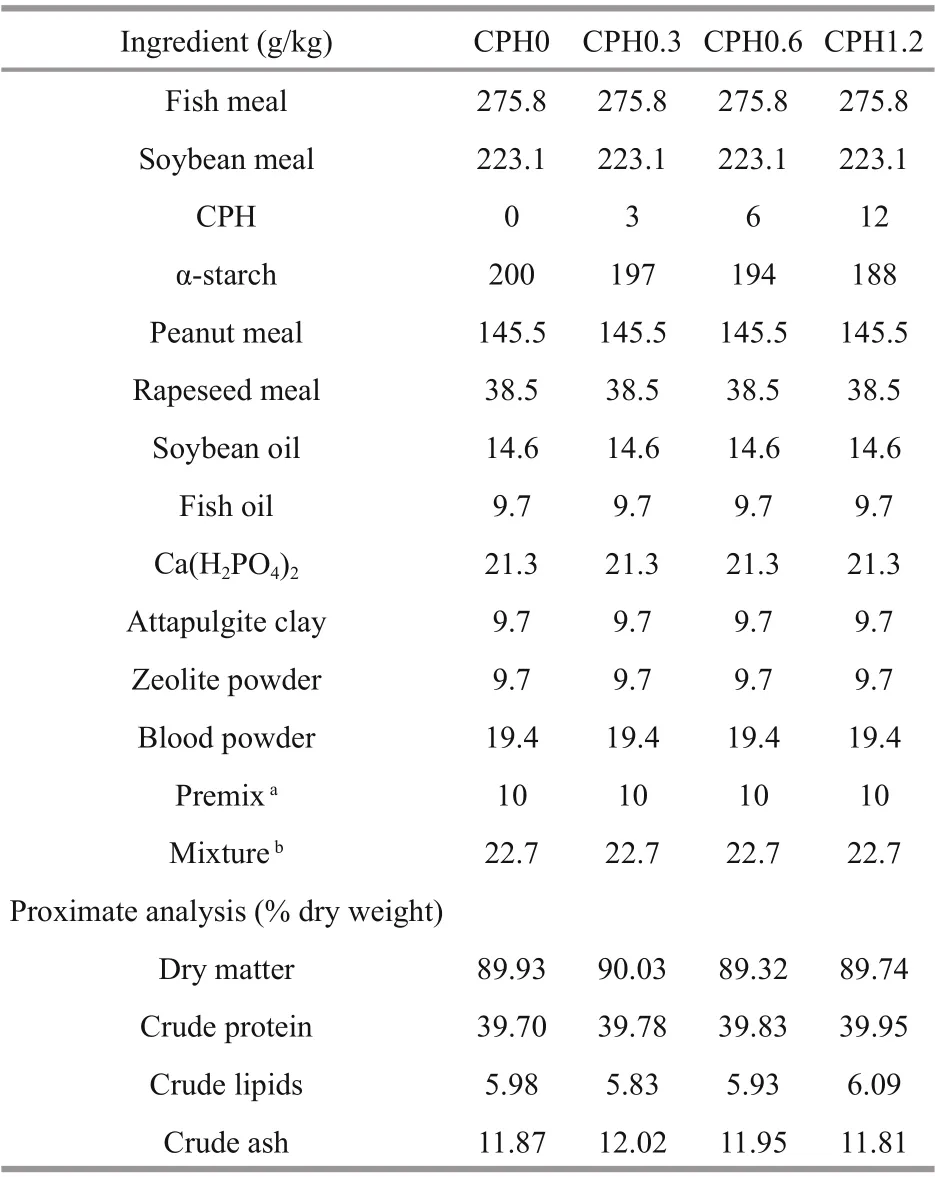
Table 1 Formulation and proximate composition of the experimental diets
2 MATERIAL AND METHOD
2.1 Experimental ingredients and diets
CPH was produced by the method of Xia et al. (2012). Cottonseed meal protein was hydrolysated by AS1.398 protease in water bath (5 h, 45°C, pH 7.0). Four difference erent CPH dosages were added to the basic diets to make the experimental diets: 0% (CPH0 group), 0.3% (CPH0.3 group), 0.6% (CPH0.6 group), and 1.2% (CPH1.2 group). Ingredients and proximate composition of the experimental diets are shown in Table 1. Fish meal, soybean meal, peanut meal, and rapeseed meal were supplemented as protein sources. Soybean oil and fi sh oil were supplemented as lipid sources, and α-starch was supplemented as carbohydrate source. All diets were prepared in laboratory. Feed ingredients were ground into fi ne powder, and then they mixed with oil and water to be dough. After that, the dough was extruded through a single-screw meat grinder extruder. These feeds were air-dried and then were cut to appropriate sizes. Last, they were stored in sealed bags at -20°C.
2.2 Crab and experimental design
Crabs were obtained from a local feeding farm in Pukou, Jiangsu Province, China. In the beginning, crabs were fed a basal diet twice a day to adopt experimental conditions for 2 weeks. Then, 160 crabs (initial weight: 51.32±0.08 g, mean±SEM) were randomly assigned into 20 cement pools (width×length×height, 2.0 m×2.0 m×0.8 m) at a density of 8 crabs per cement pool, fi ve replicates was contained in each group. Crabs were fed twice daily (07:00 am and 05:00 pm) for 60 days. The number of deaths crabs was recorded daily. During the experimental period, water temperature ranged from 23 to 30°C, pH fl uctuated between 7.2 and 7.4, and dissolved oxygen was maintained above 5.0 mg/L.
2.3 Sample collection
At the end of the feeding trial, one crab from each cement pool was sampled at random for analysis of the whole body composition. Three crabs from per replicate were anesthetized by an MS-222 (tricaine methanesulfonate, Sigma, USA) at a concentration of 150 mg/L. After that, they were weighed, respectively. Hemolymph was obtained using syringes from each crab’s second last pair of walking legs 1:1 with precooling anticoagulant solution (citrate, 26 mmol/L; glucose, 100 mmol/L; citric acid, 30 mmol/L; NaCl, 450 mmol/L; EDTA, 15 mmol/L; pH=7.2) (Li et al., 2014), with that, hemolymph was centrifuged immediately at 3 500 r/min, 4°C for 20 min. The supernatant serum was sucked up in the centrifuge tube, and then they were stored at -80°C for subsequent analysis. After that, every crab was dissected, and then obtained hepatopancreas were put in a liquid nitrogen container. Finally, they were stored at -80°C for subsequent analysis.
2.4 Perfusion experiment
Another group of crabs (mean weight 85 g) were acclimated for 2 weeks with commercial diet at 28°C in one 200-L tank with circulating water system. Crabs were starved for 24 h before perfusion.
Six crabs were randomly collected and anesthetized by an MS-222 (tricaine methanesulfonate, Sigma, USA) at a concentration of 150 mg/L, and after that, they were dissected, obtained hepatopancreas and intestine, which were used for time 0. Then, 30 crabs were divided into two groups and weighted respectively. Crabs in two groups were perfused intragastrically with either saline solution (10 mL per kg crab body weight) or CPH solution (10 mL per kg crab body weight), which a saline solution (0.9%) containing 5 mg CPH per mL. The two groups were labeled as Sham group and CPH group. After that, crabs were transferred quickly to 10 tanks (100 L each) at a density of three crabs per tank. After perfusion, crabs in each group were sampled at 3, 6, 12, 18, and 24 h, respectively. One tank was used for each sampling time so as to minimize the stress during sampling. Hepatopancreas and intestine were sampled and then were kept at -80°C for subsequent analysis of gene expression.
2.5 Parameters measurement and methods
2.5.1 Crabs growth performance
The body weight of each crab was determined at the start and end of the feeding experiment. In addition, the specifi c growth rate (SGR) and survival rate (SR) were determined using the following formula:
S pecifi c growth rate (SGR, %/d)=100×(ln Wf−ln Wi)/ t,
S urvival rate (SR, %)=(initial crab number−fi nal crab number)×100/initial crab number,
where Wfrepresented fi nal body weight (g), Wirepresented initial body weight (g), t represented the total experimental days.
2.5.2 Proximate analysis of the experimental diets and body composition
The diets and the crabs were analyzed to determine their proximate composition according to the procedures detailed by the AOAC (1984). The moisture contents of diets and crabs were estimated by using drying oven to a constant weight; ash was determined by combustion at 550°C for 4-6 h; crude protein was determined using an Auto Kjeldahl System (1030-Auto-analyzer, Tecator, Hoganas, Sweden) by using the micro-Kjeldahl method; crude lipid was determined using a Soxtec System HT (Soxtec System HT6, Tecator, Hoganas, Sweden) by solvent extraction; the energy levels of diets and crabs were determined using a Bomb Calorimeter (Parr 1281, Parr Instrument Company, Moline, IL, USA).
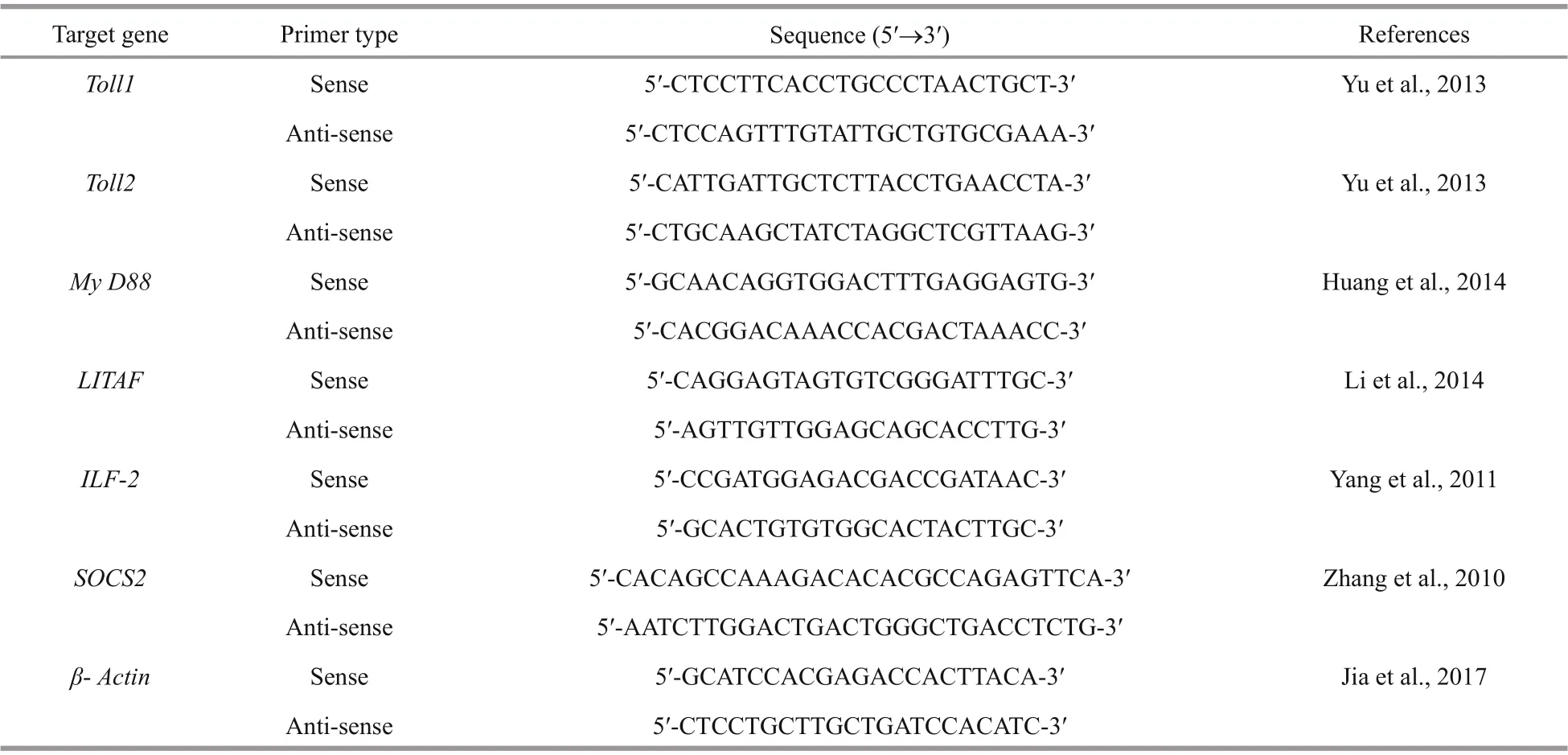
Table 2 Nucleotide sequences of the primers used to assay gene expression by real-time quantitative PCR
2.5.3 Analysis of antioxidant capabilities
Hemolymph and hepatopancreas were used to estimate the antioxidant capability of crabs. The hepatopancreas was weighed accurately and homogenized in an ice bath with nine volumes (v/w) of chilled saline in a tissue homogenizer, and then the extract was centrifuged at 9 000 r/min at 4°C for 15 min. Finally, all the supernatant was then stored at -20°C for subsequent analysis.
Total superoxide dismutase (t-SOD) activity in hemolymph was detected according to the method described by Wang and Chen (2005). Superoxide dismutase (SOD) activity in hepatopancreas was detected using the Ransod Kit (Randox, Crumlin, UK) by the ability of inhibiting superoxide radicaldependent reactions. Catalase (CAT) activity was measured according the method described by Lygren et al. (1999). Malondialdehyde (MDA) content was estimated using the thiobarbituric acid test (Satoh, 1978).
2.5.4 Immune parameters in hemolymph
Hemolymph was used to measure the activities of lysozyme (LZM), acid phosphatase (ACP) and alkaline phosphatase (ALP). The activity of LZM was assayed according to LZM kit protocol at 530 nm according to Yuan et al. (2007). The activities of ACP and ALP were measured by the disodium phenyl phosphate hydrate method (Chen et al., 2016).
2.5.5 GPT and GOT activities in hemolymph
The activities of glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) in hemolymph and their standard curves were measured by the spectrophotometer at 520 nm, then the activities of GPT and GOT were calculated according to the standard curves (Bergmeyer et al., 1978).
2.5.6 Analysis of gene expression
Total RNA was extracted using RNAiso Plus (TaKaRa, Dalian, China). The total RNA purity was detected using NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientifi c, Wilmington, DE). The RNA samples were used for the subsequent analysis only when the A260/A280 ratio between 1.8 and 2.0. The reverse transcription-PCR reaction of PrimeScriptTMRT Master Mix (TaKaRa, Dalian, China) was: 15 min at 37°C, 5 s at 85°C, and after that, 4°C thereafter. The obtained cDNA was kept at -20°C.
Real-time quantitative PCR (RT-qPCR) primers for immune-related genes are presented in Table 2. RT-qPCR was performed on a real-time PCR detection system (ABI USA-7500) using a SYBR Premix Ex Taq II kit (TaKaRa, Dalian, China). The RT-qPCR reactions were performed in a fi nal 20-μL volume, including 10 μL SYBR Premix Ex TaqⅡ (2x), 0.4 μL primer (10 μm), 0.4 μL ROX, 6.8 μL DEPC water, as well as 2 μL of cDNA template. The reactions were initially denatured at 95°C for 10 min and then 40 cycles at 95°C for 15 s, followed by annealing at 60°C for 34 s. The relative mRNA levels were calculated by the 2-ΔΔCtmethod (Livak and Schmittgen, 2001), then, β-Actin was acted as the endogenous control to normalize the mRNA expression of the immunerelated genes.
2.6 Statistical analysis
The data on feeding trial was subjected to One-way analysis of variance (One-way ANOVA) followed by Turkey’s test using the SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA). The data regarding gene expression levels in the CPH perfusion and Sham perfusion were analyzed by Two-way ANOVA for signifi cant difference erences among treatment means based on sampling time, perfusion type and their interaction. If the interaction were signifi cant ( P <0.05) difference erences, each factor was further analyzed separately by One-way ANOVA. Specifi cally, data among the difference erent treatments at each sampling time was analyzed by One-way ANOVA. Data among difference erent sampling time within each treatment was analyzed by t-test. The data are presented as mean±S.E.M.
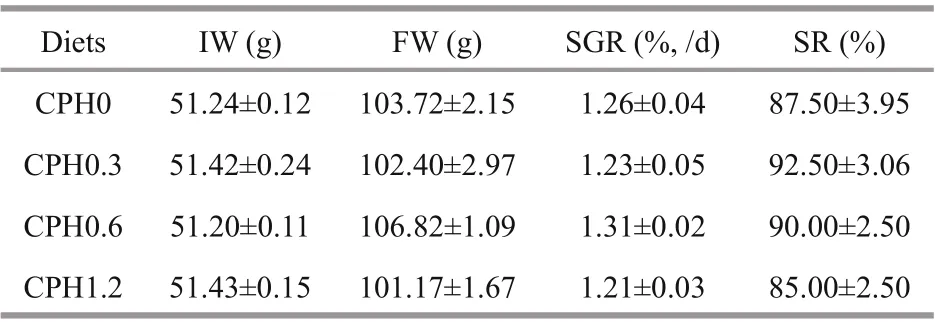
Table 3 Efference ects of dietary CPH on growth performance of crabs
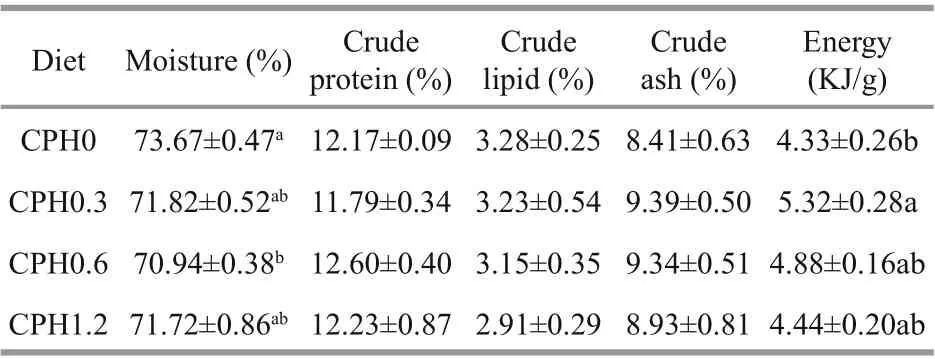
Table 4 Efference ects of dietary CPH on body composition and energy level (wet weight) of crabs
3 RESULT
3.1 Growth performance
As can be seen from Table 3, no difference erence ( P >0.05) were seen in FW, SGR and SR in crabs fed with CPH diet compared to the CPH0 group.
3.2 Whole body composition
As shown in Table 4, crude protein, lipid, and ash content all shows no difference erences ( P >0.05) among all the groups. However, moisture content was signifi cantly reduced ( P <0.05) in crabs fed with CPH0.6 diet compared to the CPH0 group, but no difference erence ( P >0.05) was observed among other supplemented diet. Similarly, energy content was signifi cantly higher ( P <0.05) in crabs fed with CPH0.3 diet compared to the CPH0 group, but no noticeable difference erence ( P >0.05) was observed among others.
3.3 The activities of SOD, CAT and the content of MDA of Chinese mitten crab
As shown in Table 5, SOD and CAT activities in hemolymph and hemolymph of crabs fed with CPH0.6 diet were signifi cantly ( P <0.05) higher than that of the CPH0 group whereas the CPH0.3 and CPH0.6 groups was not difference erent signifi cantly ( P >0.05). MDA content in hemolymph of crabs fed with CPH0.3 diet was signifi cantly lower than those of the other groups in terms of dietary CPH levels ( P <0.05). MDA content in hepatopancreas of crabs fed with CPH0.3 diet was signifi cantly ( P <0.05) lower than that of the CPH0 group whereas the CPH0.3 and CPH0.6 groups was not difference erent signifi cantly ( P >0.05).
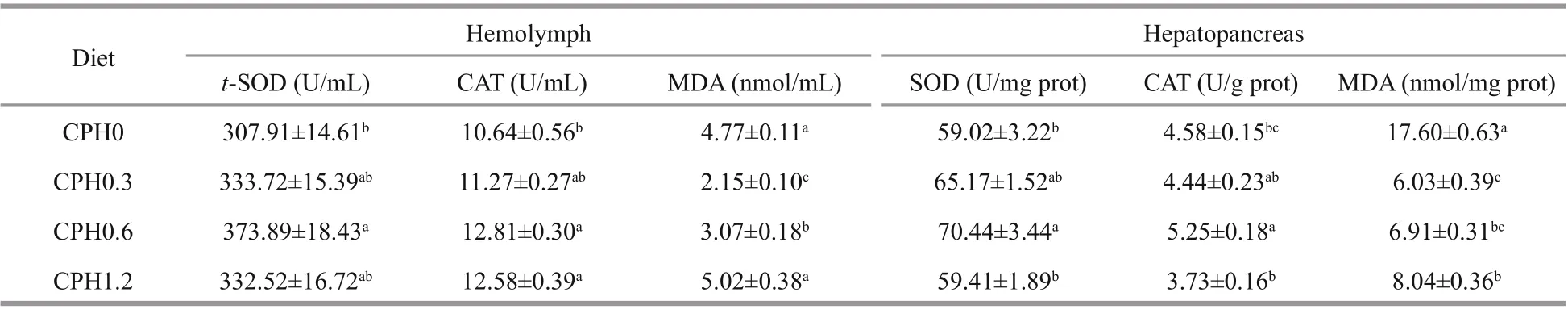
Table 5 Efference ects of dietary CPH on CAT and SOD activities and MDA content of crabs
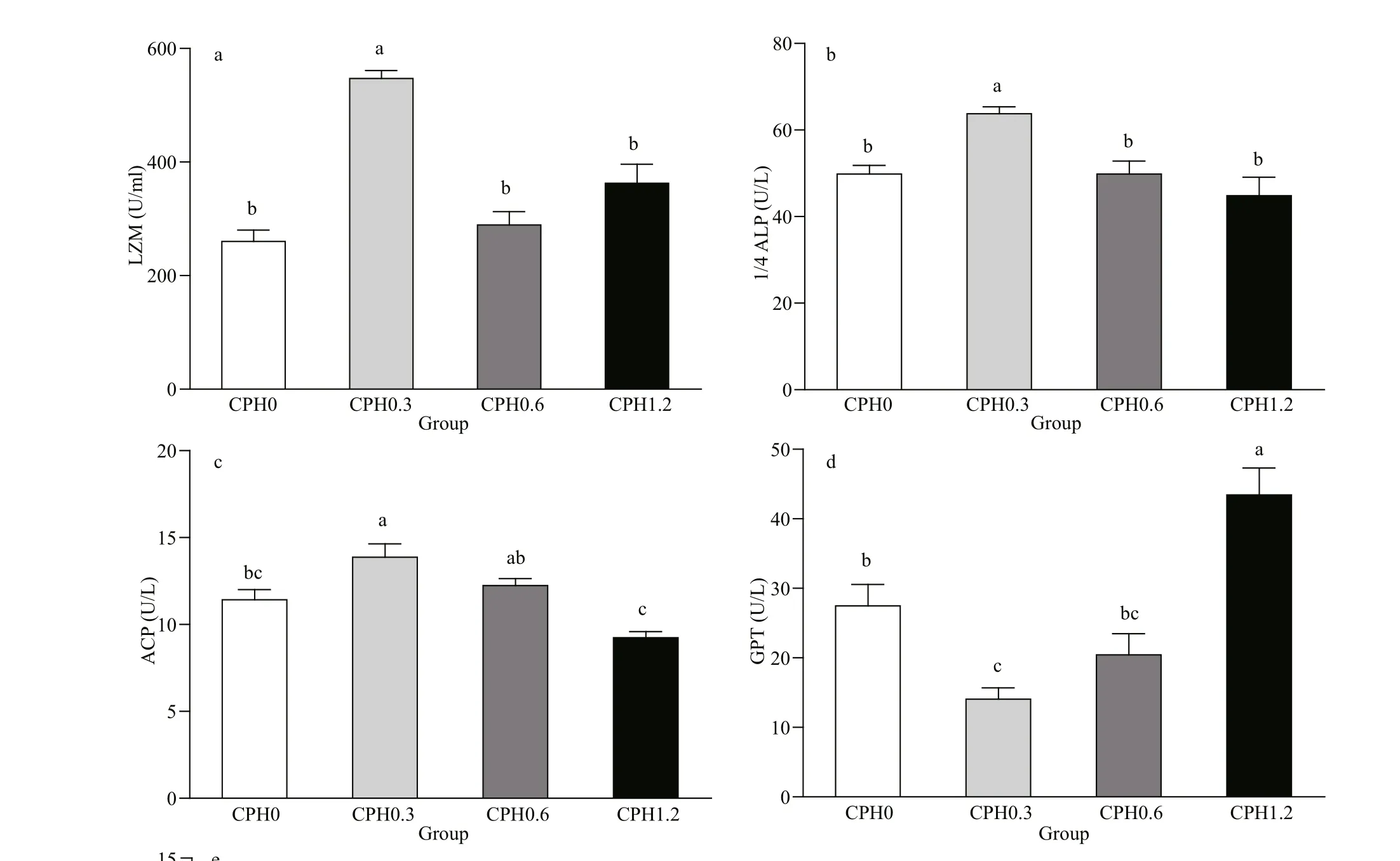
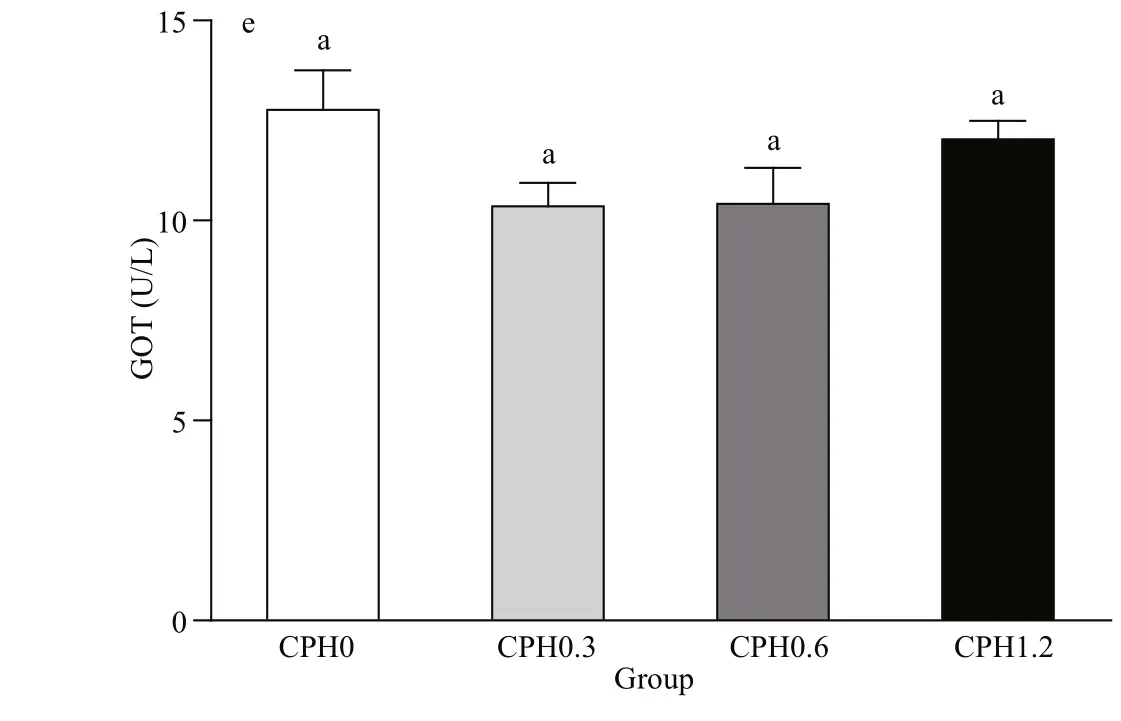
Fig.1 LZM (a), ALP (b), ACP(c), GPT (d), and GOT (e) activities in hemolymph of crabs fed diet containing different levels of CPH
3.4 The activities of LZM, ALP, ACP, GPT and GOT in hemolymph of Chinese mitten crab
As shown in Fig.1: LZM activity in hemolymph of crabs fed with CPH0.3 diet was signifi cantly ( P <0.05) higher than those of the other groups in terms of dietary CPH levels; ALP had the same tendency with LZM, and it was signifi cantly ( P <0.05) higher than those of the other groups in terms of dietary CPH levels; ACP activity in hemolymph of crabs fed with CPH0.3 diet was signifi cantly ( P <0.05) higher than the CPH0 group whereas the CPH0.3 and CPH0.6 groups was not difference erent signifi cantly ( P >0.05). GPT activity in hemolymph of crabs fed with CPH0.3 diet was signifi cantly ( P <0.05) lower than that of the CPH0 group, although there was no signifi cant difference erence between CPH0.3 and CPH0.6 groups ( P >0.05). There was no signifi cant difference erence in GOT activity among all groups ( P >0.05).
3.5 The relative expression levels of Toll1, Toll2, MyD88, LITAF, ILF- 2, and SOCS2
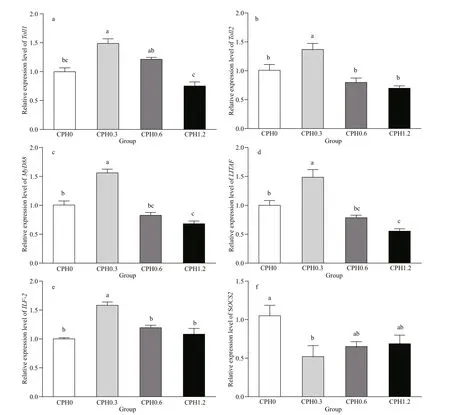
Fig.2 Relative expression levels of Toll1 (a), Toll2 (b), MyD88 (c), LITAF (d), ILF- 2 (e), and SOCS2 (f) in hepatopancreas of crabs fed diet containing difference erent levels of CPH
As shown in Fig.2, the relative expression level of Toll 1 of crabs fed with CPH0.3 diet was signifi cantly ( P <0.05) higher than that of the CPH0 group and CPH1.2 group. The relative expression level of Toll2 of crabs fed with CPH0.3 diet was signifi cantly ( P <0.05) higher than those of the other groups in terms of dietary CPH levels. The relative expression levels of MyD88 and LITAF of crabs fed with CPH0.3 diet were signifi cantly ( P <0.05) higher than those of the other groups in terms of dietary CPH levels. Similarly, the relative expression level of ILF- 2 of crabs fed with CPH0.3 diet was signifi cantly ( P <0.05) higher than those of the other groups in terms of dietary CPH levels. The relative expression levels of SOCS2 of crabs fed with CPH0.3 diet was signifi cantly ( P <0.05) lower than that of the CPH0 group.
3.6 The relative expression levels of Toll1, Toll2, MyD88, LITAF, ILF- 2, and SOCS2 after CPH perfusion
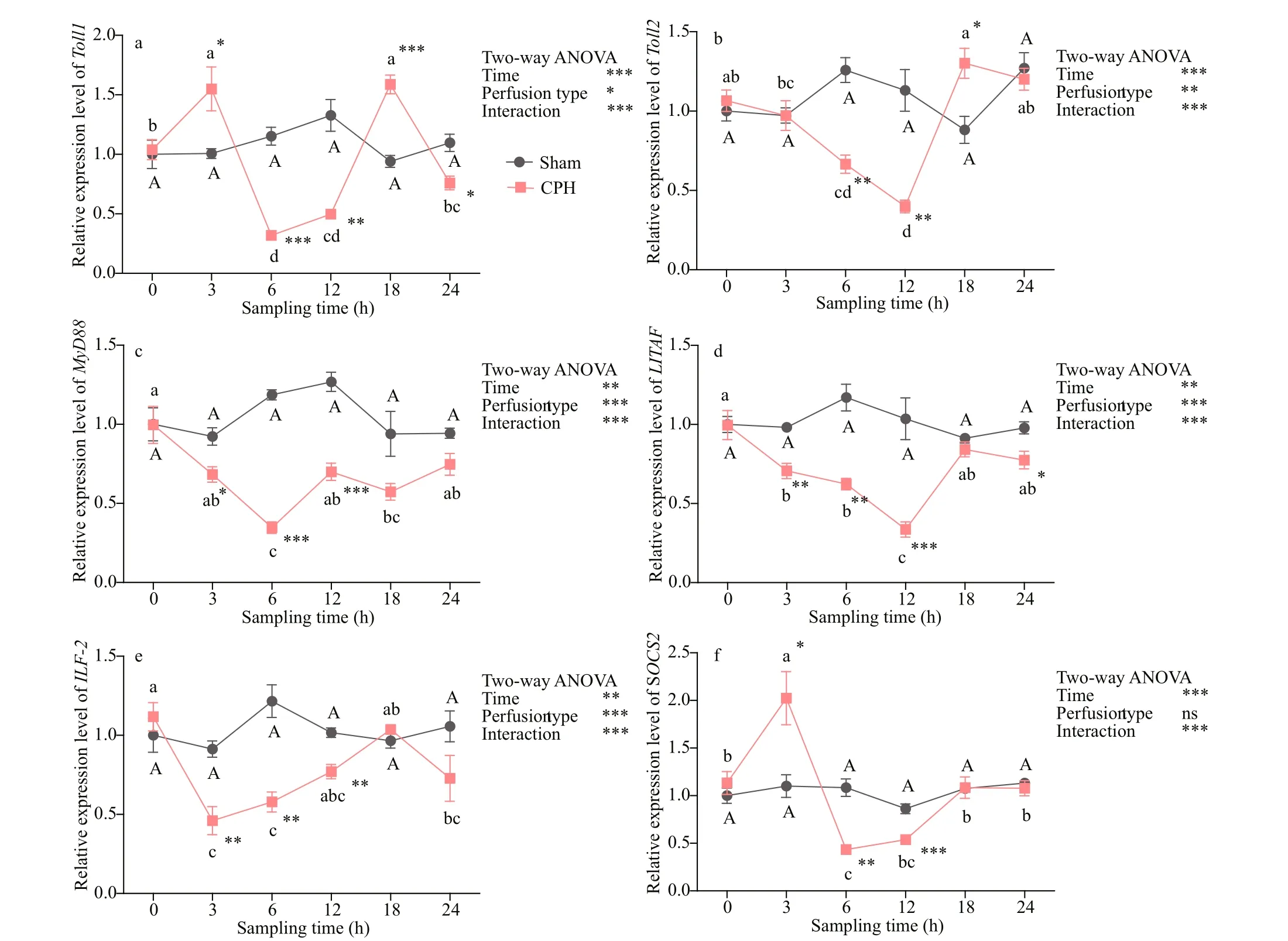
Fig.3 Relative expressions of Toll1 (a), Toll2 (b), MyD88 (c), LITAF (d), ILF- 2 (e) and SOCS2 (f) in intestine of crabs after CPH perfusion
As shown in Figs.3 & 4, after CPH perfusion, the relative expression levels of Toll1, Toll2, MyD88, LITAF, ILF- 2 in intestine and hepatopancreas were all signifi cantly ( P <0.01) afference ected by perfusion type, time and their interaction; SOCS2 expression level was signifi cantly ( P <0.001) afference ected by time and the interaction of time and perfusion type. The CPH treatment resulted in the expression levels of Toll1 at times 3 h, 18 h and Toll2 at times 18 h, 24 h in intestine signifi cantly ( P <0.05) increase after perfusion. However, they declined signifi cantly ( P <0.01) at other times. After the CPH treatment, Toll1 and Toll2 expression in hepatopancreas and MyD88 expression in intestine and hepatopancreas decreased signifi cantly ( P <0.05) with the lowest value at 6 h. LITAF expression in intestine and hepatopancreas decreased signifi cantly ( P <0.05) with the lowest value at 12 h. The expression level of ILF- 2 in intestine at time 3 h and in hepatopancreas at times 6 h decreased signifi cantly ( P <0.05) to the lowest value. After that, they increased gradually until basal level was reached compared to Sham group. The expression level of SOCS2 in intestine at time 3 h and in hepatopancreas at times 18 h increased signifi cantly ( P <0.05) to the highest value. The expression levels of all these genes did not difference erence at all times after sham treatment.
4 DISCUSSION
The growth of animal is afference ected by feed utilization rate and the nutrients deposition of animal is greatly infl uenced by the nutritional composition of feed (Sheridan and Mommsen, 1991; Duan and Plisetskaya, 1993). It is well known that crabs grow quickly only after molting (Hartnoll, 2001; He, 2005). The molting period is infl uenced by temperature, nutrition, and breeding environment (Chen et al., 1995; Saucedo et al., 2004; He, 2005). The above could explain why there was no signifi cant difference erence in SGR and SR of crabs. However, Gui et al. (2010) found that supplement of dietary CPH could increase the body weight of crucian carp ( Carassius auratus gibelio), it may be that crustacean growth only occurs during molting (He, 2005).
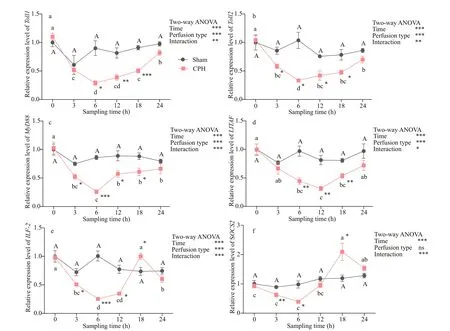
Fig.4 Relative expressions of Toll1 (a), Toll2 (b), MyD88 (c), LITAF (d), ILF- 2 (e) and SOCS2 (f) in hepatopancreas of crabs after CPH perfusion
It has been reported that the difference erences in dietary nutrients would possibly cause the difference erences in body composition (Hansen et al., 2007). In the present study, dietary supplement with 0.6% CPH could decrease the moisture content of crabs, whereas the opposite was true for crude protein content, indicating that CPH may promote the accumulation of organic matter. This result was possible that the lower molecular weight, small peptides, and free amino acids in CPH could promote protein synthesis by activating TOR signaling pathway, this needs further study. Our study also found that the energy content of crabs fed CPH0.3 diet increased signifi cantly, it might be that absorbed dietary amino acids and peptides were processed to increase energy content of total dry matters in crabs. Overall, our study suggested that CPH might have no impact on the puberty molt, but it could promote the deposition of nutrients of crabs.
The body cells produce reactive oxygen species (ROS) under normal physiological conditions (Zhang et al., 2013). However, the imbalance of generation and elimination of ROS will result in oxidative stress (Shen et al., 2010; Wu et al., 2013), then, SOD and CAT would be treated as the fi rst line of the antioxidant defense system against ROS produced in the organism (Li et al., 2007). In the study, enhancing SOD and CAT activities and reducing MDA content were seen in crabs fed diets containing CPH, suggesting that the CPH could improve the antioxidant capacity of crabs. Previous study has reported that small peptides could stimulate and activate the antioxidant capacity of animal body and increase the activities of antioxidant enzymes (Chen et al., 1998; Moure et al., 2006). So, the results of our study were probably related to the rich concentration of small peptides in CPH. Similarly, Wang et al. (2017) studied that fermented cottonseed meal could increase serum antioxidant enzymes activities in broilers. Fermented mushroom bran hydrolysate replacement fi shmeal could signifi cantly decrease the content of MDA in serum of the allogynogenetic crucian carp (Zhang et al., 2017). Adding soy protein hydrolysates in diets enhanced SOD activity and decreased MDA content in serum of juvenile starry fl ounder (Song et al., 2014). However, in the present study, high dietary CPH inclusion level might provoke oxidative stress. It may be that high level CPH could stimulate the ROS release largely resulting in the imbalance of generation and elimination. Similarly, it was reported that prawn fed with a high fermented soybean meal level diet might induce oxidative stress (Matozzo et al., 2011). Overall, our study suggested that appropriate supplement CPH could promote the antioxidant capability of crabs.
LZM is regarded as an important non-specifi c immune response indicator of crustaceans and has bactericidal activity in many studies (Yano et al., 1996; Fu et al., 2017). ACP and ALP also play an important role in crustacean non-specifi c immune defence (Matozzo et al., 2011). In the present study, the activities of LZM, ALP, and ACP of crabs in hemolymph were signifi cantly increased when dietary supplement with 0.3% CPH. This indicated that an optimum administration of CPH might improve the immune function of crabs. It might be that the small molecule peptide consisting in CPH had an important role in boosting immunity. It was similar with our results that the appropriate amount of CPH could improve the non-specifi c immunity of the Cyprinus carpio var. jian (Xia et al., 2012). Previous similar studies showed that fermented cottonseed meal could enhance immune function of yellow-feathered broilers (Tang et al., 2012), and increase serum immunoglobulin level of broilers (Wang et al., 2017). Another similar study shown that fi sh fed with soy protein hydrolysates diet could increase LZM activity signifi cantly (Song et al., 2014).
GPT and GOT can be widely found in the mitochondria of many tissues and organs, particularly in the liver cells (Zheng and Pu, 1997). In the present study, we found that the activity of GPT in the hemolymph of crabs declined as dietary CPH was 0.3%. It indicated that appropriate supplementation of CPH could protect the hepatopancreas from damage, implying that CPH had a protective mechanism to the hepatopancreas of crabs. It may be that CPH can inhibit harmful bacteria and viruses infections. Nevertheless, high concentration of CPH signifi cantly increased the activity of GPT, it might be that the hepatic cells were injured, and then the GPT transported into blood, resulting the increase of GPT activities in hemolymph (Wang et al., 2005). The reason might be that high CPH level might be harmful to hepatic cells by enhancing amino acid metabolism and producing more metabolic wastes. A similar study result appeared in juvenile starry fl ounder after feeding with soy protein hydrolysates (Song et al., 2014). Therefore, our study indicated that supplementation of moderate CPH was useful to protect hepatopancreas of crabs. This may be that the CPH could enhance immune function of crabs. Moreover, the SR of crabs in difference erent groups was consistent with these result, this further suggested that dietary CPH improved the resistance of crab by the CPH-induced immune responses.
Next, we examined the relative expressions of Toll1, Toll2, MyD88, LITAF, ILF- 2, and SOCS2 in the hepatopancreas of crabs. Tolls play an important role in eliminating extracellular invaders, and they can be regarded as the bridge linking the innate and adaptive immunity (Lin et al., 2012). LITAF is a key transcription factor in downstream of Toll signaling pathway, and it is contacted with the transcriptional regulation of cytokines dependent on MyD88 (Li et al., 2014). SOCS2 could be induced by bacteria challenge and played a key role in the immune defense response of crabs (Zhang et al., 2010). In the present study, we found that the relative expression levels of Toll 1 and Toll 2 were signifi cantly increased after feeding CPH0.3 diet. It suggested that Toll1 andToll2 might be potential receptor of CPH to regulate the downstream transcription factors. It was known that Tolls might participate in antifungal and antibacterial immune responses, and it was benefi cial to the ability of crabs to start a suitable immune response against difference erent pathogen-associated molecular patterns (Yu et al., 2013). The relative expression level of MyD88 increased signifi cantly after feeding CPH0.3 diet. This could be conjectured that the moderate supplement of CPH could promote the activation of the downstream signal factors by MyD88 in crabs. It was reported that MyD88 play a key role in activating the immune system of crabs to resist the bacterial infections (Huang et al., 2014). Dietary supplement with 0.3% CPH could increase the expression level of LITAF of crabs, the result indicated that feeding CPH0.3 diet might have a stimulating role to the downstream cytokines of Toll signaling pathway. The relative expression level of LITAF was consistent with MyD88, suggesting that the immune function of CPH on Toll signaling pathway may be shown by afference ecting its downstream cytokines through MyD88. The relative expression level of I LF- 2 increased when dietary CPH level was 0.3%, suggesting that ILF2 might induce all kinds of immune-related gene expression (Wang et al., 2006). However, the expression of SOCS2 declined after feeding CPH0.3 diet. It may be that SOCS2 was only induced after bacteria challenge. These results further suggested that dietary CPH could improve the immunity of crabs.
After that, we performed a perfusion experiment to explore further the efference ect of short-term CPH stimulant on immune gene expression of crabs. In intestine, the highest expression level of Toll1 and SOCS2 were obtained at time 3 h, indicating that the immune function of Toll1 and SOCS2 were fi rstly activated by CPH, in hepatopancreas, the highest expression level of SOCS2 was obtained at time 18 h, suggesting that the immune response in intestine was faster than in hepatopancreas, a probably reason is that the CPH fi rst acted on the intestine. Moreover, the expression level of SOCS2 was afference ected by time and the interaction of time and perfusion type, which suggested that SOCS2 could be induced by CPH and probably played an important role in immune defense responses of crabs. Evidence suggests that the transcripts encoding SOCS proteins in cells are usually at low or undetectable levels, but they can induced rapidly by cytokines and pathogen components widely in M. musculus (Zhang et al., 2010). Our results were similar to those discoveries. However, other genes expression levels declined fi rstly and then increased gradually, suggesting that these genes could not rapidly respond to CPH stimulation. The expression level of these genes decreased at CPH stimulation might indicate that this period belonged to the convalescence of crab after CPH stimulation. It took least 6 h for these genes to reanimate and then produced efference ect in innate immune responses. This is similar to the fi ndings that the expression level of SpToll in hemocytes of S cylla paramamosain dealt with poly I:C. or V. parahemolyticus (Lin et al., 2012) and the expression level of FcToll of F enneropenaeus chinensis in the early period after exposure to bacteria (Yang et al., 2008). Another reason we speculated that these genes may have additional functional roles. It was reported that certain genes were only expressed when the epithelial cells in ‘mature zone’ of the organ was damaged (Hauton et al., 2006). However, further studies should be performed to explore the reason at a deeper level.
5 CONCLUSION
In summary, this experiment showed that dietary supplementation of CPH has benefi cial efference ects on the accumulation of organic matter, antioxidant capacity, and immune response that might via the activation of immune-related genes such as Tolls and MyD88; the appropriate supplement dosage of CPH for Chinese mitten crab could be 0.3%-0.6%. However, the precise mechanism requires further study.
6 DATA AVAILABILITY STATEMENT
The data that support the fi ndings of this study are available from the corresponding author upon reasonable request.
References
Association of Oき cial Analytical Chemists (AOAC). 1984. Oき cial Methods of Analysis. 14thedn. AOAO, Washington, 1018p.
Bergmeyer H U, Scheibe P, Wahlefeld A W. 1978. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clinical Chemistry, 24(1): 58-73.
Chen H M, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. 1998. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J ournal of A gricultural and F ood C hemistry, 46(1): 49-53.
Chen J C, Lin M N, Ting Y Y, Lin J N. 1995. Survival, haemolymph osmolality and tissue water of Penaeus chinensis juveniles acclimated to difference erent salinity and temperature levels. Comparative Biochemistry and Physiology Part A: Physiology, 110(3): 253-258.
Chen Y L, Chen L Q, Qin J G, Ding Z L, Li M, Jiang H B, Sun S M, Kong Y Q, Li E C. 2016. Growth and immune response of Chinese mitten crab ( Eriocheir sinensis) fed diets containing difference erent lipid sources. Aquaculture Research, 47(6): 1 984-1 995.
Cheng H H, Jiang G Z, Zheng X C, Xu C Y, Sun C X, Zhang D D, Liu W B. 2019. Efference ects of fi ve attractants on Chinese mitten crab, Eriocher sinensis. Acta Hydrobiologica Sinica, 43(2): 395-401. (in Chinese with English abstract)
Colla G, Rouphael Y, Canaguier R, Svecova E, Cardarelli M. 2014. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Frontiers in Plant Science, 5: 448.
Duan C, Plisetskaya E M. 1993. Nutritional regulation of insulin-like growth factor-I mRNA expression in salmon tissues. Journal of Endocrinology, 139(2): 243-252.
Figueiredo-Silva C, Lemme A, Sangsue D, Kiriratnikom S. 2015. Efference ect of DL-methionine supplementation on the success of almost total replacement of fi sh meal with soybean meal in diets for hybrid tilapia ( Oreochromis niloticus × Oreochromis mossambicus). Aquacult ure Nutr ition, 21(2): 234-241.
Fu L L, Zhou G, Pan J L, Li Y H, Lu Q P, Zhou J, Li X G. 2017. Efference ects of Astragalus polysaccharides on antioxidant abilities and non-specifi c immune responses of Chinese mitten crab, Eriocheir sinensis. A quaculture I nternational, 25(3): 1 333-1 343.
Gibbs B F, Zougman A, Masse R, Mulligan C. 2004. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. F ood R esearch I nternational, 37(2): 123-131.
Gui D, Liu W B, Shao X P, Xu W N. 2010. Efference ects of difference erent dietary levels of cottonseed meal protein hydrolysate on growth, digestibility, body composition and serum biochemical indices in crucian carp ( Carassius auratus gibelio). Animal Feed Science and Technology, 156(3-4): 112-120.
Guo Z L, Qiao X, Cheng R M, Shi N N, Wang A L, Feng T T, Chen Y, Zhang F, Yu H N, Wang Y P. 2017. As-CATH4 and 5, two vertebrate-derived natural host defense peptides, enhance the immuno-resistance eき ciency against bacterial infections in Chinese mitten crab, Eriocheir sinensis. Fish & Shellfish Immunology, 71: 202-209.
Hansen A C, Rosenlund G, Karlsen Ø, Koppe W, Hemre G I. 2007. Total replacement of fi sh meal with plant proteins in diets for Atlantic cod ( Gadus morhua L.) I - efference ects on growth and protein retention. Aquaculture, 272(1-4): 599-611.
Hartnoll R G. 2001. Growth in Crustacea-twenty years on. Hydrobiologia, 449(1-3): 111-122.
Hauton C, Brockton V, Smith V J. 2006. Cloning of a crustinlike, single whey-acidic-domain, antibacterial peptide from the haemocytes of the european lobster, Homarus gammarus, and its response to infection with bacteria. M olecular I mmunology, 43(9): 1 490-1 496.
He J. 2005. Population growth characteristics of the mitten crab in ecological aquaculture ponds. Reservoir Fisheries, 25(6): 10-11, 28. (in Chinese with English abstract)
Hong Y H, Yang X Z, Yan G W, Huang Y, Zuo F, Shen Y X, Ding Y, Cheng Y X. 2017. Efference ects of glyphosate on immune responses and haemocyte DNA damage of Chinese mitten crab, Eriocheir sinensis. Fish & Shellfish Immunology, 71: 19-27.
Huang Y, Chen Y H, Wang Z, Wang W, Ren Q. 2014. Novel myeloid difference erentiation factor 88, EsMyD88, exhibits EsTube-binding activity in Chinese mitten crab Eriocheir sinensis. Developmental & Comparative Immunology, 47(2): 298-308.
Jia E T, Li Z Q, Xue Y F, Jiang G Z, Li X F, Liu W B, Zhang D D. 2017. Efference ects of dietary fructooligosaccharide on the growth, antioxidants, immunity and disease resistance of Chinese mitten crab. Aquaculture, 481: 154-161.
Jiang H B, Chen L Q, Qin J G. 2018. Fishmeal replacement by soybean, rapeseed and cottonseed meals in hybrid sturgeon A cipenser baerii ♀× A cipenser schrenckii ♂. Aquacult ure Nutr ition, 24(4): 1 369-1 377.
Kotzamanis Y P, Gisbert E, Gatesoupe F J, Infante J Z, Cahu C. 2007. Efference ects of difference erent dietary levels of fi sh protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass ( Dicentrarchus labrax) larvae. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 147(1): 205-214.
Li S, Jia Z R, Li X J, Geng X Y, Sun J S. 2014. Identifi cation and expression analysis of lipopolysaccharide-induced TNF-alpha factor gene in Chinese mitten crab Eriocheir sinensis. Fish & Shellfish Immunology, 38(1): 190-195.
Li X M, Ma Y L, Liu X J. 2007. Efference ect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J ournal of E thnopharmacology, 111(3): 504-511.
Lin Z Y, Qiao J, Zhang Y L, Guo L L, Huang H, Yan F, Li Y Y, Wang X Y. 2012. Cloning and characterisation of the SpToll gene from green mud crab, S cylla paramamosain. Developmental & Comparative Immunology, 37(1): 164-175.
Liu W B. 2005. Efference ect of Plant Protein Hydrolysates on the Growth Development of Alogynogenetic crucian and Bio-Active Valve Analysis of Plant Protein Hydrolysates. Nanjing Agricultural University, Nanjing. (in Chinese with English abstract)
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 25(4): 402-408.
Lygren B, Hamre K, Waagbø R. 1999. Efference ects of dietary pro- and antioxidants on some protective mechanisms and health parameters in atlantic salmon. Journal of Aquatic Animal Health, 11(3): 211-221.
Matozzo V, Gallo C, Monari M, Marin M G. 2011. Cellular and biochemical parameters in the crab Carcinus aestuarii after experimentally-induced stress: efference ects of bacterial injection, leg ablation and bacterial injection/leg ablation combination. J ournal of E xperimental M arine B iology and E cology, 398(1-2): 18-25.
Möller N P, Scholz-Ahrens K E, Roos N, Schrezenmeir J. 2008. Bioactive peptides and proteins from foods: indication for health efference ects. E uropean J ournal of N utrition, 47(4): 171-182.
Moure A, Domínguez H, Parajó J C. 2006. Antioxidant properties of ultrafi ltration-recovered soy protein fractions from industrial eラ uents and their hydrolysates. Process Biochem istry, 41(2): 447-456.
Muranova T A, Zinchenko D V, Kononova S V, Belova N A, Miroshnikov A I. 2017. Plant protein hydrolysates as fi sh fry feed in aquaculture. Hydrolysis of rapeseed proteins by an enzyme complex from king crab hepatopancreas. A pplied B iochemistry and M icrobiology, 53(6): 680-687.
Pauly D, Zeller D. 2017. Comments on FAOs S tate of W orld F isheries and A quaculture (SOFIA 2016). Marine Policy, 77: 176-181.
Rudnick D A, Hieb K, Grimmer K F, Resh V H. 2003. Patterns and processes of biological invasion: the Chinese mitten crab in San Francisco Bay. Basic and Applied Ecology, 4(3): 249-262.
Sánchez A, Pascual C, Sánchez A, Vargas-Albores F, Moullac G L, Rosas C. 2001. Hemolymph metabolic variables and immune response in L itopenaeus setiferus adult males: the efference ect of acclimation. Aquaculture, 198(1-2): 13-28.
Satoh K. 1978. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clinica Chimica Acta, 90(1): 37-43.
Saucedo P E, Ocampo L, Monteforte M, Bervera H. 2004. Efference ect of temperature on oxygen consumption and ammonia excretion in the calafi a mother-of-pearl oyster, Pinctada mazatlanica (Hanley, 1856). Aquaculture, 229(1-4): 377-387.
Shen W Y, Fu L L, Li W F, Zhu Y R. 2010. Efference ect of dietary supplementation with Bacillus subtilis on the growth, performance, immune response and antioxidant activities of the shrimp ( Litopenaeus vannamei). Aquac ulture Res earch, 41(11): 1 691-1 698.
Sheridan M A, Mommsen T P. 1991. Efference ects of nutritional state on in vivo lipid and carbohydrate metabolism of coho salmon, Oncorhynchus kisutch. General and Comparative Endocrinology, 81(3): 473-483.
Song Z D, Li H Y, Wang J Y, Li P Y, Sun Y Z, Zhang L M. 2014. Efference ects of fi shmeal replacement with soy protein hydrolysates on growth performance, blood biochemistry, gastrointestinal digestion and muscle composition of juvenile starry fl ounder ( Platichthys stellatus). Aquaculture, 426- 427: 96-104.
Sun R, Yue F, Qiu L M, Zhang Y, Wang L L, Zhou Z, Zhang H, Yi QL, Song L S. 2013a. The CpG ODNs enriched diets enhance the immuno-protection eき ciency and growth rate of Chinese mitten crab, Eriocheir sinensis. Fish & Shellfish Immunology, 35(1): 154-160.
Sun S M, Qin J G, Yu N, Ge X P, Jiang H B, Chen L Q. 2013b. Efference ect of dietary copper on the growth performance, nonspecifi c immunity and resistance to Aeromonas hydrophila of juvenile Chinese mitten crab, Eriocheir sinensis. Fish & Shellfish Immunology, 34(5): 1 195-1 201.
Tang J W, Sun H, Yao X H, Wu Y F, Wang X, Feng J. 2012. Efference ects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellowfeathered broilers. Asian- Australasian Journal of Animal Sciences, 25(3): 393-400.
Wang H J, Xiang L X, Shao J Z, Jia S. 2006. Molecular cloning, characterization and expression analysis of an IL-21 homologue from Tetraodon nigroviridis. Cytokine, 35(3-4): 126-134.
Wang S H, Chen J C. 2005. The protective efference ect of chitin and chitosan against Vibrio alginolyticus in white shrimp Litopenaeus vannamei. Fish & Shellfish Immunology, 19(3): 191-204.
Wang W, Gu Z F. 2002. Rickettsia-like organism associated with tremor disease and mortality of the Chinese mitten crab Eriocheir sinensis. Diseases of Aquatic Organisms, 48(2): 149-153.
Wang Y W, Deng Q Q, Song D, Wang W W, Zhou H, Wang L, Li A K. 2017. Efference ects of fermented cottonseed meal on growth performance, serum biochemical parameters, immune functions, antioxidative abilities, and cecal microfl ora in broilers. Food and Agricultural Immunology, 28(4): 725-738.
Wang Y, Xiong L, Yang K J, Wu Z, Sheng X M, Tang H F, Liu X P. 2005. Efference ect of beta-cypermethrin on GPT and GOT activities of crucian serum. Agricultural Science & Technology, 6(1): 20-23.
Wei J J, Zhang F, Tian W J, Kong Y Q, Li Q, Yu N, Du Z Y, Wu Q Q, Qin J G, Chen L Q. 2016. Efference ects of dietary folic acid on growth, antioxidant capacity, non-specifi c immune response and disease resistance of juvenile Chinese mitten crab Eriocheir sinensis (Milne-Edwards, 1853). Aquacult ure Nutr ition, 22(3): 567-574.
Wu J Q, Kosten T R, Zhang X Y. 2013. Free radicals, antioxidant defense systems, and schizophrenia. Progress in N euro- Psychopharmacology and B iological P sychiatry, 46(1): 200-206.
Xia W, Liu W B, Qiao Q S, Li G F, Zhang Y J. 2012. Efference ects of cottonseed meal hydrolysate on growth performance and biochemical indices of Cyprinus carpio var. Jian. Freshwater Fisheries, 42(1): 46-51. (in Chinese with English abstract)
Yang C J, Zhang J Q, Li F H, Ma H M, Zhang Q L, Priya T A J, Zhang X J, Xiang J H. 2008. A Toll receptor from Chinese shrimp F enneropenaeus chinensis is responsive to V ibrio anguillarum infection. Fish & Shellfish Immunology, 24(5): 564-574.
Yang J L, Wang L L, Huang M M, Wang L L, Gai Y C, Qiu L M, Zhang H, Song L S. 2011. An interleukin-2 enhancer binding factor 2 homolog involved in immune response from Chinese mitten crab Eriocheir sinensis. Fish & Shellfish Immunology, 30(6): 1 303-1 309.
Yano T. 1996. The nonspecifi c immune system: humoral defense. Fish Physiology, 15: 105-157.
Yimit D, Hoxur P, Amat N, Uchikawa K, Yamaguchi N. 2012. Efference ects of soybean peptide on immune function, brain function, and neurochemistry in healthy volunteers. Nutrition, 28(2): 154-159.
Yu A Q, Jin X K, Guo X N, Li S, Wu M H, Li W W, Wang Q. 2013. Two novel Toll genes ( EsToll1 and EsToll2) from Eriocheir sinensis are difference erentially induced by lipopolysaccharide, peptidoglycan and zymosan. Fish & Shellfish Immunology, 35(4): 1 282-1 292.
Yuan C T, Li D M, Chen W, Sun F F, Wu G H, Gong Y, Tang J Q, Shen M F, Han X D. 2007. Administration of a herbal immunoregulation mixture enhances some immune parameters in carp ( Cyprinus carpio). Fish Physiology and Biochemistry, 33(2): 93-101.
Zhang C N, Li X F, Xu W N, Jiang G Z, Lu K L, Wang L N, Liu W B. 2013. Combined efference ects of dietary fructooligosaccharide and Bacillus licheniformis on innate immunity, antioxidant capability and disease resistance of triangular bream ( Megalobrama terminalis). Fish & Shellfish Immunology, 35(5): 1 380-1 386.
Zhang D S, Zhang Y P, Liu B, Jiang Y, Zhou Q L, Wang J, Wang H L, Xie J, Kuang Q. 2017. Efference ect of replacing fi sh meal with fermented mushroom bran hydrolysate on the growth, digestive enzyme activity, and antioxidant capacity of allogynogenetic crucian carp ( C arassius auratus gibelio). Turkish Journal of Fisheries and Aquatic Sciences, 17(5): 1 039-1 048.
Zhang S, Bonami J R. 2012. Isolation and partial characterization of a new reovirus in the Chinese mitten crab, Eriocheir sinensis H Milne Edwards. Journal of Fish Diseases, 35(10): 733-739.
Zhang Y, Zhao J M, Zhang H, Gai Y C, Wang L L, Li F M, Yang J L, Qiu L M, Song L S. 2010. The involvement of suppressors of cytokine signaling 2 (SOCS2) in immune defense responses of Chinese mitten crab Eriocheir sinensis. Developmental & Comparative Immunology, 34(1): 42-48.
Zhao D, Song L, Liu R, Liang Z, Wang L, Sun M. 2016. The immunosuppressive efference ects of continuous CpG ODNs stimulation in Chinese mitten crab, Eriocheir sinensis. Invertebrate Survival Journal, 13: 34-43.
Zheng Y H, Pu F Y. 1997. Efference ect of mercury on transaminase activities of tissues in C parpio & C auratus. Journal of Southwest Agricultural University, 19(1): 41-45. (in Chinese with English abstract)
 Journal of Oceanology and Limnology2020年3期
Journal of Oceanology and Limnology2020年3期
- Journal of Oceanology and Limnology的其它文章
- List of the Most Outstanding Papers Published by CJOL/JOL in 2017-2018
- bHLH genes polymorphisms and their association with growth traits in the Pacifi c oyster Crassostrea gigas*
- Genetic variation within and among range-wide populations of three ecotypes of the Japanese grenadier anchovy Coilia nasus with implications to its conservation and management*
- Complete mitochondrial genomes of two deep-sea pandalid shrimps, Heterocarpus ensifer and Bitias brevis: insights into the phylogenetic position of Pandalidae (Decapoda: Caridea)*
- Unraveling enhanced membrane lipid biosynthesis in Chlamydomonas reinhardtii starchless mutant sta6 by using an electrospray ionization mass spectrometry-based lipidomics method*
- Behavioral responses to ocean acidifi cation in marine invertebrates: new insights and future directions*
