Culturable bacterial diversity in hypolithic and peripheral soils in the west of the Hexi Corridor desert and its influencing factors
LiFang He ,ShiWeng Li *,GaoSen Zhang ,XiuKun Wu ,BingLin Zhang ,Wei Zhang *
1.School of Chemical and Biological Engineering,Lanzhou Jiaotong University,88 West Anning Road,Lanzhou,Gansu 730070,China
2.Key Laboratory of Extreme Environmental Microbial Resources and Engineering,Northwest Institute of Eco-Environment and Resource,Chinese Academy of Sciences,Lanzhou,Gansu 730000,China
ABSTRACT Microbes inhabiting the desert respond sensitively to environmental changes and may be an indicator for changes in the desert ecosystem.Hypolithic microbial communities in the desert play a vital role in ecosystem processes such as soil for‐mation and organic matter accumulation.This study investigated and compared the culturable bacterial community struc‐ture and diversity in hypolithic and peripheral soils,and the interaction between bacteria and environmental factors.The bacteria were isolated using four different kinds of media and identified by 16S rRNA gene-sequence analysis.The num‐bers of culturable bacteria in the hypolithic and peripheral soils ranged from 3.0×104 to 3.6×105 CFU/g and from 6.5×104to 5.3×105 CFU/g,respectively,indicating that the bacteria number in peripheral soil was higher than that in hypolithic soil.A total of 98 species belonging to 34 genera were identified,among which Arthrobacter,Bacillus,and Streptomyces were found dominantly and widely distributed.The community of culturable bacteria had obvious sample specificity,and the diversity in hypolithic soil was higher than that in peripheral soil.On the regional scale,the distribution of culturable bacteria and the environmental factors showed regular changes.On the local scale,the high heterogeneity of the hypolithic environment determined the specificity of the number and species of culturable bacteria.
Keywords:Hexi Corridor;desert hypolithic and peripheral soils;culturable bacteria;diversity
1 Introduction
The desert is an extreme biotope,dry and barren,especially in gravel deserts where it is extremely diffi‐cult for organisms to survive.In this area,the ground is covered with gravel and grows few plants.The sur‐face is often desolate and lifeless(Chanet al.,2012).However,microbes survive in this extreme biotope(Friedmann and Ocampo,1976).Colonization under gravel is a known strategy for microbes to cope with environmental stresses in the desert.The gravel can protect microbes from the damage of excessive solar radiation and ultraviolet radiation,while increasing the relative humidity in the soil and ensuring the sur‐rounding stability.The lithophilous and hypolithic mi‐crobes play a key role in biogeochemical cycles and soil-environment stability in Gobi Desert environ‐ments(Bryant,2010;Chanet al.,2012).On a global scale,about 50 percent of dry land is covered by grav‐el.Hypolithic microorganisms are considered to be in‐dispensable members in this ecosystem.Therefore,many researchers have focused on the community compositions and functions of hypolithic organisms(Van Goethemet al.,2017;Wuet al.,2017).
The activities of lithophilous and hypolithic mi‐crobes promote rock weathering and accelerate soil formation and organic matter accumulation(Gorbushi‐naet al.,2008).The microbes living on the surface of carbonated rocks can respond sensitively to environ‐mental changes and have important feedback effects on the climate system through elemental circulation and mineral transformation. These microbes have been considered to be an indicator of changes in the ecological environment(Wuet al.,2017).The diversi‐ty and number of microbes in desert soil are affected by geographical location,physical and chemical fac‐tors,and climate factors(Azua-Bustoset al.,2012).Generally,the number of bacteria is negatively corre‐lated with soil depth(Liuet al.,2016)and altitude(Maieret al.,2004).The soil total organic carbon con‐tent(TOC)and total nitrogen(TN)content decreased with soil depth and the elevation increase.Studies have shown that the composition of hypolithic com‐munities exhibits a greater change than that found in barrren deserts(Cowanet al.,2011;Van Goethemet al.,2016).Other studies suggest the richness of hypo‐lithic microbial communities(Warren-Rhodeset al.,2006;Lacapet al.,2011);and bacteria are dominant in hypolithic environments,accounting for 93%of the total number of organisms(Vikramet al.,2016).The dominant bacteria phyla areActinomycetes,Proteobacteria,Chloroflexi,Bacteroidetes,andVerrucomicrobia,as well as the generaBacillusandMycobacterium,etc..The genusActinomycetes,which can ac‐count for 50%of the microbes in desert soils,gener‐ates spores,grows in a filamentous manner,and effec‐tively reduces the damage caused by drought,high temperature,and various radiations(Liet al.,2008;Bacharet al.,2012).In the desert of western China,the relative abundance ofProtebacteriareaches up to 80%of the dominant phyla(Anet al.,2013).It is known that the lithophilous and hypolithic bacteria in deserts evolve the ability to resist various radiations and adverse environmental stresses.Therefore, the lithophilous and hypolithic bacteria in deserts might be the source of novel strains and genes.
The western Hexi Corridor in Northwest China is the widely distributed Gobi Desert,where are found an extremely dry climate;windy,fluctuating tempera‐ture;intense solar radiation;and saline soil(Liuet al.,2014).This harsh habitat is considered to be a trove for discovering novel microbial reservoir.Thus,the aims of the present study are to isolate culturable bac‐teria and to ascertain the culturable bacterial commu‐nities and the change along the environmental gradi‐ent.Ten hypolithic and peripheral soil samples were collected in the gravel desert in the west of the Hexi Corridor.The numbers of culturable bacteria were counted,and the isolates were identified using the 16S rRNA gene-sequencing method.A comparative analysis of the diversity and composition of culturable bacteria among the samples was performed.The ef‐fects of environmental factors on the bacterial com‐munities were further analyzed.
2 Materials and methods
2.1 Soil-sample collection
Ten sampling sites were determined in typical grav‐el Gobi Desert in the west of Hexi Corridor(Figure 1,Table 1).Each quadrat contained three sample sites;and each sample site contained five soils,randomly col‐lected under strictly sterile procedures,with each sam‐ple taken at least 1 m away from another in the quadrat.For hypolithic bacteria isolation,the soils that adhered to the lower surface of gravels were collected.Samples were transported at low temperature and stored in a re‐frigerator at 4°C for subsequent experiments.

Figure 1 Soil-sample sites located in the west of the Hexi Corridor
2.2 Analysis of soils' physical and chemical properties
After being air-dried,the soil samples were fil‐tered using a sieve with a 2-mm aperture.The pH val‐ues,total organic carbon(TOC),and total nitrogen(TN)contents were measured according to the meth‐od described by Zhang(2007).For pH measurement,20 mL of deionized water were added to 10 g of soil and shaken for 30 min.For TOC and TN measure‐ment,10 g of soil were added to 20 mL of distilled water,followed by 30 min of shaking at 25°C,and then filtered using a 0.45-μm filter membrane.The TOC content was determined using a TOC-1020A or‐ganic carbon analyzer(Beijing Zhongyi Kexin Tech‐nology CO.,LTD.).The TN content was detected by the semi-trace Kelvin method.
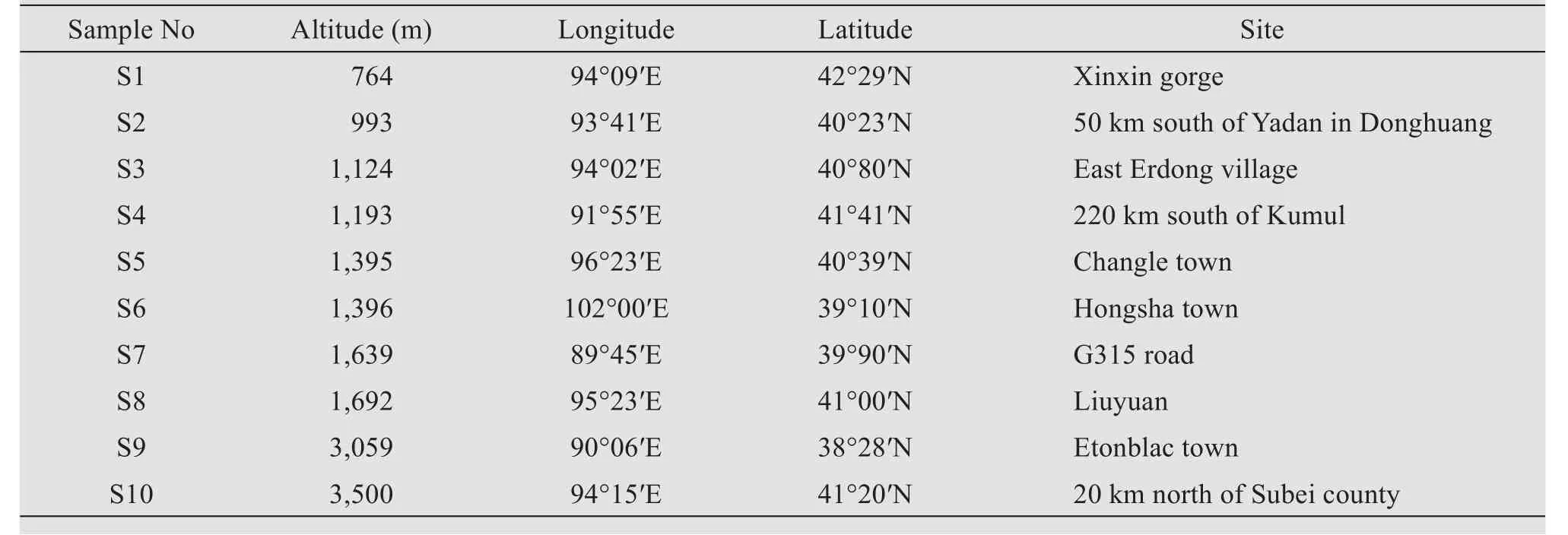
Table 1 Information about soil-sample sites in this experiment
2.3 Culture media
The following four media were used in the study.LB medium:tryptone 10 g,yeast extract 5 g,NaCl 10 g,agar 15 g,deionized water 1,000 mL,and pH 7.2.WSA medium:NH4NO30.3 g,K2HPO40.24 g,MgSO4·7H2O 0.078 g,NaCl 0.078 g,MnSO4·7H2O 0.0015 g,agar 15 g,deionized water 1,000 mL,and pH 6.8-7.0.R2A medium:tryptone 0.5 g,yeast extract 0.5 g,casein amino ac‐id 0.5 g,soluble starch 0.5 g,K2HPO40.3 g,C3H3NaO30.3 g,MnSO4·7H2O 0.05 g,agar 15 g,deionized water 1,000 mL,and pH 7.2.BG-11 medium:NaNO3/NaCl 30/20.6 g,K2HPO4·3H2O 0.80 g,Na2CO30.40 g,deion‐ized water 1,000 mL,and pH 6.8-7.0.
2.4 Bacterial isolation and counting
Bacterial strains were isolated and purified using the dilution-plate method.A soil sample(2 g)was mixed with 18 mL of normal saline(15%)and steril‐ized glass beads,followed by shaking for 30 min in a shaker at 150 r/min.An aliquot 0.2 mL of the superna‐tant was diluted in a gradient,and then plate culture was conducted in an incubator at 28°C.Each sample was used for three biological repetitions.After a set incubation period,the numbers of colonies formed on the plate were counted;and a single colony on the plate was selected and transferred to the new medium for further strain purification.
2.5 DNA extraction and 16S rRNA gene sequencing
The total DNA of the bacterial isolates was ex‐tracted using a Bacterial Genomic DNA Extraction Kit(TianGen,Baijing,China)according to its instruc‐tions.The 16S rRNA gene sequence was amplified us‐ing the primers 27F 5'-AGAGTTTGATCCTGGCT‐CAG-3'and 1492R 5'-GGTTACCTTGTTACGACITT-3').After sequencing,the sequences were aligned with the NCBI database using the BLAST tool.
2.6 Statistical analyses
Statistical analyses were performed using SPSS for Windows 12.0(SPSS Inc.,Chicago,USA).The statistically significant differences were determined by one-way analysis of variance(ANOVA),andP<0.05 was considered a significant difference.Canoni‐cal correspondence analysis(CCA)was performed us‐ing Canoco5.0.
3 Results
3.1 Properties of hypolithic and peripheral soils
The pH values of hypolithic and peripheral soils ranged from 7.4 to 8.6 and from 7.52 to 8.16,re‐spectively.Generally,the peripheral soil pH was higher than that of the hypolithic soil—with the ex‐ception of samples S2,S4,S7,and S8,where the hy‐polithic soil pH values were slightly higher than that of the peripheral soil.The TN contents of hypo‐lithic soils and peripheral soils ranged from 24.2 to 73.2 mg/kg and from 23.7 to 61.7 mg/kg,respectively.The TOC contents of hypolithic and peripheral soils ranged from 466.6 to 1,023.9 mg/kg and from 524.3 to 1,010.6 mg/kg,respectively.These results indicate a general trend,i.e.,the hypolithic soil contained high‐er TN and TOC contents relative to the peripheral soil(Table 2).
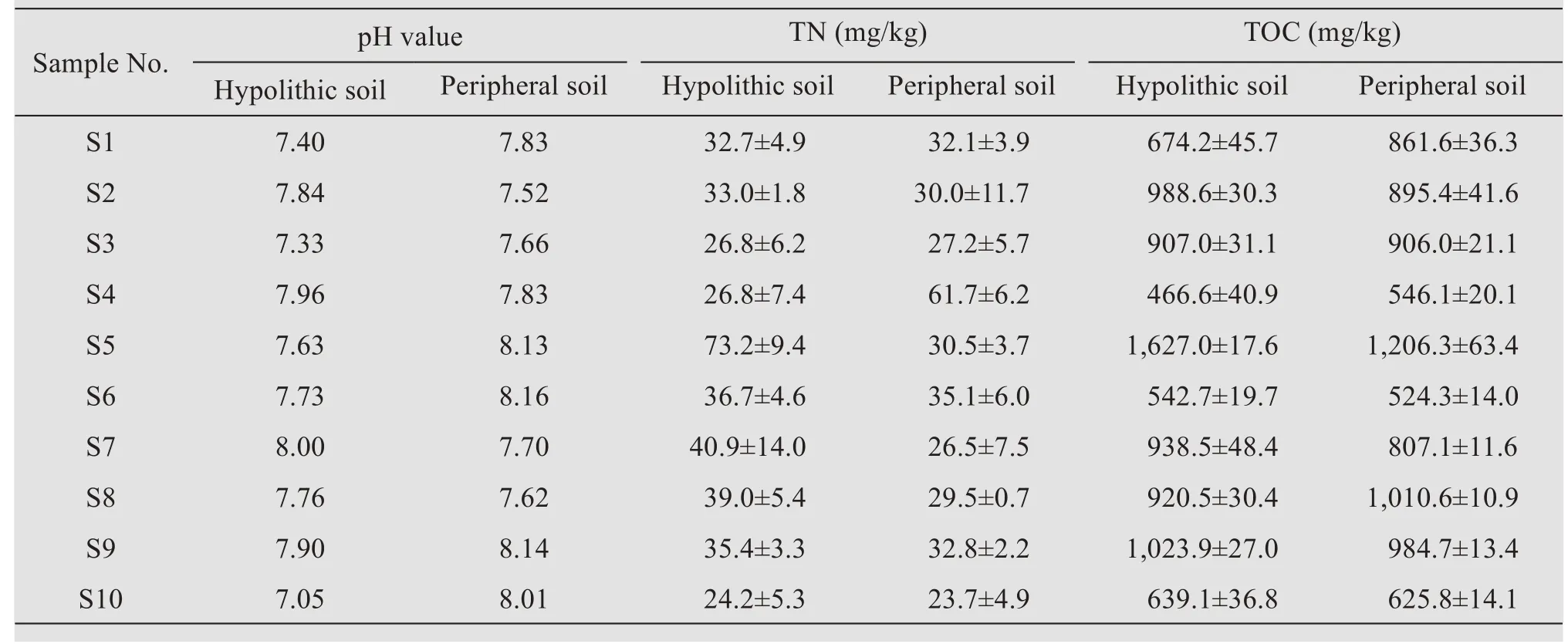
Table 2 Chemical properties of the soils
3.2 Culturable bacterial numbers and variations
The numbers of culturable bacteria varied signifi‐cantly across the sampling sites.The total bacterial number in the hypolithic soils and peripheral soils ranged from 3×104to 3.6×105and from 6.5×104to 5.3×105,respectively.The bacterial numbers in the peripheral soils were significantly higher than those in the hypolithic soil samples S1,S2,S5,S7,S8,and S10.However,the bacterial numbers in the hypolith‐ic soil samples S3,S4,S6,and S9 were significantly higher than those in the peripheral soil(Figure 2).Obviously,the changes in the numbers of culturable bacteria suggest the heterogeneity of the microhabi‐tats in the desert.The higher number of culturable bacteria in the peripheral soil may be due to the ex‐ogenous bacteria input.The analysis showed that the difference in bacterial numbers between the two kinds of soil was positively correlated with the con‐tents of TN and TOC but not significantly correlated with pH values.
3.3 Community composition of culturable bacteria
Based on the morphological characteristics of the bacterial colonies on the plates,a total of 150 strains were isolated and purified from all soil samples.The alignment of 16S rRNA gene sequences showed that the bacterial isolates were assigned to three bacterial phyla—Actinobacteria,Firmicutes,and Proteobacte‐ria;34 genera;and 98 species(Table 3).Among them,67 and 31 bacterial species were identified from the hypolithic and peripheral samples,respec‐tively.Arthrobacter,Kocuria,Pseudomonas,Agrococcus,Planomicrobium,Streptomyces,andBacilluswere the common genera in both kinds of samples.Nesteriaceae,Modestobacter,Gordonia,Blastococcus,Georgenia,Brevibacterium, andKineococcuswere isolated from the hypolithic soils.Neorhizobium,Nocardiopsis,Saccharothrix,Enterobacter,Microbacterium,Dietzia,Microbacterium,andKribbellawere isolated from the peripheral samples.Arthrobacter,Bacillus,andStreptomyceswere the wide‐spread genera in both kinds of samples.Obviously,the composition of culturable bacterial communities in the two kinds of soils has sample-site specificity.These results indicate that the diversity of culturable bacteria in the hypolithic soil is higher than that in the peripheral soil,suggesting that the harsh condi‐tions such as drought and intense radiation in the pe‐ripheral soil reduced the diversity of culturable bacte‐ria.The heterogeneity of a hypolithic soil determines the uniqueness of the culturable bacterial community in it,evidence that habitat determines the bacterial community structure.
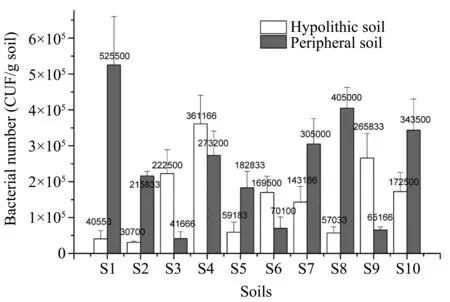
Figure 2 Numbers and variation of culturable bacteria in hypolithic and peripheral soils

Table 3 List of culturable bacterial species isolated from hypolithic and peripheral soils
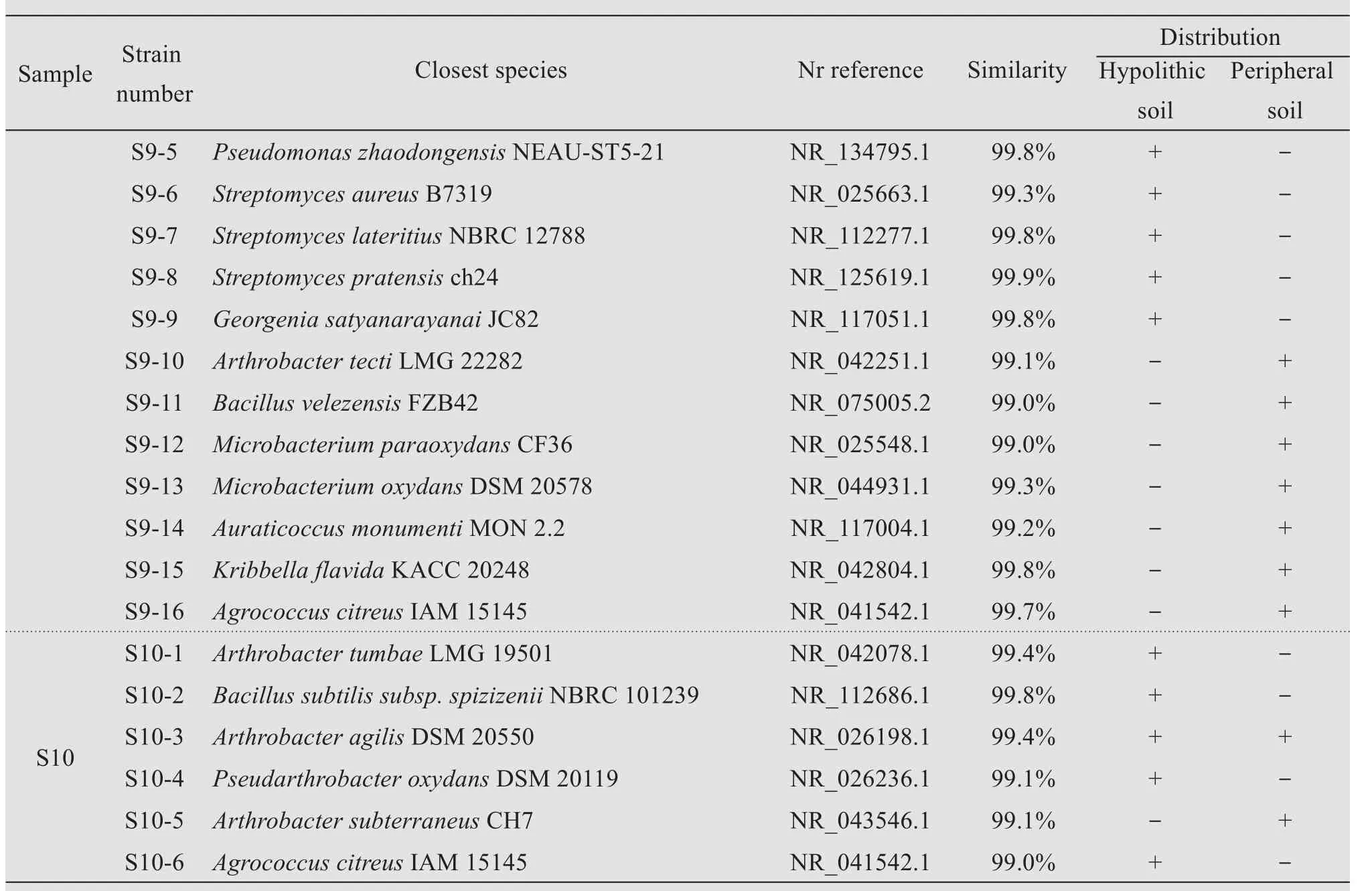
Table 3 List of culturable bacterial species isolated from hypolithic and peripheral soils
3.4 Abundance and distribution of culturable bacteria
Statistical analysis showed that the number of most bacterial species ranged from 20 to 80 CFU/g soil,suggesting low abundances of culturable bacteria in the soils.Among the hypolithic bacteria,the spe‐cies with higher incidence are 8Streptomycessp.,5Bacillussp.,4Athrobactersp.,Planomicrobium okeanokoites,Kocuria rosea,andBlastococcus endophyticus.Arthrobacter tumbae,Arthrobacter agilis,andBacillus tequilensisappeared in 5,4,and 3 soil samples,respectively.The other species appeared in no more than one soil sample(Figure 3).Among the peripheral bacteria,the species with higher abundance are 7Arthrobactersp.,4Streptomycessp.,Auraticoccus monumenti,Microbacterium oxydans,Microbacterium hibisci,andKocuria rosea.The culturable bacteria iso‐lated from the peripheral soil samples mostly have sample-site specificity(Figure 4).
3.5 The relationship between culturable bacteria and environmental factors
The canonical correspondence analysis(CCA)showed that the TOC,TN,and elevation of the hypolithic soil samples decreased from east to west,while the TOC in‐creased from south to north.The numbers of culturable bacteria showed an increasing trend from east to west and negative correlation with the altitude,TN,and pH values. The samples obviously clustered into three groups—S1,S2,and S3;S4,S5,S7,S8,S9,and S10;and S6—by the environmental factors such as eleva‐tion,TN,and pH values in the CCA figure(Figure 5a).The isolated bacteria are also classified into three cate‐gories.In the peripheral soil samples,TN and pH val‐ues showed decreasing trends from south to north and from east to west,and positive correlation with altitude.The TOC contents showed increasing trends from south to north and from east to west.The number of cultur‐able bacteria showed a decreasing trend from south to north and from east to west,and positive correlation with TN and pH values.The peripheral soil samples can be divided into four groups—S9 and S4;S6 and S8;S3,S5,and S10;and S7 and S2(Figure 5b).The above results indicate that the relationships among the soil physicochemical properties and the number of cul‐turable bacteria and environmental factors are complex in the soils of heterogeneous habitats with spotty distri‐bution.The number and species of culturable bacteria were mainly affected by the niche,indicating that the environmental difference determined the specificity of culturable bacteria.On a large scale,the changes of en‐vironmental factors have a certain spatial variation rule but little influence on the hypolithic soil bacteria.
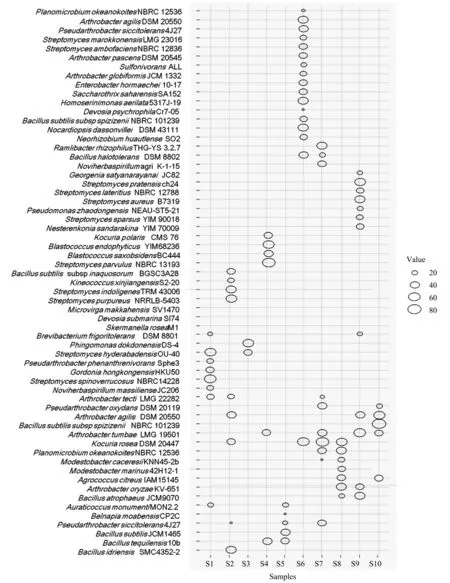
Figure 3 Variation in bacterial number and species distribution in the hypolithic soil samples.Bubbles are sized according to the observed number of bacterial species

Figure 4 Variation in bacterial number and species distribution in the peripheral soil samples.Bubbles are sized according to the observed number of bacterial species
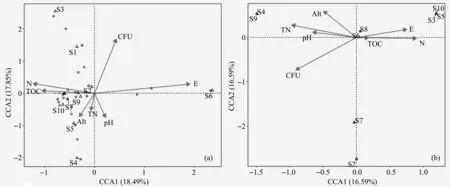
Figure 5 Canonical correspondence analysis(CCA)among the soils,species,and environmental factors:(a)Hypolithic soils;and(b)peripheral soils
4 Discussion
In this study,we isolated and analyzed culturable bacteria in the hypolithic and peripheral soils in the west of the Hexi Corridor.The number of culturable bacteria ranged from 104to 105CFU/g.Along the vari‐ation of sample sites,the numbers of culturable bacte‐ria changed irregularly in the hypolithic and peripher‐al soils.The numbers of hypolithic bacteria were much lower than those of the peripheral soil at most sample sites.Hypolithic bacterial diversity frequently exceeds that of lithic communities,in spite of lower microbial biomass(Caryet al.,2010).Recent phylo‐genetic surveys have shown significant differences in hypolithic and surface soil community composition(Makhalanyaneet al.,2013).It is generally believed that the distribution of desert microbes directly de‐pends on environmental factors (Pasternaket al.,2013).Studies show that water is the driving force for the difference of community diversity in hot and cold deserts in China(Warrenrhodeset al.,2007;Pointinget al.,2010).Besides,hypolithic colonization rates can be up to 80%,with all quartz rocks over 20 g be‐ing colonized on the seaward face of the Coastal Range.This finding strongly suggests that hypolithic microbial communities can survive with fog as the main regular source of moisture.A model has been ad‐vanced in which the development of the hypolithic communities under quartz stones relies on a positive feedback between fog availability and the higher ther‐mal conductivity of the quartz rocks,which results in lower daytime temperatures at the quartz-soil inter‐face microenvironment(Azua-Bustoset al.,2011).That means hypolithic microbial communities can use all the environmental factors in their surroundings to escape stress and survive safely.Although the hypo‐lithic soil is exposed to low solar radiation,high hu‐midity,and lower pH as compared to the peripheral soil,the contents of TN and TOC showed irregular changes in the two kinds of soils.The number of cul‐turable bacteria was not significantly correlated with the soil types and their chemical properties.This find‐ing implies that the high heterogeneity of the hypolith‐ic habitat determines the numbers and species of bac‐teria living in it.
In the present study,a total of 98 species of cultur‐able bacteria assigned to 34 genera was isolated from the hypolithic and peripheral soils from 10 sampling sites.The dominant and widespread genera wereArthrobacter,Bacillus,andStreptomyces;and most spe‐cies appeared in only one of the sample sites,suggest‐ing high sample-site specificity. Results obtained from northern Australia showed that distance was the main factor affecting the hypolithic microbial commu‐nity composition(Christianet al.,2017).The number of bacterial species in hypolithic soils is significantly higher than that in peripheral soils,suggesting that the hypolithic habitat has relative superiority over the pe‐ripheral habitat(Schlesinger and Mahall,2003).Our results imply that the high heterogeneity of the hypo‐lithic biotope determines the cultivatable bacterial community structure.The high number of bacteria in hypolithic soil may be due to a number of possible reasons,such as positive synergistic ecological inter‐actions between species or proposed mechanisms in which lithobionts serve as reservoirs of terrestrial bio‐ta(Pointinget al.,2009).In the harsh biotope of the desert,the adaptability of microbes determines their abundance and distribution.ArthrobacterandBacillusbacteria have a strong ability to endure drought stress and have high abundance in arid and desert soil(Hauschildet al.,2017).In the present study,these two genera andStreptomyceswere the culturable bac‐teria occurring in high abundance and widespread dis‐tribution.Streptomycesbacteria have been shown to be the dominant bacterial group in the saline alkali soil in the Hexi Corridor(Liet al.,2015).Ultraviolet radiation is a primary threat to microbes living in the desert.Bacillusbacteria,which produce spores resis‐tant to radiation damage,have evolved to become the dominant bacteria in the Hexi Corridor.
Cyanobacteria-dominated hypolithic communities have been described in cold deserts,as well as in hot deserts (Warren-Rhodeset al., 2006); but in this study,the bacteria living in the desert soil also have been considered as an important subject for further ex‐ploration. For example,ArthrobacterandBacillusbacteria can play a key role in soil-element circulation(Zhanget al.,2012).Sphingomonas xenophagacan degrade a wide range of aromatic compounds and syn‐thesize valuable extracellular biopolymers(Quet al.,2013). TheNesterenkoniabacterium was initially found in a high-salt environment,indicating that it has resistance to such conditions(Chenet al.,2004).Planomicrobiumbacteria,which have a ability to secrete lipase(Zhuet al.,2009),are often isolated from ex‐treme environments such as volcano mud(Liet al.,2010).Geodermophilicstrains,regarded as one of the pioneer organisms in extreme environments, have been used in the research on stress-resistance mecha‐nisms,desertification control,and environmental res‐toration(Sunet al.,2015).The pigments secreted by bacterial cells have been proved to be related to bacte‐rial resistance to stresses.For example,Chroococcopsissp.isolated from surface soil in the Atacama Des‐ert can produce pigments that absorb ultraviolet radia‐tion(Doseet al.,2001).Most of the bacterial isolates in the present study were pigmented,suggesting a tol‐erance to adverse environments that may be worthy of further study.In conclusion,the present study identi‐fied the culturable bacteria in the desert hypolithic and peripheral soils in the west of Hexi Corridor.The majority of the bacterial isolates possess the ability to cope with the harsh biotope in this area and may be an important bacterial subject for further study and po‐tential application.
Acknowledgments:
This study was financially supported by the National Nat‐ural Science Foundation of China(31870479,31570498,and 41801045)and the Key Foreign Cooperation Projects of the Bureau of International Cooperation of Chinese Academy of Sciences(131B62KYSB20160014).
 Sciences in Cold and Arid Regions2020年1期
Sciences in Cold and Arid Regions2020年1期
- Sciences in Cold and Arid Regions的其它文章
- Characteristics of permafrost degradation in Northeast China and its ecological effects:A review
- Aeolian processes on sandy desertification of an alpine meadow:A wind tunnel experiment
- A case study on Landscape Component Niche based on Landscape Pattern Indices:Yanchi,Ningxia Province,China
- Soil-moisture dynamics and tree-water status in a Picea crassifolia forest,Qilian Mountains,China
