Oncometabolic surgery:Emergence and legitimacy for investigation
Won Jun Kim,Yeongkeun Kwon,Chang Min Lee,Seung Hyun Lim,Yong Li,Junjiang Wang,Weixian Hu,Jiabin Zheng,Gang Zhao,Chunchao Zhu,Wei Wang,Wenjun Xiong,Quan Wang,Mingjie Xia,Sungsoo Park
1Korea University College of Medicine,Seoul 136-701,Republic of Korea;2Division of Foregut Surgery,Korea University College of Medicine,Seoul 136-701,Republic of Korea;3Department of General Surgery,Guangdong Provincial People’s Hospital,Guangdong Academy of Medical Sciences,Guangzhou 510080,China;4Department of Gastrointestinal Surgery,Shanghai Jiao Tong University School of Medicine,Renji Hospital,Shanghai 200025,China;5Department of Gastrointestinal Surgery,Guangdong Provincial Hospital of Chinese Medicine,Guangzhou 510120,China;6Department of Gastrointestinal Surgery,The First Bethune Hospital of Jilin University,Changchun 130021,China
Abstract Studies on morbid obesity have shown remarkable improvement of diabetes in patients who have undergone bariatric operations.It was subsequently shown that these operations induce diabetes remission independent of the resultant weight loss;as a result,surgeons began to investigate whether operations for gastric cancer(GC)could have the same beneficial effect on diabetes as bariatric operations.It was then shown in multiple reports that followed that certain operations for GC were able to improve or even cure type 2 diabetes mellitus(T2DM)in GC patients.This finding gave rise to the concept of“oncometabolic surgery”,in which a patient diagnosed with both GC and T2DM undergo a single operation with the purpose of treating both diseases.With the increasing incidence of T2DM,oncometabolic surgery has the potential to improve the quality of life and even extend survival of many GC patients.However,because the GC patient population and the bariatric patient population are wildly different and because different GC operations have different properties,the effect of oncometabolic surgery must be carefully assessed and engineered in order to maximize benefit and avoid harm.This manuscript aims to summarize the findings made so far in the field of oncometabolic surgery and to provide an outlook regarding the possibility of oncometabolic surgery being incorporated into standard clinical practice.
Keywords:Stomach neoplasms;bariatric surgery;diabetes mellitus;metabolic syndrome;gastric bypass
Introduction and history of oncometabolic surgery
Gastric cancer(GC)—adenocarcinoma of the stomach—is the most commonly diagnosed cancer in East Asia and the sixth most commonly diagnosed cancer worldwide(1,2).While various treatment modalities are used for different stages of GC,radical resection with total or subtotal gastrectomy remains the mainstay of treatment to prolong survival in early GC patients(3,4).In East Asia,where the GC prevalence is the highest,the introduction of national screening programs has allowed discovery of more patients in earlier stages of the disease;this has led to a significant decline in GC mortality in recent years(5,6).For earlier-stage patients,the 5-year survival rate has reached over 90% in some countries,with even higher disease-specific survival rates(7-9).Consequently,medical care that can improve the quality of life in these patients’status postgastrectomy is becoming increasingly important,and clinicians have shifted their focus somewhat to characterizing and addressing the patients’quality of life after surgery(10,11).In this respect,control of chronic medical conditions in postoperative GC patients is becoming an important aspect of holistic care for these patients.
Almost serendipitously,bariatric surgery—whose procedures are remarkably similar to surgery for GC treatment—has shown effectiveness in ameliorating these chronic medical conditions(12).Initially,bariatric surgery was used to treat morbid obesity,but it rapidly became clear that this approach was also highly effective for treating chronic comorbidities of obesity,such as hypertension,dyslipidemia,and type 2 diabetes mellitus(T2DM).The effect on T2DM was particularly prominent,and it was shown that glycemic control was achieved independent of the weight-loss induced by the operation.This discovery gave rise to the concept of“metabolic”surgery(13).The efficacy of metabolic surgery for treating T2DM has been remarkable,with a 2009 meta-analysis of 4,070 diabetic patients showing diabetes resolution in 78.1% and improvement in 86.6% of patients(14).Such promising results have prompted physicians to include surgery in the arsenal of treatment for T2DM.
In more recent years,based on the similarities between GC surgery and bariatric/metabolic surgery,surgeons have hypothesized that GC surgery could also have a beneficial effect on patients’glycemic control and have investigated the effect of GC surgery on T2DM.Indeed,analyses of patients who underwent gastrectomy for GC demonstrated that these patients,similar to bariatric patients,experienced improvement of T2DM after surgery(15-18).This gave rise to the concept of“oncometabolic surgery”(19),in which a patient diagnosed with both GC and T2DM undergoes a single operation with the simultaneous goal of successful removal of the malignancy and achievement of glycemic control.This dual-purpose surgery not only has the potential to treat two illnesses with a single operation,but could also prolong the survival of certain patients,as comorbid T2DM confers a higher mortality on GC patients(20).
It has been shown that traditional methods of surgery for GC already induced benefits in terms of glycemic control,but the degree of glycemic improvement differed according to the operative technique.Procedures can be modified,based on the principles of bariatric surgery,to maximize the metabolic benefits.Therefore,although still a developing concept,by prompting surgeons to select and modify traditional procedures of GC surgery purposefully,oncometabolic surgery offers the potential of treating an oncologic condition,whilst simultaneously improving the quality of life of the patients by ameliorating a chronic metabolic condition with debilitating consequences.
Mechanism of action
Both bariatric surgery and GC surgery involve resection of the stomach and rerouting of food passage through the gut.However,one key difference is that,bariatric surgery patients,in whom the concept of metabolic surgery was first validated,undergo much more significant weight-loss postoperatively.Because T2DM is a comorbidity of obesity,improvement of T2DM after bariatric surgery was originally thought to be a secondary effect of weight-loss.It was also shown that amelioration of T2DM correlated with the degree of weight-loss experienced by the patients after surgery.However,although it is true that weight-loss is an important component of improving glycemic control in bariatric surgery patients,multiple studies have subsequently shown that glycemic control precedes any significant weight-loss(12,21,22),suggesting that mechanisms independent of weight-loss play a role in T2DM control.
Several theories have been devised to explain this phenomenon,particularly with regard to Roux-en-Y gastric bypass(RYGB),which has traditionally been the procedure of choice for bariatric surgery patients(23).One example,“the foregut hypothesis”proposes that bypass of the duodenum induces changes in levels of postprandial hormones,which in turn improve glucose control by increasing release of and sensitivity to insulin(24).The hormones involved in this“entero-insular axis”include glucagon-like peptide 1(GLP-1)and ghrelin(25,26).Another theory,known as the“hindgut hypothesis”suggests that early contact of nutrients with the distal portion of the small intestine induce an antidiabetic effect by increasing GLP-1,which increases insulin release and blunts postprandial hyperglycemia by slowing gastric emptying(27-29).
Although these two theories are the best-known,evidence suggests that the mechanism by which metabolic surgery facilitates glucose control is not likely to be limited to only these two hypothesized pathways.A study in rats showed that the Roux limb of RYGB-treated rats underwent hyperplasia and hypertrophy and,in response to undigested nutrient exposure,this limb displayed increased usage and disposal of glucose(30).Another investigation in mice demonstrated that sleeve gastrectomy(SG),a bariatric procedure that has recently become more popular,exerts its metabolic effect by regulating metabolic signaling through bile acids and nuclear receptor FXR(farsenoid-X receptor,or NR1H4),rather than through weight-loss(31).Yet another study found that reduced hepatic gluconeogenesis also plays a role in positively impacting glucose control after SG(32,33).Other proposed mechanisms include decreased glucose transport through sodiumglucose co-transporter 1 in the intestines,reduced branched-chain amino acids in circulation,and alterations in the gut microbiome(34).In reality,it is most likely that all of these mechanisms work in concert to induce glycemic control.
These mechanisms illustrate the complex relationship between the gut and glucose metabolism,which has not been fully appreciated until the effects of gut surgery on T2DM was witnessed.The surgeon’s role is to understand the surgical physiology behind various metabolic operations and to select and utilize various procedures to ensure the best outcome for the patient.The different types of operations and techniques are discussed in the following section.
Operative technique
The three main types of bariatric surgery are RYGB,SG and biliopancreatic diversion/duodenal switch(BPD/DS).RYGB and BPD/DS involve resection of the stomach and rerouting of the food passage to bypass the duodenum(i.e.,the duodenum is severed from the stomach,and the jejunum/ileum is attached directly to the outlet of the stomach),enabling both restriction of food intake and malabsorption of food within the gut.SG is a simpler operation that involves only resection of the stomach with no rerouting of food passage through the gut.
In gastrectomy for GC,the initial part involves resection of the stomach,as either total gastrectomy or subtotal gastrectomy:the extent of resection is determined by the location of the tumor.Total gastrectomy is followed by reconstruction with Roux-en-Y esophagojejunostomy[Roux-en-Y total gastrectomy(RYTG)],while subtotal gastrectomy is followed by either Billroth I(BI)or Billroth II(BII)reconstruction.RYTG and BII reconstructions are similar to RYGB and BPD/DS,as they restore gut continuity after stomach resection in a way that bypasses the duodenum.BI reconstruction,on the other hand,is similar to SG and does not involve a duodenal bypass.
In the bariatric surgery population,BPD/DS,RYGB and SG all improve glycemic control.A notable finding is that SG,despite not involving any duodenal bypass and thus not being able to employ many of the glucose-lowering mechanisms described in the previous section(including the foregut and the hindgut theory),has been shown to result in significant improvement in glycemic control,at a level comparable to RYGB(35).However,in GC populations,the procedures that involve duodenal bypass(i.e.,RYTG and BII)have shown far superior glycemic outcomes than BI,which does not involve duodenal bypass(18,36-39).This difference is likely because GC patients have lower baseline body mass index(BMI)than bariatric surgery patients,and as a result,the weight-loss component of glucose control improvement is minimal in these patients.Thus,they rely more heavily on the effect of duodenal bypass to improve their glucose homeostasis.This finding is reflected well by the meta-regression analysis by Kwonet al.,which illustrated that populations that experience less reduction in BMI are more likely to benefit from BII than from BI reconstruction(16).
In summary,among the existing procedures for GC,total gastrectomy with Roux-en-Y reconstruction and subtotal gastrectomy with BII reconstruction yield the best outcomes for glycemic control.It therefore stands to reason that,although diabetic status has not previously been a consideration in selection of operative procedures for GC,surgeons now treating GC patients who also suffer from T2DM should take this metabolic difference between the operation types into account.
Furthermore,in addition to the traditional GC surgery methods,an altogether new operative method is also being attempted.This new type of procedure strives to mimic the maximization of nutrient malabsorption of the bariatric RYGB procedure.RYTG for GC usually results in a 30−40 cm biliopancreatic limb and a 40−50 cm alimentary limb,thus a combined length of 70−90 cm of bypassed proximal jejunum(40).In RYGB,on the other hand,in order to induce a maximum malabsorptive effect,the alimentary limb and the biliopancreatic limb are 100−150 cm and 30−60 cm long,respectively(41).This principle of reconstruction with long alimentary and biliopancreatic limbs to reduce the common segment where nutrient absorption takes place has been applied to RYTG and has led to the development of a“long-limb bypass reconstruction”,in which the alimentary and biliopancreatic limbs are elongated.This new type of procedure has shown superiority over BII reconstruction in diabetes control one year after surgery,in a retrospective study involving 226 patients(42).Although whether long-limb bypass reconstruction is truly superior to standard-length reconstruction in RYTG remains to be investigated in future studies,it is currently a promising operative technique for oncometabolic surgery that should be considered along with RYTG and subtotal gastrectomy with BII reconstruction.
Patient selection
Oncometabolic surgery borrows many concepts from bariatric surgery,but one key pitfall is that the patient populations for the respective surgeries differ markedly from each other.While bariatric surgery is aimed at morbidly obese patients of all ages that are fit for surgery,candidate patients for oncometabolic surgery are generally older,frailer,and lighter.Therefore,these special characteristics must be considered when selecting patients who are most likely to benefit from oncometabolic surgery.The most important difference between oncometabolic surgery patients and bariatric patients is weight.Weightloss is an important contributing factor to metabolic improvements in bariatric surgery.Indeed,this is reflected by the fact that the ABCD score,a patient classification system developed to predict the likelihood of glycemic improvement after metabolic surgery,includes preoperative BMI as an important predictive factor(43).Because of the lower BMI in GC patients,they are less likely to benefit from the metabolic benefits of weight-loss that are experienced by bariatric patients.This calls into question whether metabolic surgery would be effective enough in GC patients to justify setting metabolic goals.To address this question,multiple studies on non-obese GC patient populations with a mean BMI ranging in the mid-twenties have investigated the impact of GC surgery on glucose control and have confirmed that metabolic surgery does indeed improve T2DM in these patients(18,36,37,39,44).At the same time,however,some studies also showed that preoperative BMI or perioperative BMI changes are significantly correlated with T2DM improvement(19,36,39),suggesting that GC patients in the lowest BMI ranges may benefit less or may not benefit at all from metabolic surgery.More data from larger studies investigating the metabolic effect of such surgery in relation to baseline BMI are needed to obtain a better guide for clinical decision-making.Meanwhile,surgeons must keep this relationship in mind during patient selection for oncometabolic surgery.Furthermore,weight also impacts the postoperative care and nutritional strategy after oncometabolic surgery.For example,obese GC patients’status postsurgery are followed up with dietary measures to reinforce weight-loss,similar to bariatric patients.However,patients with BMIs in the range of normal to overweight may not benefit from the same postoperative nutritional approach;on the contrary,they may even require the opposite type of care to prevent excessive weight-loss and to maintain a healthy weight.Research on nutritional care and weight control for GC patients following surgery is lacking,and this will likely be an important topic of future research in the field of oncometabolic surgery.
Another important consideration is age.GC patients are generally older than bariatric patients.Not only does this confer a higher risk for an invasive operation in general,but age also has implications for T2DM.While all T2DM patients have impaired glucose control,the cause can be different for each patient:decreased insulin sensitivity in the peripheral tissue,reduced pancreatic β-cell function,and abnormal hepatic glucose metabolism are some of the pathophysiologic mechanisms that can cause T2DM.Depending on which mechanism is the main pathophysiology underlying the T2DM in each patient,the effectiveness of metabolic surgery can vary.Older patients,particularly those of Asian descent,are more likely to have pancreatic β-cell dysfunction as the major factor underlying their T2DM,whereas young obese patients are more likely to have decreased insulin sensitivity in the peripheral tissue and impaired hepatic glucose metabolism as the major cause of their T2DM.This difference can impact the patient’s likelihood of benefitting from metabolic surgery.This relationship is reflected by the fact that age is a negative predictive factor for T2DM remission after surgery in both the ABCD and DiaRem scoring systems,which were devised to calculate the probability of T2DM remission after metabolic surgery in bariatric patients(43,45).This difference in T2DM pathophysiology must be taken into consideration in clinical decision-making and in further research.
Perhaps even more important than age,the“severity of T2DM”in each patient is also a predictive factor for T2DM improvement after metabolic surgery and is thus a critical patient selection criterion.Studies investigating patient characteristics versus the likelihood of T2DM remission after gastrectomy almost unanimously showed that patients who have had T2DM for a longer duration,patients requiring insulin therapy,or patients who have higher preoperative glycated hemoglobin(HbA1c)levels are significantly less likely to note glycemic benefits from surgery(15,18,19,36,39,40,44).The ABCD and DiaRem scoring systems also respectively incorporate duration of T2DM and treatment with insulin or drugs other than metformin as negative predictive factors of the success of metabolic surgery(43,45).This strongly suggests that patients with more“severe”diabetes are less likely to benefit from metabolic surgery,possibly due to the decrease in pancreatic β-cell function over the heavier accumulation of hyperglycemic stress.This evidence indicates that a shorter duration of T2DM is an important patient selection criterion and also advocates earlier intervention to ensure optimal metabolic outcomes.
From an oncologic perspective,patients most likely to benefit from oncometabolic surgery are those with long life expectancies,i.e.,patients who are diagnosed with GC at earlier stages.These patients will require long-term management of their T2DM after surgery to remove the GC,and therefore,oncometabolic surgery should be more seriously considered as an option for these patients.Figure 1shows a potential treatment algorithm for GC patients with T2DM if oncometabolic surgery is incorporated into standard practice.This may be used to alter the most popular guidelines currently in use today,which propose endoscopic mucosal dissection for treatment of GC found in the earliest stages i.e.,cT1aN0M0,≤2 cm in size,differentiated,and without ulceration(3,4).Because these patients undergo only endoscopic dissection and no further resection under these traditional guidelines,it is reasonable to expect that SG could serve as an adequate alternative for these patients if they have T2DM.For oncometabolic patients with more advanced diseases,surgery based on long-limb bypass reconstruction is proposed.
Efficacy of oncometabolic surgery
The reported efficacy of oncometabolic surgery—i.e.,its ability to improve glycemic status after surgery—varies widely from study to study.The American Diabetes Association(ADA)defines remission of diabetes as follows:complete remission(CR)is a return to fasting glucose<100 mg/dL(5.6 mmoL/L)or normal HbA1c(<6.0%)for at least 1 year in the absence of antidiabetic medication,and partial remission(PR)is a sub-diabetic hyperglycemia with fasting glucose of 100−125 mg/dL(5.6−6.9 mmoL/L)or HbA1c of 6.0%−6.5% for at least 1 year in the absence of antidiabetic medication(46).Studies that have reported on the efficacy of GC surgery on T2DM have inconsistently employed the ADA definition of diabetes remission,butTable 1shows the efficacy of RYTG,subtotal gastrectomy with BI,subtotal gastrectomy with BII,and long-limb reconstruction surgeries as reported in various studies,with the results portrayed in terms of the ADA definition(i.e.,the remission percentages presented inTable 1may be different from those presented in the original paper,as we display the remission rates in terms of the ADA definition,regardless of the criteria used by the authors themselves).For BII,the rate of remission ranges from 1.0% to 72.8%,and for RYTG,from 27.3% to 90.5%.Such wide variability is most likely due to the different follow-up periods and study designs in each investigation.When presenting T2DM remission rates,most studies also employed their own definition of diabetes improvement,which are less strict,e.g.,a decrease in the number of diabetic medication or a decrease in fasting plasma glucose or HbA1c levels.Based on these definitions,the rate of diabetes improvement is much higher and often in excess of 50% for BII and RYTG.Regardless of the definition used,the general trend is that,among the conventional GC surgery methods,RYTG has the best efficacy in inducing T2DM remission.There is less data on the efficacy of the more-recently introduced long-limb bypass reconstruction,but preliminary prospective data of 30 patients who underwent this operation showed ADA-based T2DM remission in 30% and a general improvement in a further 20% of patients(40).Moreover,a retrospective cohort study of 226 patients demonstrated statistically significant superiority of long-limb bypass reconstruction over traditional BII reconstruction(42).The exact efficacy of this new procedure still needs to be confirmed in larger prospective trials.
It is notable,although expected,that diabetes remission and improvement rates are lower than those for bariatric populations.This is most likely due to the characteristics of the patient populations described in the previous section(lower BMI,older age,less pancreatic β-cell function).
To date,studies on the efficacy of oncometabolic surgery have almost solely focused on T2DM remission.Future investigations into the effect of oncometabolic surgery on the microvascular and macrovascular complications of diabetes are necessary,as these are the main disease entities that afflict the patient.Furthermore,the impact of oncometabolic surgery on survival would also be noteworthy,as there is evidence that diabetes is a risk factor for mortality in GC patients(20)and that cure of diabetes after metabolic surgery is correlated with a higher 5-year survival rate(17).While it must be remembered that this evidence does not yet suggest a causative effect between diabetes remission and mortality,it provides a reasonable basis for the belief that oncometabolic surgery may confer an overall survival benefit in GC patients.Hence,there is a need for longitudinal investigations of the impact of oncometabolic surgery on mortality.
Risks of oncometabolic surgery
The technique of oncometabolic surgery does not differ in any significant extent from standard gastrectomy for GC or from bariatric operations;therefore,the risk profile of oncometabolic surgery is unlikely to be higher than that of standard total/subtotal gastrectomy or RYTG.
The only exception to this is long-limb bypass reconstruction.Because of the enhanced degree of malabsorption with this new procedure,nutrition is a potential issue for these patients.This nutritional concern about long-limb bypass reconstruction has been investigated in 20 patients who underwent the operation.The results showed that there was no increased incidence of anemia,iron deficiency,and vitamin B12 deficiency after
the operation;however,median vitamin B12 levels were lower in these patients.Clinicians therefore need to pay close attention to vitamin B12 levels during follow-up of patients who undergo oncometabolic surgery with longlimb bypass reconstruction(50).
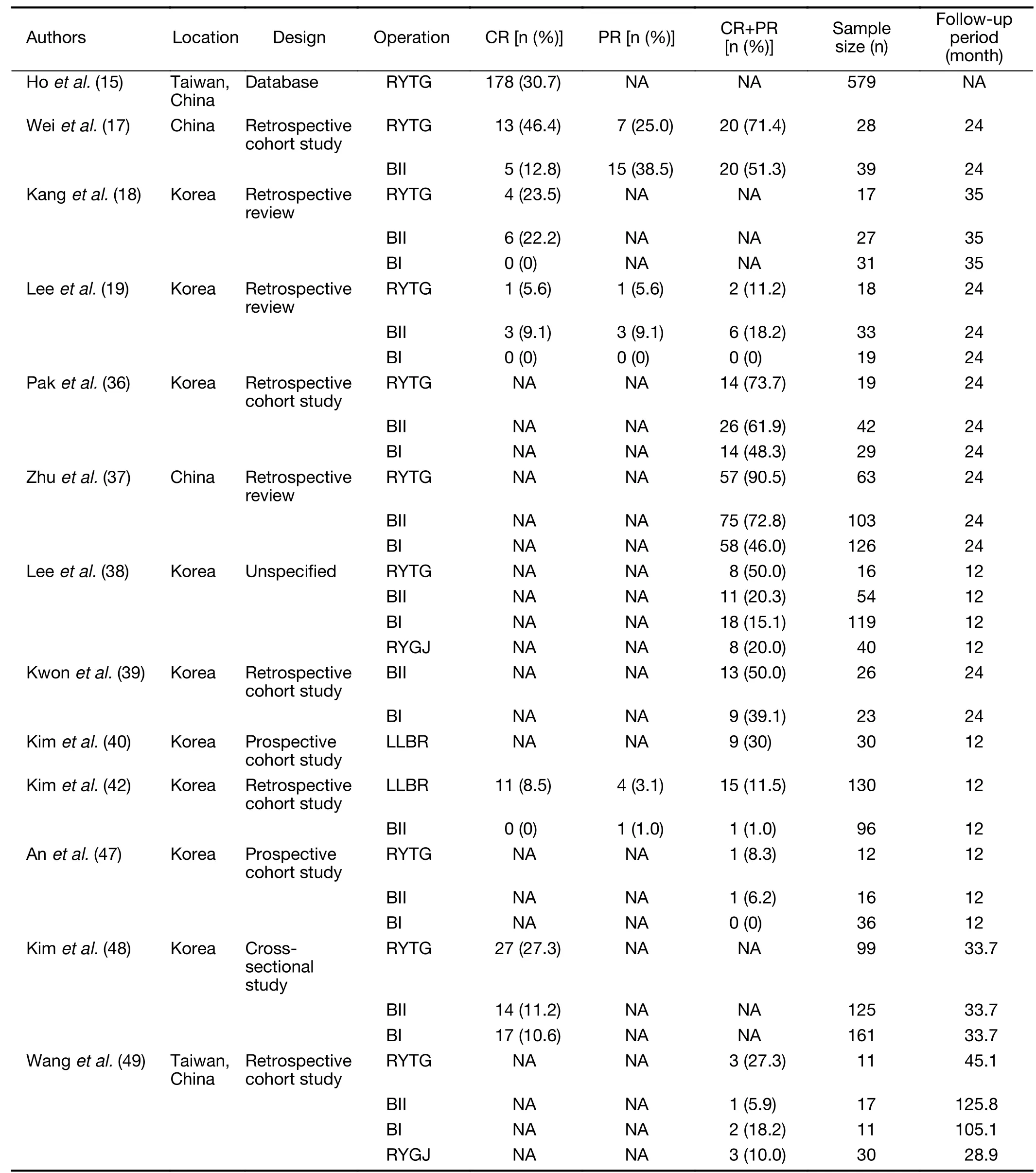
Table 1 Efficacy of oncometabolic surgery
Possibility of pure metabolic surgery?
With the success of bariatric surgery,clinicians have begun to discuss the possibility of a cure for diabetes(46).This surgical treatment of diabetes,however,has been limited to patients with high BMI.The original indications for metabolic and bariatric surgery outlined by the American Society for Metabolic and Bariatric Surgery are 1)BMI≥40 kg/m2or 2)BMI≥35 kg/m2and an obesity-related comorbidity,such as T2DM.For patients of Asian heritage,the Asia-Pacific Bariatric Surgery Group has recommended surgical treatment in patients with 1)BMI>37 kg/m2or 2)BMI>32 kg/m2with diabetes or two other obesity-related comorbidities(51).These guidelines have been challenged in recent years,with the demonstration of equivalent benefit of metabolic surgery for T2DM patients with BMI<35 kg/m2in multiple studies(34).A new international guideline by the second Diabetes Surgery Summit,now incorporated into the ADA Standards of Diabetes Care 2020,recommends that metabolic surgery be considered as a standard therapy for appropriate candidates with inadequately controlled T2DM and BMI>30 kg/m2or BMI>27.5 kg/m2for Asian individuals(52).
Oncometabolic surgery provides an opportunity to research the impact of metabolic surgery on patients with even lower BMI.This research will help to elucidate the physiology of diabetes in non-obese or only mildly obese patients and may provide new insights into the development and treatment of T2DM.Investigation of the enteroinsular axis in these patients may also reveal novel targets for diabetes therapy.Furthermore,it may even lead to the possibility of pure metabolic surgery in a highly select group of non-obese T2DM patients.This would entail new predictive systems for T2DM remission identifying in these patients,as the prediction systems that were designed for obese patients(43,45,53)have been shown to be inapplicable to patients in the lower BMI range(54).This will also necessitate discovery of new parameters that predict a patient’s likelihood to benefit from metabolic surgery,such as the visceral fat proportion of a patient(44).Discovery and characterization of enough parameters to define a non-obese patient population that may benefit from metabolic surgery may be possible through the study of patient populations undergoing oncometabolic surgery.
Conclusions
Bariatric surgery,originally intended as a treatment for obesity,has shown the unexpected benefit of ameliorating metabolic conditions,most notably T2DM.Because operations for GC are similar to bariatric operations,the metabolic effects of GC surgery have been investigated,and it was promptly revealed that GC surgery induces similar benefits of T2DM remission and improvement,even though the majority of these patients are not obese.This gave rise to the field of oncometabolic surgery,in which the surgeon actively selects from and modifies traditional GC operations to treat both GC and T2DM in a single operation.Oncometabolic patient populations are different from traditional GC patients and bariatric populations,but they have seen successful results in terms of achieving glycemic control.Because the study of oncometabolic surgery is in its infancy,further research is likely to provide more insights into its efficacy and patient population characteristics.Furthermore,because these operations have been performed on non-obese individuals,this surgery could be used to investigate the possibility of“pure metabolic surgery”,i.e.,surgery for treatment of T2DM in non-obese,non-GC patients.
Acknowledgements
None.
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2020年2期
Chinese Journal of Cancer Research2020年2期
- Chinese Journal of Cancer Research的其它文章
- Evaluation and reflection on claudin 18.2 targeting therapy in advanced gastric cancer
- Data mining-based model and risk prediction of colorectal cancer by using secondary health data:A systematic review
- Nomogram for prediction of pathologic complete remission using biomarker expression and endoscopic finding after preoperative chemoradiotherapy in rectal cancer
- Phase 1/2 study of concurrent chemoradiotherapy with weekly irinotecan hydrochloride for advanced/recurrence uterine cancer:A multi-institutional study of Kansai Clinical Oncology Group
- p16/Ki-67 dual-stained cytology used for triage in cervical cancer opportunistic screening
- Analysis and external validation of a nomogram to predict peritoneal dissemination in gastric cancer
