miR-203基因启动子区甲基化与肥胖膝关节骨性关节炎患者滑膜炎症水平相关性研究
李建伟 张丽娜 张英民 康少英 魏萌

[摘要] 目的 研究滑膜組织miR-203基因启动子区异常甲基化状态与肥胖膝关节骨性关节炎(KOA)关节液促炎因子水平相关性。 方法 KOA患者滑膜组织及关节液取自2018年1月~2019年1月于河北省邯郸市中心医院行膝关节人工关节置换的36例患者,体重指数(BMI)≥28.0 kg/m2的患者纳入肥胖组(23例),18.0 kg/m2≤BMI≤23.9 kg/m2的患者纳入正常对照组(13例)。甲基化特异性PCR(MSP)检测滑膜组织miR-203的基因启动子区甲基化状态。通过定量聚合酶链反应(qPCR)检测miR-203在滑膜组织的表达水平;应用qPCR检测miR-203在肥胖组和正常对照组中的表达水平;采用Pearson相关分析探讨miR-203与BMI,关节液中肿瘤坏死因子-α(TNF-α)、白细胞介素-1β(IL-1β)、白细胞介素-6(IL-6)的相关性。 结果 肥胖组滑膜组织中miR-203基因启动子区呈异常低甲基化状态,正常对照组则呈部分低甲基化状态。与正常对照组比较,肥胖组滑膜组织中miR-203的表达水平升高,差异有统计学意义(P < 0.05)。肥胖组关节液中IL-1β、IL-6、TNFα的相对表达均高于正常对照组,差异均有统计学意义(均P < 0.05)。miR-203基因启动子区甲基化水平与人群BMI和关节液中IL-1β、IL-6、TNF-α均呈负相关(均P < 0.05);miR-203表达水平和与人群BMI和关节液中IL-1β、IL-6、TNF-α均呈正相关(均P < 0.05)。 结论 KOA患者滑膜组织miR-203基因启动子区低甲基化与肥胖KOA患者BMI及关节液促炎因子水平密切相关,miR-203可作为肥胖KOA炎症的重要调控因素及KOA治疗的潜在靶标。
[关键词] 肥胖;膝关节骨性关节炎;微小RNA; miR-203;甲基化
[中图分类号] R684.3 [文献标识码] A [文章编号] 1673-7210(2020)04(c)-0032-05
Study on the correlation between the methylation of miR-203 gene promoter region and the level of synovitis in obese patients with knee osteoarthritis
LI Jianwei1 ZHANG Li′na2 ZHANG Yingmin1 KANG Shaoying1 WEI Meng1
1.Department of Orthopedics, Handan Central Hospital, Hebei Province, Handan 056000, China; 2.Department of Cardiology, Handan Central Hospital, Hebei Province, Handan 056000, China
[Abstract] Objective To study the correlation between abnormal methylation of miR-203 gene promoter region in synovial tissues and the level of joint fluid proinflammatory factors in obese knee osteoarthritis (KOA). Methods Synovial tissue and joint fluid of KOA patients were taken from 36 patients who underwent knee arthroplasty in Handan Central Hospital in Hebei Province from January 2018 to January 2019. Patients with body mass index (BMI)≥28.0 kg/m2 were included in the obesity group (23 cases), and patients with 18.0 kg/m2≤BMI≤23.9 kg/m2 were included in the normal control group (13 cases). Methylation specific PCR (MSP) was used to detect the methylation status of the gene promoter region of miR-203 in synovial tissues. The expression level of miR-203 in synovial tissues was determined by quantitative polymerase chain reaction (qPCR). qPCR was used to detect the expression levels of miR-203 in the obese group and the normal control group. Pearson correlation analysis was used to investigate the correlation between miR-203 and BMI, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in joint fluid. Results In the synovial tissues of the obese group, the promoter region of miR-203 gene was abnormally hypomorphic, while in the normal control group, it was partially hypomorphic. Compared with the normal control group, the expression level of miR-203 in synovial tissues of the obesity group was increased, and the difference was statistically significant (P < 0.05). The relative expressions of IL-1β, IL-6 and TNF-α in the joint fluid of the obese group were higher than those of the normal control group, with statistically significant differences (all P < 0.05). The methylation level in the promoter region of miR-203 gene was negatively correlated with population BMI and the IL-1β, IL-6 and TNF-α in the joint fluid (all P < 0.05). The expression level of miR-203 was positively correlated with population BMI and the IL-1β, IL-6 and TNF-α in the joint fluid (all P < 0.05). Conclusion Hypophalation of miR-203 gene promoter in synovial tissues of KOA patients is closely related to BMI and levels of joint fluid proinflammatory factors in obese KOA patients, and miR-203 can be used as an important regulatory factor for inflammation of obese KOA and a potential target for treatment of KOA.
[Key words] Obesity; Knee osteoarthritis; microRNA; miR-203; Methylation
肥胖诱导包括膝关节骨性关节炎(knee osteoarthritis,KOA)在内多种疾病[1],而且肥胖患者体内炎性因子失衡很可能与KOA的发生相关。研究表明,膝关节滑膜组织释放的炎性因子升高能引发关节软骨基质退变[2]。另外,微小RNA(microRNA,miRNA)的基因启动子区异常甲基化状态在多种疾病中起重要的作用[3]。miR-203在脂肪代谢及软骨细胞的炎性损伤中扮演重要调控角色[4],且已有研究表明miR-203启动子区域的低甲基化与类风湿性关节炎(rheumatoid arthritis,RA)患者的滑膜炎症密切相关[5]。然而,少见miR-203在KOA疾病和肥胖相关疾病中的研究。本研究初步研究了肥胖KOA患者滑膜组织中miR-203的基因启动子区异常甲基化状态分别与KOA患者体重指数(body mass index,BMI)、滑膜肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素-1β(interleukin-1β,IL-1β)、白细胞介素-6(IL-6)的相关性。为进一步探讨miR-203在肥胖KOA中的作用与机制提供线索。
1 材料与方法
1.1 临床样本
KOA患者滑膜组织及关节液取自河北省邯郸市中心医院(以下简称“我院”)于2018年1月~2019年1月行膝关节人工关节置换的36例K-L评分Ⅳ级的KOA患者。按照2003年中国肥胖工作组制定的《中国成人超重和肥胖症预防和控制指南》[7]的建议,将BMI≥28.0 kg/m2的患者纳入肥胖组,18.0 kg/m2≤BMI≤23.9 kg/m2的患者纳入正常对照组。其中正常对照组13例,肥胖组23例。组织及关节液RNA稳定试剂处理后,-80℃保存。KOA的诊断符合《骨关节炎诊疗指南(2018年版)》的KOA诊断标准[6]。本研究经我院医学伦理委员会审查通过。所有患者签署知情同意书。
1.2 基因组DNA提取和重亚硫酸盐修饰
应用Universal GenomicDNA Extraction KitVer 3.0试剂盒(TaKaRa Bio Inc.;货号:DV811A)提取滑膜组织的DNA。紫外-可见分光光度法测定DNA纯度(1.8 1.3 甲基化特异性PCR(methylation specific PCR,MSP) The University of California,Santa Cruz′s Genome Technology Center用来搜索miR-203的基因序列。应用Methprimer (http://www.urogene.org/index.html)扫描miR-203的基因序列发现4个CpG岛。BDGP(http://fruitfy.org:9005/seq_tools/promoter.html)扫描miR-203的基因序列发现3个启动子区评分>0.9,分别位于115~173、941~977、1726~1736 bp。经过对比,确定胞嘧啶—磷酸—鸟嘌呤(CpG)岛位于miR-203的基因启动子区[8]。 甲基化产物长度124 bp,非甲基化产物长度125 bp。反应产物应用TaKaRa Taq Hot Start Version试剂盒(Takara Bio Inc.;货号:DR007A)进行PCR扩增。反应条件为:94℃ 5 min;94℃ 30 s;58℃ 30 s;72℃ 30 s,40个循环;72℃孵育10 min。结果观察:5 μL PCR产物经琼脂糖凝胶电泳溴化乙锭染色后于紫外线照射下直接观察。 1.4 实时定量PCR 按TianGen试剂盒说明书步骤提取滑膜组织总RNA,抽提好的RNA按照TaKaRa(PrimeScript@RT reagent kit;货号:RR047A)试剂盒的反转录体系进行反转录,将反转录产物4℃保存。参照Power UpTM。SYBR Green Master Mix PCR试剂盒(Thermo Fisher Scientific;货号:4309155)说明,以上述反转录cDNA为模板进行miR-203的定量聚合酶链反应(quantitative polymerase chain reaction,qPCR)扩增。采用2-ΔΔCt法计算,以人U6基因作为内参,计算miR-203的相对表达量。 1.5 酶联免疫吸附试验(enzyme-linked immunosorbent assay,ELISA) 按照制造商的说明書使用酶联免疫试剂盒测定(R&DSystems,Minneapolis,MN)关节液中IL-1β、IL-6、TNF-α的浓度。所有实验重复至少3次。 1.6 统计学方法 采用SPSS 19.0对所得数据进行统计学分析,计量资料采用均数±标准差(x±s)表示,采用两样本t检验。计数资料采用百分率表示,采用χ2检验。采用Pearson相关系数分析法对miR-203表达量(2-ΔΔCt)与BMI、IL-1β、IL-6、TNF-α进行相关性分析。以P < 0.05为差异有统计学意义。
2 结果
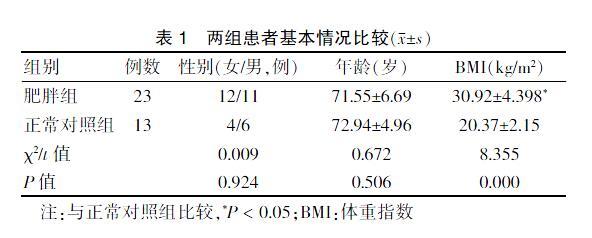
2.1 两组患者基本情况比较
共纳入KOA患者共36例,肥胖组中男11例,女12例;正常对照组中男6例,女7例。两组患者男女比例、年龄比较,差异无统计学意义(P > 0.05);两组患者BMI比较,差异有统计学意义(P = 0.000)。见表1。
表1 两组患者基本情况比较(x±s)
注:与正常对照组比较,*P < 0.05;BMI:体重指数
2.2 肥胖KOA患者滑膜组织中miR-203基因启动子区呈异常低甲基化状态
肥胖组滑膜组织中miR-203基因启动子区呈异常低甲基化状态,正常对照组则呈部分低甲基化状态。见图1。
M:高甲基化;U:低甲基化
图1 滑膜组织中甲基化特异性PCR反应结果
2.3 miR-203在KOA患者滑膜组织中表达情况
肥胖组滑膜组织中miR-203的表达水平(4.56±0.26)高于正常对照组滑膜组织中miR-203的表达水平(1.00±0.07),差异有统计学意义(t = 49.891,P = 0.000)。
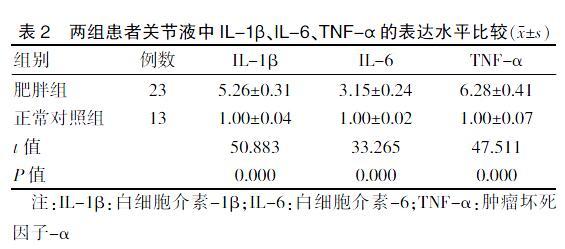
2.4 两组患者关节液中IL-1β、IL-6、TNF-α的表达水平比较
肥胖组关节液中IL-1β,IL-6,TNFα的相对表达均高于正常对照组,差异均有统计学意义(均P < 0.05)。见表2。
表2 两组患者关节液中IL-1β、IL-6、TNF-α的表达水平比较(x±s)
注:IL-1β:白细胞介素-1β;IL-6:白细胞介素-6;TNF-α:肿瘤坏死因子-α
2.5 滑膜组织中miR-203基因启动子区甲基化状态和miR-203水平与关节液中促炎因子相关性
miR-203基因启动子区甲基化水平与人群BMI和关节液中IL-1β、IL-6、TNF-α均呈负相关(均P < 0.05);miR-203表达水平和与人群BMI和关节液中IL-1β、IL-6、TNF-α均呈正相关(均P < 0.05)。见表3。
3 讨论
肥胖是一种机体脂肪组织总含量过多和局部含量增多及分布异常的慢性代谢性疾病。肥胖脂肪组织中的促炎因子IL-1β、IL-6、TNF-α升高[9-10]。有学者认为,肥胖是一种“慢性低度炎症状态”,可诱导包括KOA在内多种疾病[11]。膝关节滑膜组织释放的炎性因子升高与关节软骨基质退变密切相关,因此需要深入探索其发生发展的分子机制,为探明肥胖和KOA的发生机制提供新的研究思路。
miRNA调控细胞的免疫、炎症、发育、增殖、分化、凋亡等生理活动及恶性肿瘤等疾病的发生发展[12-15]。因此,控制miRNA的表达可能发挥重要作用。研究表明,遗传学调控方式是最关键的miRNA表达调控机制之一[16]。在肥胖和炎症相关疾病发生发展过程中,功能性的miRNA的基因启动子区异常升高或降低甲基化状态可起重要作用[17]。本研究结果显示,肥胖KOA患者滑膜组织中miR-203的基因启动子区甲基化水平降低,而其在滑膜組织中表达增高。与本研究类似的是,RA滑膜成纤维细胞中miR-203基因启动子区同样呈现低甲基化[5]。以上研究提示,基因启动子区甲基化水平降低和miR-203的表达增高在关节滑膜炎症发展过程中扮演重要角色。
研究发现,与正常个体比较,肥胖者体内更易于产生IL-1β,IL-6,IL-18及TNF-α[5,18]。这些因子可能对肥胖相关疾病有重要影响[19-20]。早期研究表明,肥胖对软骨代谢有较大影响[21],且IL-1β在OA患者体内被高度诱导,并且与BMI正相关[22]。本研究肥胖组滑膜液中3种促炎因子IL-1β、IL-6、TNF-α水平高于正常对照组,与以上研究结果一致。提示体重的增加对于骨关节腔内炎性水平有正向影响。另外,最新研究表明miR-203调节肥胖脂肪组织代谢,且miR-203可以抑制抗炎信号IFN-γ[23]。本研究显示miR-203基因启动子区甲基化状态与BMI和促炎因子IL-1β、IL-6、TNF-α均呈负相关,而miR-203表达升高与BMI和促炎因子IL-1β、IL-6、TNF-α均呈正相关,说明miR-203可能在肥胖KOA滑膜中扮演促进炎症的作用,且调节机制可能是miR-203基因启动子区的低甲基化水平。类似的是,RA患者滑膜成纤维细胞中miR-203被上调[5];另外,RA滑膜成纤维细胞的高水平炎症可能通过miR-203基因启动子区域的低甲基化来调节,而直接的证据也表明miR-203表达改变能调节滑膜细胞基质金属蛋白酶MMP-1和促炎因子IL-6水平[5]。以上研究提示miR-203基因启动子区甲基化调节的miR-203水平上调与肥胖KOA患者关节腔内炎性水平密切相关。
综上所述,miR-203可作为KOA疾病的重要调控因子。滑膜组织中miR-203表达上调,且与BMI呈正相关,提示miR-203在肥胖KOA疾病防治中的潜在作用。但仍需进一步设计体外和体内实验探明miR-203在肥胖KOA疾病中的作用和下游调控机制。
[参考文献]
[1] 安琪儿,张帅,江文宇,等.促进老年退行性骨关节炎软骨内源性修复的化合物研究进展[J].现代生物医学进展,2019(2):383-388.
[2] 朱瑜琪,王智耀,张帅,等.细胞因子与膝骨关节炎关节软骨损伤的修复[J].中国组织工程研究,2017,21(36):5873-5878.
[3] Oshima G,Poli EC,Bolt MJ,et al. DNA methylation controls metastasis-suppressive 14q32-encoded miRNAs [J]. Cancer Res,2019,79(3):650-662.
[4] Wang Z,Chi X,Liu L,et al. Long noncoding RNA maternally expressed gene 3 knockdown alleviates lipopolysaccharide-induced inflammatory injury by up-regulation of miR-203 in ATDC5 cells [J]. Biomed Pharmacother,2018, 100:240-249.
[5] Tavasolian F,Abdollahi E,Rezaei R,et al. Altered expression of microRNAs in rheumatoid arthritis [J]. J Cell Biochem,2018,119(1):478-487.
[6] 王弘德,李升,陳伟,等.《骨关节炎诊疗指南(2018年版)》膝关节骨关节炎部分的更新与解读[J].河北医科大学学报,2019,40(9):993-995,1000.
[7] 中国肥胖问题工作组.中国成人超重和肥胖症预防与控制指南(节录)[J].营养学报,2004,26(1): 1-4.
[8] Briem E,Budkova Z,Sigurdardottir AK,et al. MiR-203a is differentially expressed during branching morphogenesis and EMT in breast progenitor cells and is a repressor of peroxidasin [J]. Mech Develop,2019,155:34-47.
[9] Poret JM,Souza-Smith F,Marcell SJ,et al. High fat diet consumption differentially affects adipose tissue inflammation and adipocyte size in obesity-prone and obesity-resistant rats [J]. Int J Obes (Lond),2018,42(3):535-541.
[10] 赵会娟,尹晓琳,王园园,等.iNKT细胞在肥胖脂肪组织慢性炎症发生中的作用[J].医学研究与教育,2018, 35(1):19-24.
[11] Jiang LF,Fang JH,Wu LD. Role of infrapatellar fat pad in pathological process of knee osteoarthritis:Future applications in treatment [J]. World J Clin Cases,2019,7(16):2134-2142.
[12] Bots STF,Hoeben RC. Herpesvirus microRNAs for use in gene therapy immune-evasion strategies [J]. Gene Ther,2017,24(7): 385-391.
[13] Nejad C,Stunden HJ,Gantier MP. A guide to miRNAs in inflammation and innate immune responses [J]. FEBS J,2018,285(20):3695-3716.
[14] Xue H,Li MX. MicroRNA-150 protects against cigarette smoke-induced lung inflammation and airway epithelial cell apoptosis through repressing p53:MicroRNA-150 in CS-induced lung inflammation [J]. Hum Exp Toxicol,2018,37(9):920-928.
[15] Lin ZY,Chen G,Zhang YQ,et al. MicroRNA-30d promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer [J]. Mol Cancer,2019,18(1):122.
[16] Vaccaro TDS,Sorrentino JM,Salvador S,et al. Alterations in the MicroRNA of the Blood of Autism Spectrum Disorder Patients:Effects on Epigenetic Regulation and Potential Biomarkers [J]. Behav Sci(Basel),2018,8(8):pii:E75.
[17] Mansego ML,Garcia-Lacarte M,Milagro FI,et al. DNA methylation of miRNA coding sequences putatively associated with childhood obesity [J]. Pediatr Obes,2017,12(1):19-27.
[18] Fu Y,Huebner JL,Kraus VB,et al. Effect of Aging on Adipose Tissue Inflammation in the Knee Joints of F344BN Rats [J]. J Gerontol A Biol Sci Med Sci,2016, 71(9):1311-1140.
[19] Orekhov AN,Nikiforov NG,Elizova NV,et al. Tumor Necrosis Factor-α and CC Motif Chemokine Ligand 18 Associate with Atherosclerotic Lipid Accumulation In situ and In vitro [J]. Curr Pharm Design,2018,24(24):2883-2889.
[20] Yaribeygi H,Atkin SL,Sahebkar A. Interleukin-18 and diabetic nephropathy:A review [J]. J Cell Physiol,2018, 234(24):5674-5682.
[21] Votava L,Schwartz AG,Harasymowicz NS,et al. Effects of dietary fatty acid content on humeral cartilage and bone structure in a mouse model of diet-induced obesity [J]. J Orthop Res,2019,37(3):779-788.
[22] Clockaerts S,Bastiaansen-Jenniskens YM,Runhaar J,et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review [J]. Osteoarthritis Cartilage,2010,18(7):876-882.
[23] Guo X,Zhang Z,Zeng T,et al. cAMP-MicroRNA-203-IFNγ network regulates subcutaneous white fat browning and glucose tolerance [J]. Mol Metab,2019,28:36-47.
(收稿日期:2019-11-11 本文編辑:顾家毓)

