Phase 1/2 study of concurrent chemoradiotherapy with weekly irinotecan hydrochloride for advanced/recurrence uterine cancer:A multi-institutional study of Kansai Clinical Oncology Group
Satoshi Takeuchi,Haruo Kuroboshi,Taisuke Mori,Kimihiko Ito,Eiji Kondo,Tsutomu Tabata,6,Yoshio Itani,Ryuji Kawaguchi,Kyosuke Takeuchi,Toshinori Soejima0,,Ryohei Sasaki,0
1Department of Obstetrics and Gynecology,National Hospital Organization Kobe Medical Center,Kobe 6540155,Japan;2Division of Gynecologic Oncology,Department of Gynecology,Women Health Care,Kobe Tokushukai Hospital,Kobe 6500017,Japan;3Department of Obstetrics and Gynecology,Kyoto Prefectural University of Medicine,Kyoto 6028566,Japan;4Department of Obstetrics and Gynecology,Kansai Rosai Hospital,Amagasaki 6608511,Japan;5Department of Obstetrics and Gynecology,Mie University Graduate Medical School Faculty of Medicine,Tsu,5148507,Japan;6Department of Obstetrics and Gynecology,Tokyo Women’s Medical University,Tokyo 1628666,Japan;7Department of Obstetrics and Gynecology,Nara Prefectural Nara Hospital(Nara Prefectural General Medical Center),Nara 6308581,Japan;8Department of Palliative Care,Palliative Care Center of Yao-city Hospital,Osaka 5810069,Japan;9Department of Obstetrics and Gynecology,Nara Medical University,Nara 6348521,Japan;10Department of Radiation Oncology,Kobe University Graduate School of Medicine,Kobe 6500017,Japan;11Department of Radiation Oncology,Hyogo Ion Beam Medical Center Kobe Proton Center,Kobe 6500047,Japan
Abstract Objective:Concurrent chemoradiotherapy using cisplatin was thought to be standard treatment for squamous cell carcinoma of cervix,but it had not been effective for adenocarcinoma.Concurrent chemoradiotherapy using irinotecan hydrochloride(CPT-11)had been effective for colorectal cancer,thus,we chose CPT-11 as a candidate for gynecologic adenocarcinoma.To evaluate the maximum tolerated dose(MTD)of weekly CPT-11 with external pelvic radiotherapy,a phase 1/2 study was conducted according to modified Fibonacci method.Methods:Eligible patients were advanced uterine cancer with measurable diseases[performance score(PS):0−2].Study period was from August 1st,2002 to December 31st,2008.The starting dose level(DL)of CPT-11 was 30 mg/m2(DL1)given weekly for 4 weeks.Subsequently,dose escalation was scheduled in 10 mg/m2 increments to 60 mg/m2(DL4).The fixed radiotherapy consisted of whole pelvic 1.8 Gy/d,once a day in weekday for five weeks and it amounted to 45 Gy(25 fractions)in total.Results:Seventeen patients were enrolled.As for toxicities,one(1/17:5.9%)grade(G)4 neutropenia lasting 7 days had been seen in DL4.G2 diarrhea was identified in 35.3%(6/17)of the patients,and 11.8%(2/17)G3 diarrhea was observed in DL3 and DL4.Thus,the MTD of CPT-11 was defined as dose of 60 mg/m2.The recommended dose was decided as 50 mg/m2.The response rate was 88.2%[9 complete response(CR),3 partial response(PR),3 stable disease(SD),2 not evaluable(NE)].Disease control rate at 1 month after treatment completion was 100% but distant metastases were found in 24%(4/17)in longer outcome.Conclusions:MTD was 60 mg/m2 and recommended dose was set as 50 mg/m2.This concurrent chemoradiation using weekly CPT-11 was feasible at 50 mg/m2,and it might be effective even in adenocarcinoma of the uterus.
Keywords:Concurrent chemoradiotherapy(CCRT);irinotecan hydrochloride(CPT-11);cervical adenocarcinoma;endometrial cancer
Introduction
In Japan,the recommendation of vaccination of human papillomavirus(HPV)by the Ministry of Health,Welfare,and Labor has been suspended,thus the number of patients with cervical cancer has increased in conjunction with lower receiving ratio of Pap smear test as 37.3%−42.3% in 2016(1).Especially,the issues of uterine cervical cancer are not only the increase of the disease in adolescents and young adults generation(AYA generation)but also the percentage of adenocarcinoma of cervix which has been increased in comparison with that of squamous histology[squamous cell carcinoma(SCC)](2).As for diagnosis in cervical adenocarcinoma,it is very difficult to detect in early stage by yearly screening of Pap smear test.Even if cervical adenocarcinoma is detected at earlier stages,it causes ovarian metastases more frequent than that of SCC(3,4).Furthermore,because of its dependency on estrogen in proliferation,the treatment of cervical adenocarcinoma with fertility preservation in AYA generation has been restricted to very early stage such as IB1 stage with small volume disease.It is well known that cervical adenocarcinoma has been life-threatening disease because of its biologically malignant character,such as resistance to usual radiotherapy(RT)including concurrent chemoradiotherapy(CCRT)with cisplatin or systemic chemotherapy(CT)(5,6).Cervical adenocarcinoma was not homogeneous as well,which consists of at least 5 subtypes,such as endometrioid type,endocervical type,mucinous intestinal type,adeno-squamous type and gastric morphology and immunophenotype,which is called“adenoma malignum”or“minimal deviated adenocarcinoma”.The endometrioid type is sensitive for chemotherapy like corpus cancer,on the other hand,gastric type shows apparently poorer prognosis than that of other subtypes because of chemo-resistance(7).In Japan,5 year-survival of SCC and adenocarcinoma of cervix showed 58.7% and 40.0%(P<0.005,Kaplan-Meir,Tukey-Cremer analysis),respectively in stage III(8).
As for treatments for advanced cervical cancer,the efficacy of CCRT has been elucidated by many randomized trials in SCC(9-13),and it was endorsed by the National Cancer Institute(NCI)-recommendation of CCRT in patients with cervical cancer stage over IB2(14).
To cure or control cervical adenocarcinomas was an urgent issue for us.To date,in spite of the improvement of CT or CCRT in SCC,survival superiority of the CT and CCRT in the treatment of cervical adenocarcinoma and endometrial cancer has not been identified.In order to improve the prognosis of advanced cervical adenocarcinoma and endometrial cancer,the new effective regimen of CCRT must be explored.
Iirinotecan hydrochloride(CPT-11)was considered as a good partner with CCRT,because of its sensitizing effect of irradiation(15-20).It is well known that CPT-11 is a semisynthetic analog of camptothecin,originally isolated from the ornamental treeCamptotheca acuminata.CPT-11 is transformed to an active metabolite,7-ethyl-10-hydroxycamptothecin(SN-38)by carboxyl esterase which is mainly found in the liver,bowel mucosa,and tumor tissue(21),and plays an essential role in the cytocidal activity and toxicity of the parent compound(22).Irinotecan has been used for cervical cancer in Japan and its response rates of the disease were reported from 9.1% to 29.3%(22,23).As for enhancing radio-sensitivity,fundamental experiment showed the elevation of sensitivity of irradiationin vitrofor colorectal cancer(17).As for clinical use,the CCRT with irinotecan was reported to be useful in the treatment for advanced rectal cancer in CCRT setting(24).Thus,CPT-11 seems to become one of the candidates as an agent used for cervical cancer especially for cervical adenocarcinoma which showed resistance to CCRT with cisplatin.We conducted a phase 1/2 study for uterine cancers to determine the feasibility and MTD of concurrent use with irradiation in patients with uterine cancers(SCC,cervical adenocarcinoma,and endometrioid cancer).
Materials and methods
Study design
This study was prospective,exploring,non-randomized,interventional dose finding study and was registered in University Hospital Medical Information Network(UMIN)(No.UMIN000000148).
Radiotherapy was fixed as extra pelvic irradiation with/without high-dose rate brachytherapy for pelvic lesion and 45 Gy for para-aortic lesion.Dose of CPT-11 was escalated from 30 mg/m2to 60 mg/m2in 10 mg/m2incremental dose,and the maximum tolerated dose(MTD)and recommended dose[one dose level(DL)down of MTD]was assessed by modified Fibonacci method.The study period was from Aug 1st,2002 to Dec 31st,2008.
This study was approved by institutional review board(IRB)of each institution in Kansai Clinical Oncology Group(KCOG),and written informed consent was obtained from each accrued patient.The study followed the current guidelines of the International Conference on Harmonization for good clinical practice and the Declaration of Helsinki.
Patients and methods
Eligibility criteria were as follows:1)unresectable advanced/recurrent uterine cancers(cervical cancer/endometrial cancer)that are pathologically diagnosed,adenocarcinoma was preferable.SCC and endometrioid cancer were also allowed because fully efficacy of this treatment had not been reported,and adverse events(AEs)would not be different between cervical adenocarcinoma and SCC because of the same radiation field and the same dose and dosage;2)the disease must be measurable and the response must be evaluated according to the response criteria of solid tumor(RECIST)version 1.0;3)regional lymph-nodes(and para-aortic nodes below 326b1)metastases are allowed as measurable lesions but no distal metastases in other organs(lung metastasis or brain metastasis,or distal lymph nodes upper than the 326b1)were required;4)prior treatments were allowed except RT and/or CT by topoisomerase 1 inhibitor;5)the interval of the prior therapy must be over 4 weeks(including with biological response modifier);6)patient’s age must be 20−75 years old at informed consent;7)Eastern Cooperative Oncology Group(ECOG)performance status scores(PS)must be 0−2;8)patients have a life expectancy greater than 3 months;and 9)no severe dysfunctions in major organs(bone mallow,heart,lung,liver,kidney)were necessary.All patients signed informed consent forms that were approved by the participating institution’s IRB before screening.
Exclusive criteria were as follows:1)patients who had received prior RT and the irradiation field would be overlapped;2)patients who had other active progressive cancers,or active aggressive infection diseases,over grade 2 diarrhea,ileus,interstitial pneumonitis,massive ascites or pleural fluid retention;3)patients who had been pregnant or were lactating were excluded for fear of teratogenicity or bone marrow suppression of baby due to placental transition or milk transfer of the SN-38,an active form of CPT-11;or 4)patients who had received CT with CPT-11 before or had hypersensitivity to CPT-11.
Protocol treatment
RT
In pelvic region,RT was used external irradiation:Irradiation was delivered using 3-dimensional(3D)-conformal radiation(a dose of 45 Gy,1.8 Gy×25 fractions).Brachytherapy was used with high-dose rate,6 Gy for 3 or 4 times by Remote After Loading System(RALS).In para-aortic region,RT was used as an additional treatment if there are metastases at para-aortic lesions.Para-aortic RT was the same dose of pelvic external irradiation at a dose of 45 Gy(1.8 Gy×25 fractions)from upper plane of 12th thoracic bone to lower plane of 4th lumbar bone in 8 cm width with 4-field box technique.
CT
CPT-11(Yakult Honsha,Tokyo,Japan,and Daiichi-Pharm.,Tokyo,Japan)was administered by intra venous administration via peripheral venous access once a week,during earlier period the week(from on Monday to winthin on Wednesday),for 4 times during irradiation(Figure 1,Table 1,2).The agent was diluted into 250−500 mL of saline and administered in 90 min,on d 1,d 8,d 15,and d 22.Only one skip was allowed and if skipped one administration,additional one administration must be performed on d 29,during the period of external RT.
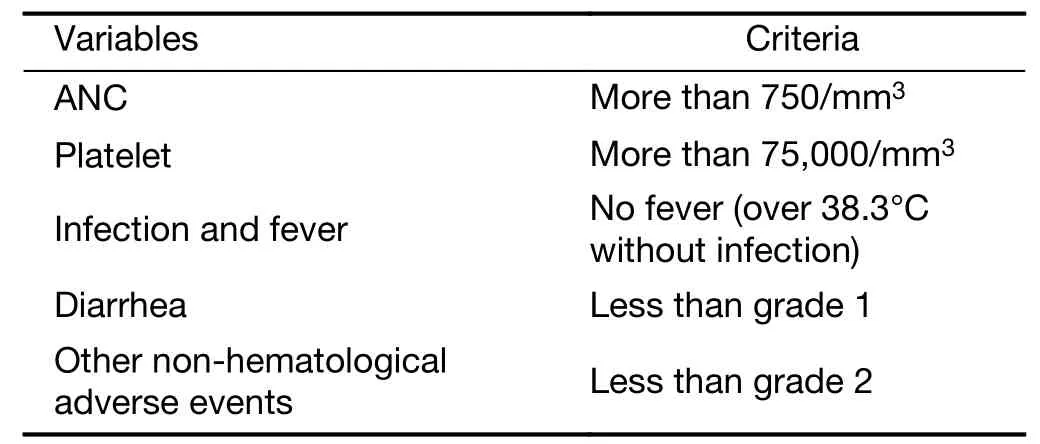
Table 1 Criteria of skip of chemotherapy
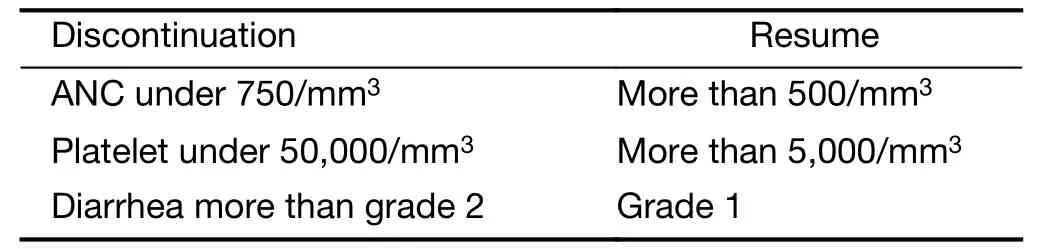
Table 2 Discontinuation of radiotherapy
Sequential two skips were dealt with as discontinuation of protocol treatment due to dose limiting toxicity(DLT).The starting DL of CPT-11 was 30 mg/m2(DL1)given weekly for 4 weeks.Subsequently,dose escalation was scheduled in 10 mg/m2increments to 60 mg/m2(DL4).During the treatment of dose level 2(DL2)in 2005,the pharmaceutical alert concerning to metabolic enzymes for SN-38(metabolite from CPT-11 and having most anticancer bio-activity),uridine diphosphate glucuronosyltransferase 1A1(UGT1A1)polymorphism,further 3 patients’cohort was added(25).
Supportive medication
According to anti-emetic agents of the second levels of American Society of Clinical Oncology(ASCO)emetic guideline,that is,domperidone 10 mg t.i.d.per os and infusion of weak steroid as hydrocortisone sodium phosphate 200 mg/body with 100 mL of saline were used as pre-medication.
As for hematological toxicities,grade(G)4 toxicity without absolute neutrophil counts(ANC)was dose limiting toxicity(DLT).In ANC,G4 and lasting 7 d were used as pre-medication.
Non-hematologically,any G4 toxicity and unknown G3 toxicity were regarded as DLTs.Especially,G3 diarrhea was regarded as DLT.Administration skip was performed if there was no recovery to G2 neutropenia,G2 thrombocytopenia,and G1 diarrhea from worse status.
Granulocyte-colony stimulating factor(GCSF)could be used according to ASCO guideline.Supportive treatments of anti-diarrhea including prophylactic use,such as loperamide and herbal medicine,hangeshashintou(Tsumura,Tokyo,Japan)could be allowed,but not intestinal alkalization.
Endpoints
Primary endpoint was to decide the MTD of this treatment protocol according to modified Fibonacci method.The recommended dose was defined as one DL down of MTD.Secondary endpoints were detection of toxicity profiles,tumor-response rate(evaluated by RECIST version 1.0),progression-free survival(PFS)and overall survival(OS).
Safety was evaluated in all patients being received at least one course of the study by assessment of AEs,clinical laboratory test results,physical examinations,and vital signs.AEs and laboratory values were graded according to the NCI-CTC version 2.0.All patients were followed up until recovery from toxicity and OS was observed until relapse or progression of the disease.
Statistical analysis
The toxicity profiles were assessed in frequency and severity in dose escalation manner and MTD was decided according to modified Fibonacci method.The response rate was calculated according to RECIST version 1.0.As for the survival analyses,the Kaplan-Meier method(logrank test)with Greenwood’s formula was used by SAS STATview 5.0(SAS Institute Inc.,Cary,USA).For the purpose of calculating OS,failure was defined as death of any cause.For the purpose of calculating disease-free survival(DFS,DFS is equal to PFS),the period from the date of obtaining informed consent to the date of the first appearance of loco-regional failure and/or distant failure of the disease was defined.All efficacy endpoints were measured from study entry to date of first failure or last follow-up visit for censored patient.
Results
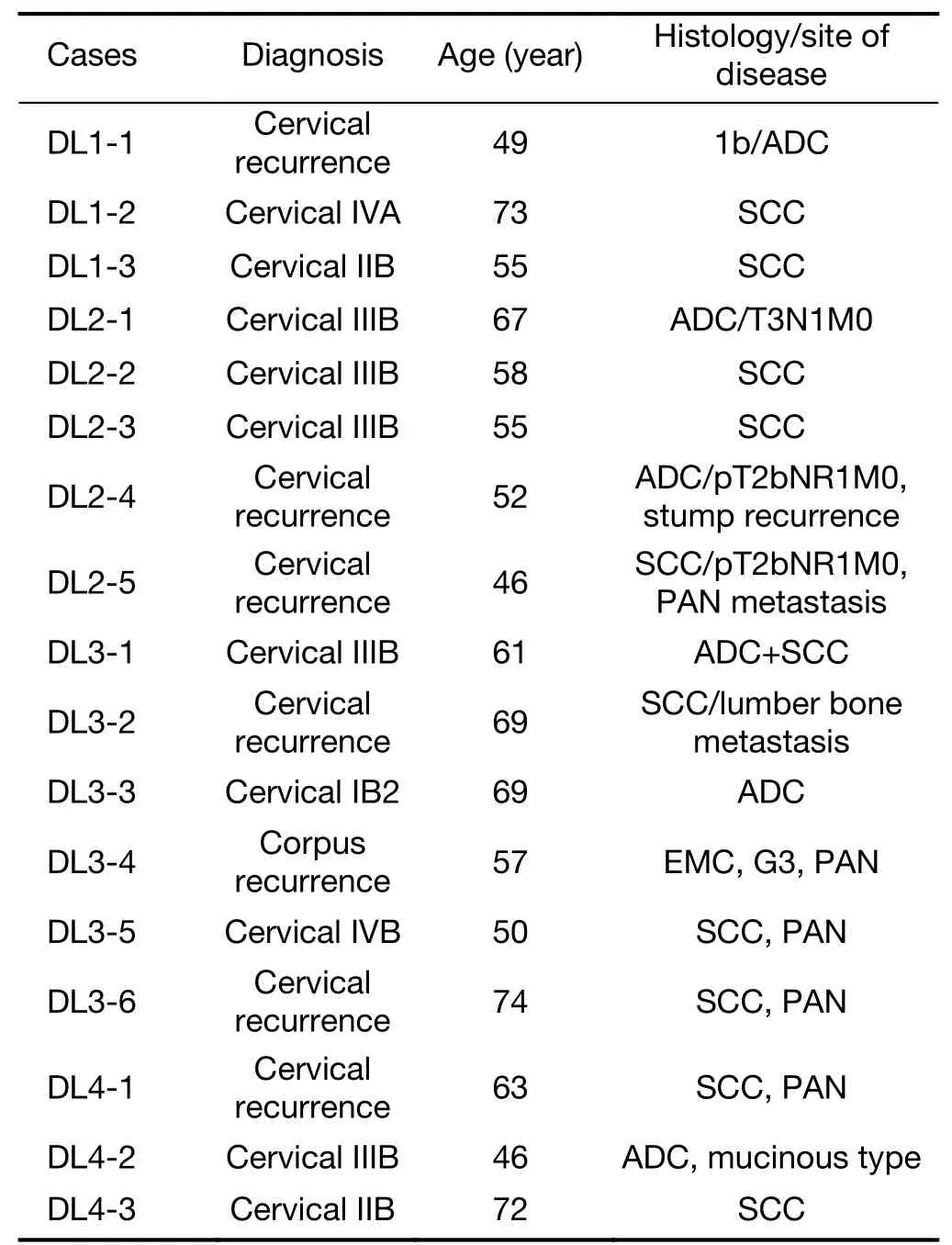
Table 3 Patients’accrual and characteristics
Patients’accrual diagram and results of Fibonacci are demonstrated inFigure 2.Figure 2demonstrates diagrams of overall accrual and result of DLT occurrence.Both DL1 and DL2 showed no DLT occurrence.During DL3,the second patient(DL3-2)showed DLTs(G3 diarrhea,G3 nausea,and G3 abdominal pain)and further three patients were accrual and they did not show any DLT.At DL4,first patient showed no DLTs.The second patient showed G3 abdominal pain and G3 diarrhea.The third patient showed G4 leukopenia and neutropenia lasting for more than 1 week.According to modified Fibonacci,the MTD was decided as DL4 60 mg/m2.Precise characteristics of patients are shown inTable 3.The median age was 58(range,46−74)years old.At the third patient had accrued in DL1,further entry was halted until the third patient finished her protocol treatment and identified all adverse event at one month after of treatment.From DL2,the accrual patients number changed from 3 to 6 to identify the DLTs.Because,in 2005,pharmatheutical alert of CPT-11 concerning to polymorphism aboutUGT1A1was announced in United States,however,the invader assay had not available in Japan during this period,we could not evaluate the polymorphism ofUGT1A1*6 and/or *28,so that double cohort system was used.As a result,there were no DLTs detected both in DL2 and DL3 patients.The 4th level(DL4)of dose patients,there was 2 DLTs out of 3 patients were detected and the MTD has decided as DL4 as 60 mg/m2.
Patients’clinical characteristics are shown inTable 3.Among seventeen patients in total,adenocarcinoma occupied 7(41.2%).All patients had unresectable and measurable bulky tumors and seven were recurrence.
Toxicity and AEs
As for hematologic toxicities,G3 and G4 neutropenia were detected in 3 patients(17.6%)and one G4 deserved as DLT in DL4.Grade 3 anemia was detected in 5 patients(29.4%)and no G3,G4 thrombocytopenia was detected.As for non-hematologic AEs,G3 diarrhea was detected in 2 patients(both 1 patient in DL3 and DL4)and both patients suffered from G3 abdominal pain at the same time.In total,5 patients(29.4%)were detected as G2 diarrhea but they all recovered to G1 during secession of administration of CPT-11.As for nausea and vomiting,5 patients complained over G1 nausea/vomiting,and 1 patient of DL3 needed admission and treatment(Table 4).The evaluation of DLT demonstrated that one out of six patients was in DL3(50 mg/m2),and two out of three in DL4(60 mg/m2).
Responses of treatment
Responses shown inTable 5are 9 complete response(CR)+3 partial response(PR)+3 stable diseases(SD)/17(88.2%)in all cases.The result showed that no progressive disease(PD)was detected.Disease control rate which was defined as CR,PR,SD per total cases were 88.2%.5/6(83.3%)in adenocarcinoma,10/11(90.9%)in SCC.Local control was completed in higher than DL2(40 mg/m2).Two cases of DL4 did not complete the protocol treatment and changed to RT alone,and they were not evaluable(NE)for response.
Pelvic lesions of this case achieved CR(DL3-1)are shown inFigure 3A(pre-treatment)andFigure 3B(after one month of treatment completion).
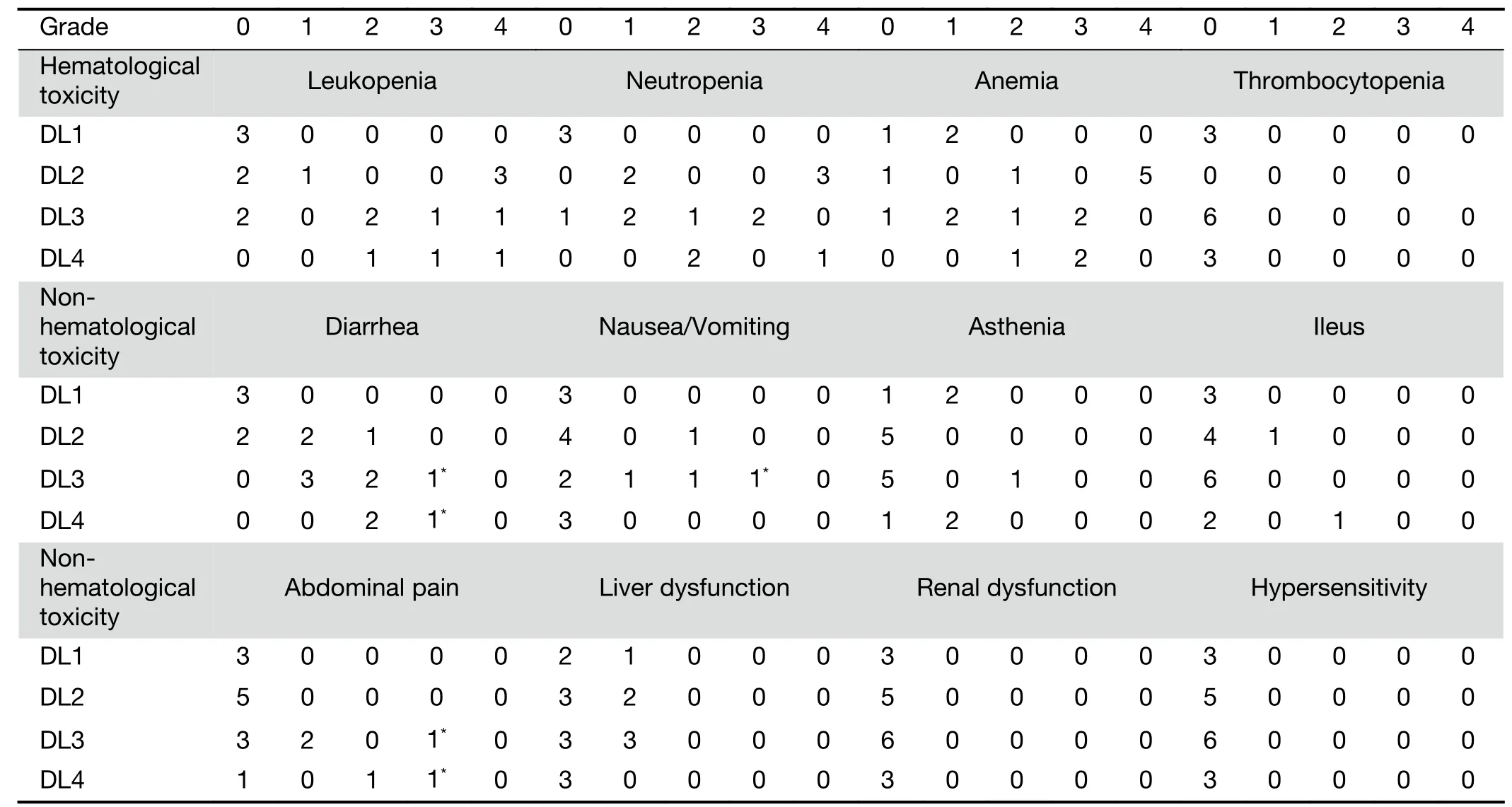
Table 4 Toxicity profiles assessed by NCI-CTCAE version 3.0
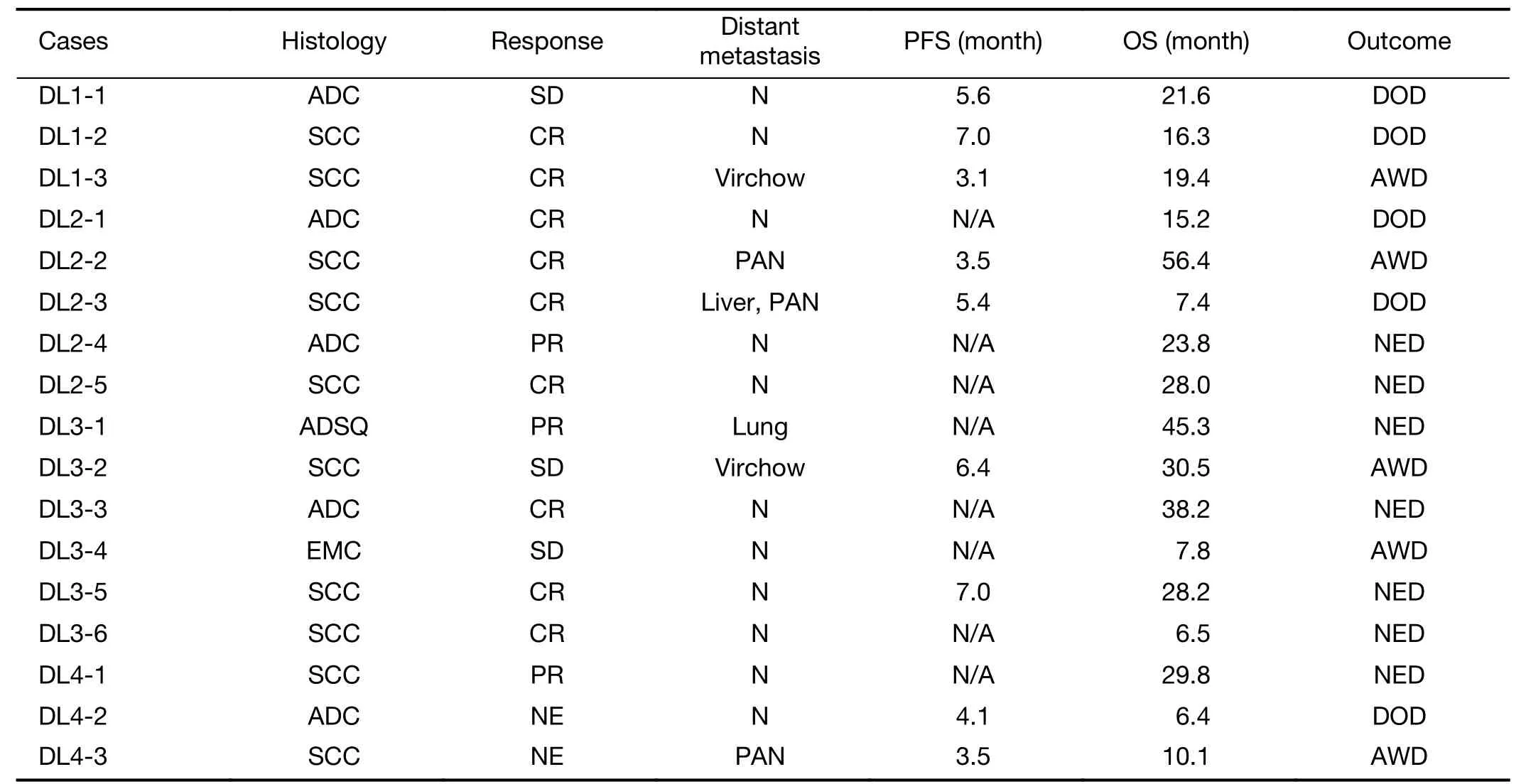
Table 5 Response of treatment
The median progression-free survival(mPFS)time and overall survival(mOS)time were 6.7[95% confidence interval(95% CI):6.1−16.8]months and 29.4(95% CI:13.6−28.7)months,respectively.The Kaplan-Meier curves are shown inFigure 4A,B.Histological subtype stratification analyses demonstrate that SCC showed longer PFS than adenocarcinoma but there are not significant differences by log-rank method(Figure 4C).There are no significant differences in OS(Figure 4D).
Discussion
It is an important issue to control the progression of unresectable uterine cancer.SCC is relative sensitive to both CT and RT,but for cervical adenocarcinoma and endometrial cancer,no effective treatment was found in CT,RT,and combination of CT and RT including CCRT.
We conducted this phase 1/2 study of CCRT using CPT-11 to resolve such issue.As a result of our study,the concurrent setting of CPT-11 and external RT was safe and effective at a dose of 50 mg/m2weekly administration for 4 or 5 weeks.The management of AE concerning diarrhea maybe necessary.We used herbal medicine,hangeshashintou(Tsumura,Tokyo,Japan)during irradiation period in prophylactic manner.Neutropenia was the second DLT of this protocol,but severe AE was not detected.This study was performed before checking rule according toUGT1A1polymorphism,single nucleoside polymorphism(SNP)in *6 and *28.The reports ofUGT1A1SNPs had been emerging during the trial period,in over DL2,patients’accrual was modified from 3 patients to 6 patients in one cohort.As a result,in DL2,five patients out of six were feasible,then dose escalation was advanced to DL3.In DL3,one patient experienced G3 diarrhea,G3 nausea/vomiting,and G3 abdominal pain.They were all recovered without complications by secession of administration of CPT-11.Eventually,only one patient showed DLT out of six patients,then trial had moved on DL5.In DL5,two patients showed DLTs out of three patients,and the MTD was decided as DL5,60 mg/m2.The AEs were G3 diarrhea and G3 abdominal pain in different patients.
This study was not a randomized study,so the case number was very small.Further analyses of stratifications such as the differences between SCC and adenocarcinoma had apparent biases,and it should be kept in mind as a reference,and of course,it was not statistically significant.Further study of randomized phase 2/3 in unresectable adenocarcinoma of uterus must be warranted.
The administration of CPT-11 must be early of the week because its active form of SN-38 concentration keeps effective level for three days due to re-uptake from intestine as so-called enterohepatic circulation.From this fact,some studies reported the effectiveness of twice administrations of a week of CPT-11in vivoanalyses using mice,but CPT-11 induces gastrointestinal toxicity including nausea/vomiting and anorexia.Twice a week administration would not be good for patients’quality of life.
From the pharmacokinetic point of view,alkalization of intestine did not decrease both concentration and area under the curve(AUC)(26).Reduction of diarrhea and neutropenia will be controlled by alkalization of intestine as well(27).However,the latter study was a retrospective study and there were no pharmacokinetic analyses and the relation of SN-38 AUC and neutropenia remains unclear.It must be elucidated the mechanism of reduction of neutropenia in prospective pharmacokinetic study.Actually,if two major AEs were suppressed,more escalation of CPT-11 dosage might be achieved and peripheral concentration and full-dose intensity(CPT-11 300 mg/m2/4 weeks)can be maintained.
In our study,CPT-11 plays a role of sensitizer in local irradiation area,and does not affect distal metastases.Actually,in this dose setting,systemic effect was not obtained,and recurrence at distal lesions had been detected in most of lower dose than MTD(60 mg/m2)(Table 4).In order to eliminate the distal micro-metastases,it would be necessary to dose escalation up to 100 mg/m2under fully prophylactic premedication intestinal alkalization or additional anti-cancer agents such as 5-fluorouracil(5-FU)or tegafur/gimeracil/oteracil(TS-1R).
As other combination with CCRT,paclitaxel and carboplatin had been reported that the combination showed better OS in adenocarcinoma than SCC,but the limitation of this study was that this study was retrospective,and the biases of patient numbers were limited(28).
Conclusions
This phase 1/2 study of CCRT using CPT-11 was feasible and effective in this cohort of patient.The recommended dose was 50 mg/m2weekly administration for 4 times during irradiation period.Further prospective randomized phase 2/3 study using CCRT with cisplatin,CCRT with paclitaxel/carboplatin doublet and CCRT with CPT-11 might be mandatory.Secondly,P1 dose escalation study using intestinal alkalization should be conducted concomitant withUGT1A1SNP examination.
Acknowledgements
This trial was partially supported by grant of Japanese Foundation for Multidisciplinary Treatment of Cancer(JFMC)for Cancer Research in 2005(26 th).
Footnote
Conflicts of Interest:These authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2020年2期
Chinese Journal of Cancer Research2020年2期
- Chinese Journal of Cancer Research的其它文章
- Evaluation and reflection on claudin 18.2 targeting therapy in advanced gastric cancer
- Oncometabolic surgery:Emergence and legitimacy for investigation
- Data mining-based model and risk prediction of colorectal cancer by using secondary health data:A systematic review
- Nomogram for prediction of pathologic complete remission using biomarker expression and endoscopic finding after preoperative chemoradiotherapy in rectal cancer
- p16/Ki-67 dual-stained cytology used for triage in cervical cancer opportunistic screening
- Analysis and external validation of a nomogram to predict peritoneal dissemination in gastric cancer
