Nomogram for prediction of pathologic complete remission using biomarker expression and endoscopic finding after preoperative chemoradiotherapy in rectal cancer
Hyuk Hur,Min Soo Cho,Woong Sub Koom,Joon Seok Lim,Tae Il Kim,Joong Bae Ahn,Hoguen Kim,Nam Kyu Kim
1Division of Colon and Rectal Surgery,Department of Surgery;2Department of Radiation Oncology;3Department of Radiology,Research Institute of Radiological Science;4Department of Internal Medicine,Institute of Gastroenterology;5Department of Medical Oncology;6Department of Pathology,Severance Hospital,Yonsei University College of Medicine,Seoul 03722,Korea
Abstract Objective:The aim of this study is to develop a nomogram for prediction of pathologic complete remission(pCR)after preoperative chemoradiotherapy(CRT)for rectal cancer.Methods:mRNA expression levels of seven molecular markers[p53,p21,Ki-67,vascular endothelial growth factor(VEGF),CD133,CD24,CD44]were measured by reverse transcriptase polymerase chain reaction(RTPCR)in 120 rectal cancers.Endoscopic findings of clinical complete remission(cCR)and biologic variables were used to construct nomogram in the training group(n=80),which was validated in the validation group(n=40).Results:mRNA expression levels of four markers(p53,p21,Ki67,CD133)correlated with pCR(24/80,30.0%)in the training group.Low expression of p53 and/or high expression of p21,Ki67 and CD133 showed greater pCR rate.pCR was shown in 18(69.2%)of 26 cases showing endoscopic cCR in the training group.Higher pCR rate was demonstrated in lower tumor location than middle tumor(19/49,38.8% vs.5/31,16.1%).A nomogram for prediction of pCR was developed from the multivariate prediction model using these six variables,which showed good discrimination ability in the training group[area under the curve(AUC)=0.945]and validation group(AUC=0.922).The calibration plot showed good agreement between actual and predicted pCR in both patient groups.Conclusions:Nomogram for assessment of pCR can be useful for making treatment decisions after CRT according to predicted responses.
Keywords:Rectal cancer;chemoradiotherapy;treatment response;molecular marker;endoscopy;nomogram
Introduction
Most expert groups and treatment guidelines have adopted preoperative chemoradiotherapy(CRT)as the preferred method to improve oncologic outcomes and allow performance of sphincter-preserving surgery through tumor downstaging in patients with advanced middle or low rectal cancer(1).The ideal outcome of CRT is complete response,in which the tumor is totally replaced by scar.The rate of pathologic complete remission(pCR)after preoperative CRT ranges from 12% to 16% for standard CRT(2,3)and can increase to 24%−30% with advanced regimens and targeted chemotherapy agents(4,5).Patients with significant response to CRT are expected to show better oncologic outcomes,and those with pCR potentially might even avoid major surgery(6,7).
To date,a number of tumor proteins are being studied as candidate predictive markers for prediction of response to CRT in rectal cancer.These proteins can be easily assessed by immunohistochemical(IHC)analysis or reverse transcriptase polymerase chain reaction(RT-PCR).In previous study,we demonstrated a scoring system based on IHC analysis of four tumor proteins(p53,VEGF,p21 and Ki67)that accurately predicted pCR in middle and low rectal cancer(8).Furthermore,we validated the significance of these biomarkers and provide more quantitative data by RT-PCR for comprehensive analysis of potential predictive markers(9).
It has also been suggested that endoscopic finding of gross tumor after preoperative CRT can be used as a tool for evaluation of tumor response,and this is supported by some studies with favorable outcomes(10,11).
Each modality is not enough as a predictive tool individually;therefore,an integrated use of various evaluation tools might provide comprehensive information about CRT response and yield better predictive outcomes.The combination of gross tumor finding and numerous potential molecular markers should provide a good predictive model for individualized treatment strategy.
This study aimed to develop a nomogram for prediction of pCR after preoperative CRT in rectal cancer based on mRNA expression of molecular markers measured by RTPCR and endoscopic findings.
Materials and methods
Patients
A total of 120 consecutive patients with rectal cancer were enrolled prospectively between May 2016 and April 2019.The inclusion criteria were histologically proven primary rectal adenocarcinoma clinically staged as T2N(+)M0 or T3−4NanyM0 and planned for preoperative CRT and curative resection.
Eighty patients were designated to the training group for development of the nomogram in serial order for prediction of pCR.For external validation of the nomogram,consecutive 40 patients were designated to the validation group in sequence of treatment time.
All patients underwent preoperative staging(clinical Tand N-staging)by magnetic resonance imaging(MRI).Clinical N(+)stage was defined as the presence of regional lymph nodes larger than 10 mm or with spiculated shape.Single experienced gastrointestinal radiologists reviewed all of the MRI studies and calculated the tumor volume.During this process,the tumor margin was manually traced,and the summation of each crosssectional image was processed on a workstation using imaging software(Aquarius workstation;TeraRecon,San Mateo,USA).The radiologists were blinded to clinical data and permanent pathologic results.
After inclusion and before starting preoperative CRT,all patients underwent rigid sigmoidoscopy and two-punch biopsy from the visibly deepest area of the tumor,which was preserved in frozen status and further used for RTPCR analysis.Low and middle rectal cancers were defined as lower tumor edge located<5 cm and 5−10 cm,respectively,from the anal verge by rigid sigmoidoscopy.
Endoscopy was performed before preoperative CRT and 4 weeks after completion of preoperative CRT.We defined the endoscopic findings of tumor after preoperative CRT and evaluated the treatment response.
This study was approved by the Institutional Review Board(IRB)for the protection of human subjects.
Preoperative CRT and surgery
All patients completed preoperative CRT in full and underwent curative surgery at 8 weeks after completion of CRT.
Radiation therapy was delivered using dual-photon linear accelerators at an energy level of 6-MV/10-MV.Longcourse radiation therapy included 25 fractions of 1.8 Gy each delivered to the pelvis over a period of 5 weeks(5 d per week),resulting in a total radiation dose of 45 Gy,followed by a 5.4-Gy boost targeting the primary tumor.
Chemotherapy was administered to all patients with two types of regimen:5-fluorouracil with leucovorin or xeloda only.Two cycles of intravenous bolus injection of 5-fluorouracil(425 mg/m2/d)and leucovorin(20 mg/m2/d)were administered for 5 d during the first and fifth weeks of radiation therapy.Xeloda was continuously administered orally at a dose of 1,450 mg/m2/d twice daily during the radiation therapy period.
Quantitative real-time PCR
Tissue samples which have tumor content more than 90%were analyzed.Total RNA was isolated from frozen samples and used for RT-PCR.We extracted total RNA with an RNeasy plus mini kit(Qiagen,Valencia,USA)according to the manufacturer’s instructions.cDNA was synthesized using a Transcriptor first-strand cDNA synthesis kit(TaKaRa Bio Inc.,Otsu,Japan)and 1 μg of total RNA.We conducted real-time PCR in duplicate using TaqMan master mix(Applied Biosystems,Foster City,USA)and the Applied Biosystems ViiA7 Real-Time PCR System.Seven biomarkers(P53,P21,Ki67,VEGF,CD133,CD24,CD44)were chosen as candidates from our previous research findings(8)and published data,and mRNA expression levels were investigated.mRNA expression levels ofTP53(assay ID Hs01034249_m1),CDKN1A(assay ID Hs00355782_m1),MKI67(assay ID Hs01032443_m1),vascular endothelial growth factor A(VEGFA)(assay ID Hs00900055_m1),PROM1(assay ID Hs01009250_m1),CD44(assay ID Hs01075861_m1)andCD24(assay ID Hs03044178_g1)were normalized to that of the glyceraldehyde-3-phosphate dehydrogenase(GAPDH)housekeeping gene(assay ID Hs02758991_g1).We performed relative quantification using the QuantStudio software v1.2(Applied Biosystems,ThermoFisher Scientific,USA).Expression level of each mRNA was indicated by ΔCt compared to expression ofGAPDH(ΔCt=target Ct—GAPDH Ct).The quantity of mRNA in pCR tissue relative to that in non-pCR tissue was calculated from the relative ratios of 2−ΔCtbetween the two conditions.Lower ΔCt and higher 2−ΔCtvalues indicate higher expression of each mRNA.
Endoscopic findings
Endoscopic gross tumor findings after preoperative CRT were classified according to morphologic characteristics.No visualization of tumor,white scar,or red scar were defined as clinical complete remission(cCR),whereas ulcerations and remaining masses of any size were defined as non-cCR(Figure 1).The endoscopic findings were interpreted by two independent endoscopists who were blinded to the patients’clinical information.The consistency of the endoscopic findings between the two independent observers was greater than 90%.In cases of disagreement,the endoscopic finding was determined by consensus.
Assessment of tumor response to CRT and histopathology
A single pathologist reviewed all of the surgical specimens.The 7th American Joint Committee on Cancer(AJCC)TNM staging manual was used.T-downstaging was observed if pathologic T-stage(pT)was lower than clinical T-stage(cT)in the same patient.In patients who initially presented with clinically positive lymph nodes[cN(+)],Ndownstaging was defined as conversion to pathological lymph node negative status[pN(−)].The tumor response to preoperative CRT was evaluated using the Mandard’s tumor regression grade(TRG)(12).Tumors classified as TRG 1 or TRG 2 were considered to be responders,whereas tumors staged as TRG 3−5 were defined as nonresponders.pCR was defined as no viable tumor(TRG 1)with no lymph node involvement(pN0).
Statistical analysis
Fisher’s exact or χ2tests were used for categorical data analysis.Two-sidedt-test or Mann-Whitney test was used for continuous variables.The significance between each variable and pCR was analyzed using univariate logistic regression analysis and selected variables were used for subsequent multivariate logistic regression analysis.The final multivariate model was developed for the pCR prediction model and tested by Hosmer-Lemeshow goodness of fit test for evaluation of prediction ability.A nomogram for prediction of pCR was developed using the multivariate model with predictive variables.The discrimination and calibration performance were quantified for evaluation of the mode performance.Discrimination is the predictor’s ability to distinguish between pCR and nonpCR.Discrimination ability was measured by the area under a receiver operating characteristic(ROC)curve;a higher ROC area under the curve(AUC)means a better discriminatory power.An AUC of 0.50 means that the test is as good as random chance for discriminating an outcome,whereas an AUC of 1.0 means perfect discrimination of the rest(sensitivity and specificity of 100%).In general,the model is considered relatively good for values greater than 0.75.Calibration is the agreement between actual probability and predicted probability of pCR produced by the model.This was evaluated with a calibration curve,where patients were grouped by predicted pCR and then plotted as actual versus predicted pCR.We used the bootstrapping resampling method(300 repetitions)to obtain relatively unbiased estimates and to assess internal validation.Both discrimination and calibration were evaluated in the training and the validation set.All statistical analyses were performed using IBM SPSS statistics(Version 20;IBM Corp.,New York,USA)and R software version 3.0.1(the R foundation for statistical computing,Vienna,Austria).Values of P<0.05 were considered statistically significant.
Results
Patient characteristics and clinical variables
A total of 120 consecutive patients were included in this study.Detailed patient characteristics for the training and validation sets are listed inTable 1.Before starting treatment,the majority of patients in the training set presented with advanced T-stage and N-stage:cT3−T4 stage was diagnosed in 97.5% and cN(+)in 81.2% of patients(Table 1).
In the training set,good response to preoperative CRT(TRG 1 and TRG 2)was observed in 50.0% of patients,and pCR was revealed in 30.0% of patients.The clinical and pathologic characteristics of patients in the validation set and training set were similar(Table 1).
Univariate analysis of different clinical variables in the training set revealed that tumor location in the lower rectum was significantly associated with pCR and TRG response.Tumors located in the lower rectum demonstrated significantly higher rates of pCR and TRG 1−2 than middle rectal tumors(Table 2).
Endoscopic findings and correlation with pCR
Endoscopic findings demonstrated a significant correlation between cCR and pCR or TRG 1−2 in the training set.pCR was shown in 18(69.2%)of 26 cases defined as endoscopic cCR.Non-pCR was shown in 48(88.9%)of 54 cases defined as endoscopic non-cCR.Endoscopic findings demonstrated 75.0% sensitivity and 85.7% specificity for prediction of pCR(Table 2).
Analysis of mRNA expression according to preoperative CRT responses
In the training set,the mRNA expression levels of the studied biomarkers were not significantly different between patients who did or did not develop T-or N-downstaging.However,mRNAs of four biomarkers(p53,p21,Ki-67 and CD133)were differentially expressed between patients who had pCR and those who did not develop pCR,as well as between responders to CRT(TRG 1−2)and nonresponders(TRG 3−4).Responders to CRT and patients with pCR demonstrated significantly higher ΔCt and lower 2−ΔCtof p53 mRNA,but lower ΔCt and higher 2−ΔCtof mRNA of p21,Ki-67,and CD133(Table 3).
Logistic regression model for prediction of pCR
In univariate and multivariate logistic regression analyses of the training set,tumor location,endoscopic findings,low expression of p53 mRNA,and high expression of p21,Ki67,CD133 mRNA were significantly associated with pCR(Table 4).These variables were selected for further analysis as possible predictors of pCR to CRT.
Using logistic regression analysis,prediction models for pCR were suggested based on these six clinical and biological variables(Table 5).The mRNA expression of each biomarker was presented as a ΔCt value.Of three prediction models,model 3 with all six variables demonstrated the highest AUC.The Hosmer-Lemeshow goodness of fit test was not significant(P=0.984),indicating good fit of this model.
Nomogram for prediction of pCR
The prediction model including p53,p21,Ki67,CD133,endoscopy and tumor location to predict the probability of pCR was visually represented by a nomogram(Figure 2).From each variable’s value,a vertical line can be drawn to the top point row and points for each variable are assigned.The sum of each variable’s value indicates the total points.A vertical line is drawn downward from the total points row to obtain the probability of pCR.According to the nomogram,patients with total points of 210,240 and 270 had estimated pCR probability of 10%,50% and 90%,respectively.Figure 3A,Bdemonstrate the ROC curve and calibration plot of the nomogram in the training set.Area under the ROC curve of the multivariate model was 0.945[95% confidence interval(95% CI):0.900−0.989]with good significance.The calibration plot showed good agreement between predicted and actual probability.In theexternal validation set,the AUC was 0.922(95% CI:0.841−0.999)with good significance.The calibration plot of predicted and actual probability showed good correlation in low and high probability areas.However,it appeared to have poor correlation in the middle probability area because the intermediate probability could be ambiguous for binary variables(Figure 3C,D).
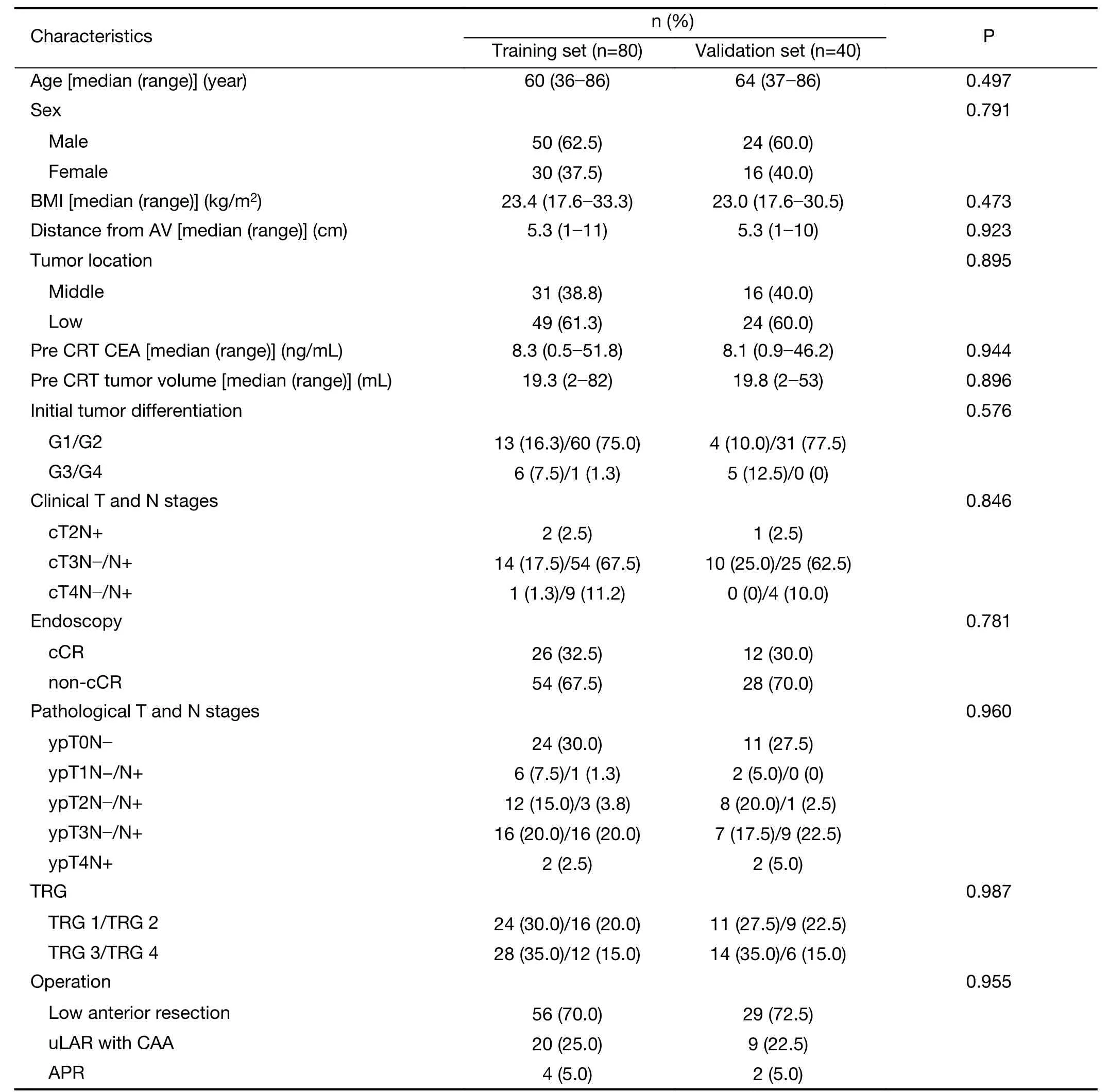
Table 1 Patient characteristics
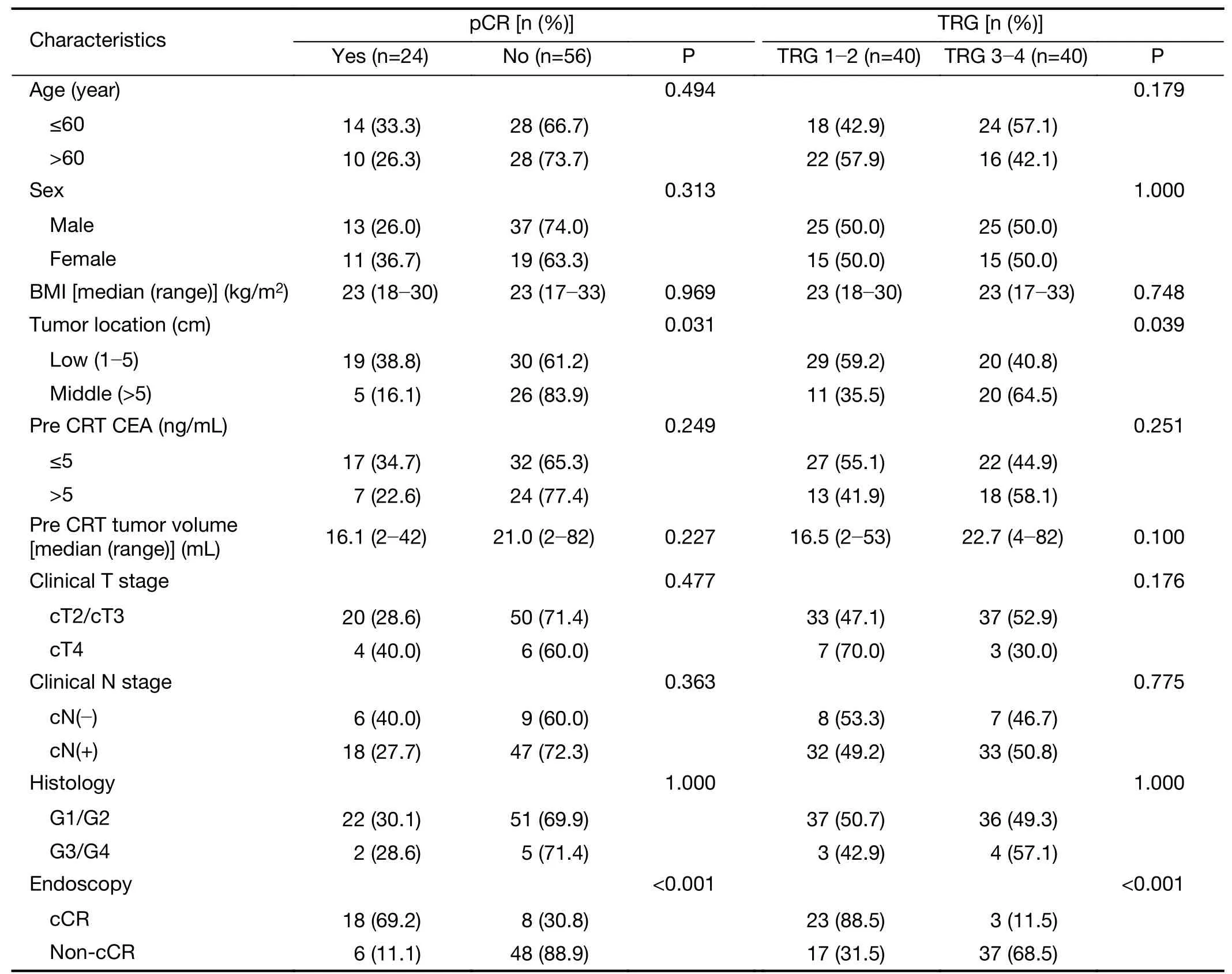
Table 2 Univariate analysis of clinical variables associated with pCR and TRG in training set(n=80)
Discussion
After preoperative CRT,various tumor responses are presented from complete remission to still remaining tumors.Complete remission is shown in about 10%−30%of patients,and 60% show a partial tumor response and T and N downstaging,although some tumors show a poor response and no downstaging.If CR can be predicted,less invasive surgery or close follow-up may be considered to avoid radical resection in some cases(13).
Numbers of diagnostic tools have been investigated and proposed for assessment and prediction of responses to CRT.For gross evaluation of tumor response after preoperative CRT,endoscopy,MRI and positron emission tomography(PET)have been investigated and demonstrated variable results.Furthermore,numbers of molecular markers have been studied for assessment and prediction of tumor response to preoperative CRT in rectal cancer.However,the evidence is insufficient,and these potential predictive markers cannot be used in clinical practice.Numbers of candidates still remain a hope for the future.
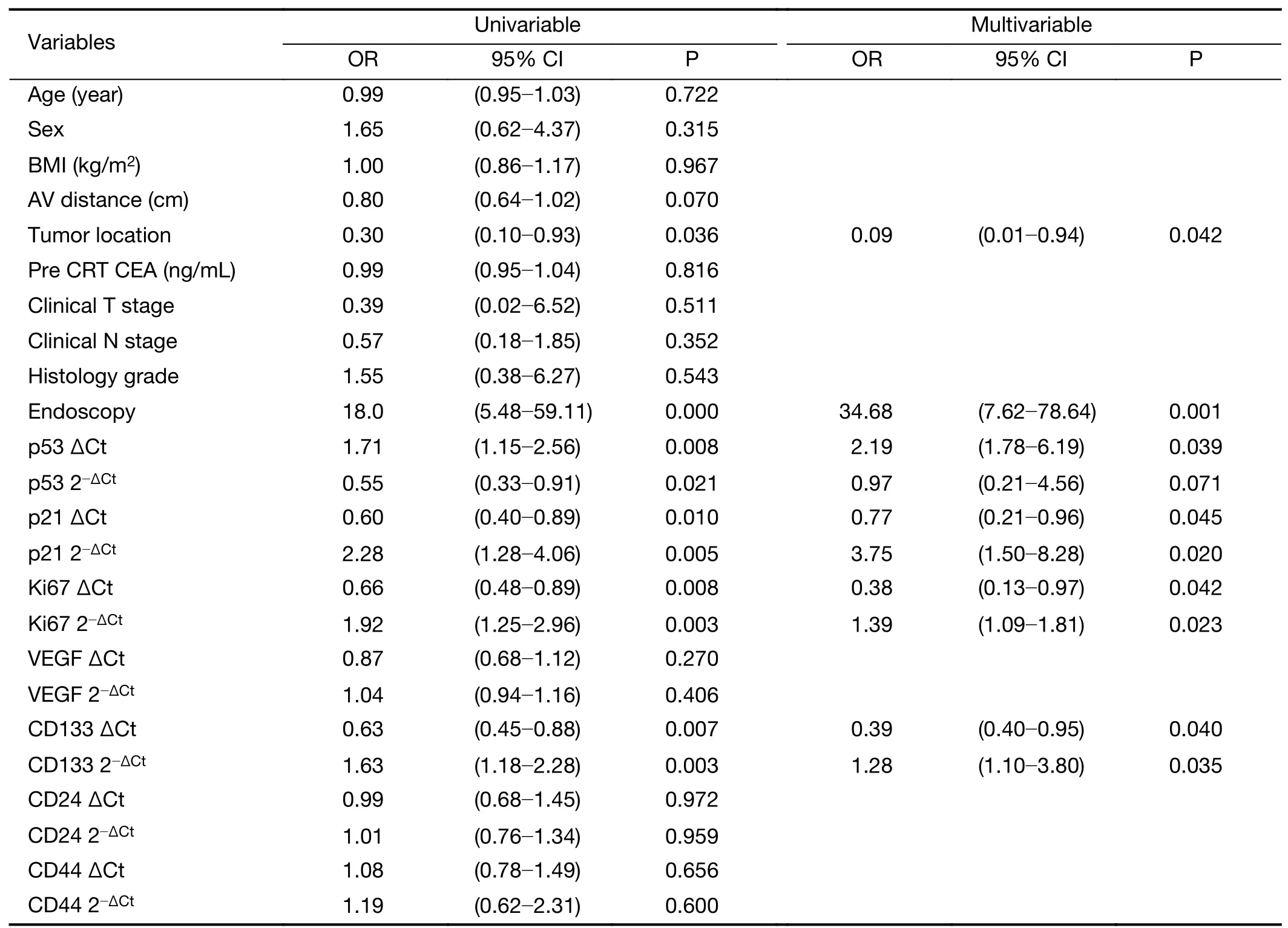
Table 4 Logistic regression analysis of clinical variables and biomarker expression in tumor tissue by RT-PCR for assessment of pCR in training set(n=80)
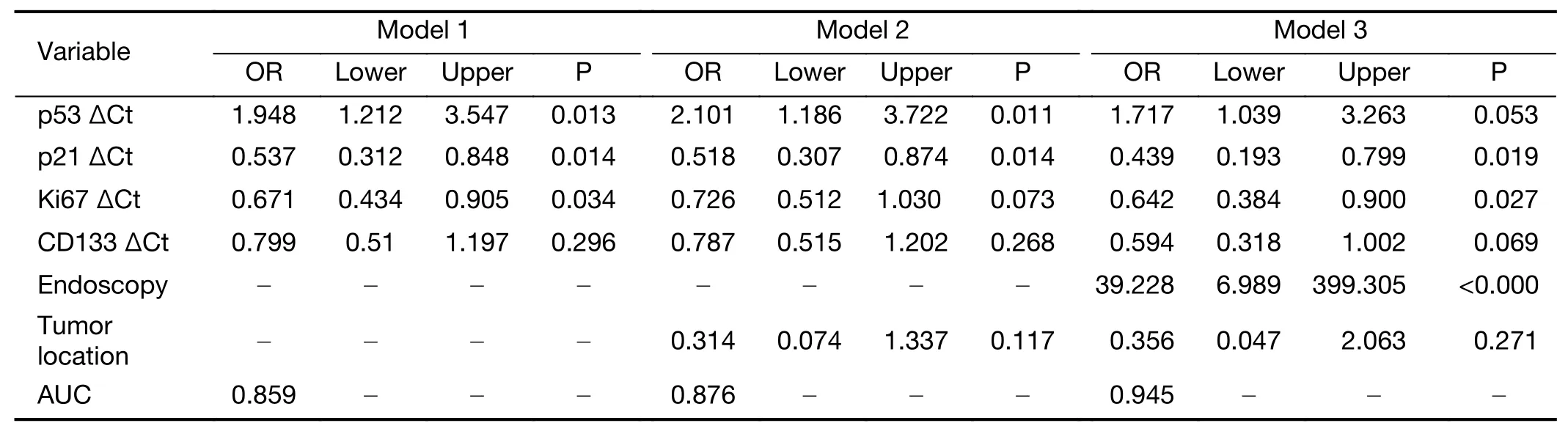
Table 5 Logistic regression model using biomarker expression in tumor tissue by RT-PCR and endoscopic findings for assessment of pCR in training set(n=80)
In the present study,we developed a nomogram combining endoscopic findings of morphometric tumor changes and mRNA expression levels of four biomarkers(p53,p21,Ki67 and CD133)by quantitative RT-PCR to predict pCR in rectal cancer patients receiving preoperative CRT.This predictive model was externally validated and showed good performance in terms of calibration and discrimination.We believe that this comprehensive nomogram is useful for pCR prediction and could provide guidance for individualized treatment.
Previously,we reported a scoring system that used the levels of expression of p53,VEGF,p21 and Ki67 measured by IHC analysis(8).These proteins were chosen from a panel of 12 markers as having a significant correlation between expression level and pCR in 81 patients.Although this technique is widespread and easily available,real-time RT-PCR is known as a more reliable and reproducible method that allows quantification of gene expression(14).Therefore,we evaluated mRNA expression levels of 7 candidate biomarkers and 4 biomarkers(p53,p21,Ki67 and CD133)showed the significant correlation with pCR(8).In this study,we added endoscopic findings as a clinical tool for evaluation of morphometric changes of the tumor after preoperative CRT.The classification according to morphologic characteristics should be generalized in largescale series.However,gross tumor response findings would be valuable assessment modalities for prediction of pathologic tumor response.In our study,we demonstrated the importance of endoscopic evaluation of primary tumor after preoperative CRT for rectal cancer.Although the number of patients studied is rather small,one of the strengths of this study is its prospective nature.In contrast to our previous study,all consecutive patients in the study period were included,thus overcoming the influence of selection bias inherent in retrospective studies.
In addition to p53,VEGF,p21 and Ki67 from our previous study(8),three cancer stem cell proteins(CD133,CD24,CD44)were selected as potential candidate biomarkers.
Among the significant biomarkers identified in the present study,tumor protein p53 is probably the most extensively studied biomarker in this field,and a number of studies have shown that the presence of p53 mutation correlates inversely with pCR,although this association was not significant in all studies(8,15-17).The results of our present investigation confirm the findings from our previous research(8)and other research groups(16,17)that low expression of mutant p53 is predictive of tumor response to CRT and pCR.
The biomarkers p21 and Ki67 are related to inhibition of the cell cycle and high proliferative activity of tumor cells,respectively.However,their clinical significance in rectal cancer is still unclear as controversial data have been reported to date(18-23).Our present work is consistent with our previous IHC study and showed that high expression of p21 and Ki67 is strongly associated with pCR.
Cancer stem cells show the resistance to chemotherapy and radiotherapy.CD133,CD44,and CD24 are wellknown colorectal cancer stem cell markers.Therefore,these cancer stem cell markers have been investigated to determine the correlation with the treatment response to CRT in patients with rectal cancer(24-31).CD133 expression indicates the existence of cancer stem cells,and Sprengeret al.reported that a high expression level of CD133 in rectal cancers treated with preoperative CRT demonstrated a high recurrence rate and poor survival outcomes(32).Another stem cell marker CD24 was also reported to be related with invasiveness and differentiation in colorectal cancer(26).Huhet al.investigated 13 molecular markers and only highly measured CD44 mRNA level in pretreatment biopsies was significantly associated with poor tumor response after CRT.Otherwise,CD133 and other stem cell markers demonstrated no significant association with tumor response(24).In contrast,in our study,high expression of CD133 mRNA was associated with better response to CRT and higher pCR rate.Currently,the association between colorectal cancer stem cell markers and CRT response is not firmly established.Numbers of studies presented the possibility and need for further investigation.
In this study,endoscopic findings after preoperative CRT were significantly correlated with the pathologic tumor response.However,endoscopic findings alone lead to false positive and negative predictions.In the total set of 120 patients,26(68.4%)of 38 patients with endoscopic CR showed pCR,and 12(31.6%)patients showed non-pCR.Of 35 patients showing pCR in 120 patients,26(74.3%)patients were endoscopic CR and 9(25.7%)patients were not CR.More comprehensive approach with various tools is needed to improve the accuracy of tumor response prediction.The endoscopic and pathologic CR group showed a lower p53 and higher p21,Ki67,CD133 expression than the endoscopic CR and non-pCR group.Of 82 patients with endoscopic non-CR,nine showed pCR,and 73 showed non-pCR.The endoscopic and pathologic non-CR group showed higher p53 and lower p21,Ki67,CD133 expression than the endoscopic non-CR and pCR group(Table 6).Therefore,the endoscopic findings couldbe complemented by inclusion of biomarker expression to reduce false results.
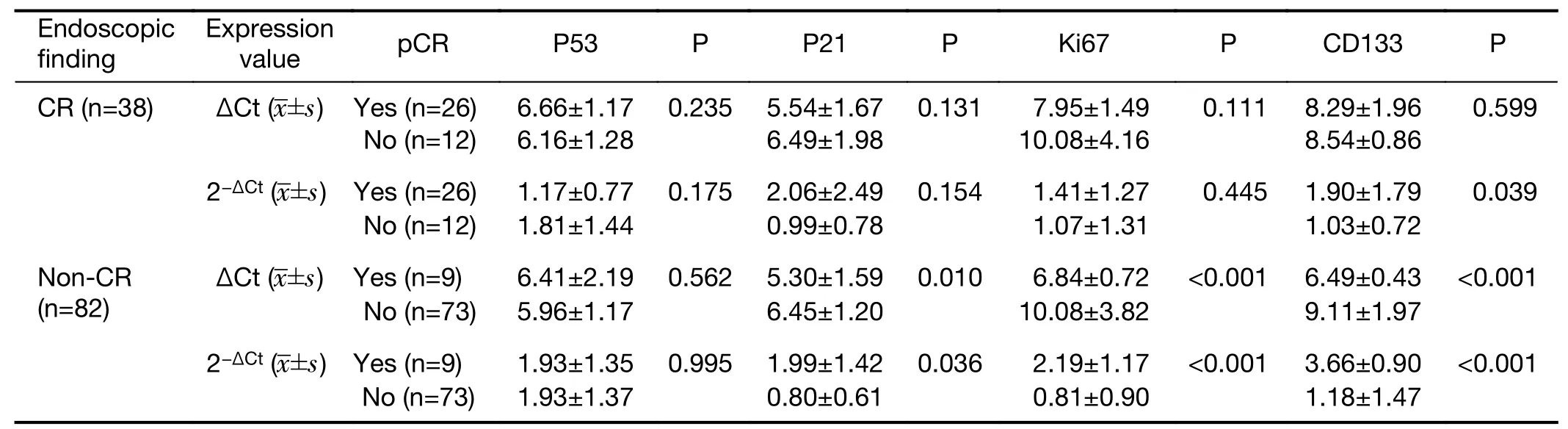
Table 6 Relative quantity of biomarker mRNA in tumor tissue according to pCR and non-pCR in endoscopic CR and non-CR groups(N=120)
To date,various models or nomograms for prediction of CRT response in patients with rectal cancer have been presented from several studies(33-35).Van stiphoutet al.(36)suggested a nomogram predicting pCR after CRT for locally advanced rectal cancer using clinical features and early sequential18F-FDG PET-CT imaging.The pCR rate was 21.4% in the training set(n=112)and 23.1% in the validation set(n=78).The nomogram was presented based on the selected predictive values including cT-stage,cNstage,response index of SUVmean,and maximum tumor diameter during treatment.The AUC was calculated to evaluate the model performance.AUC were 0.78 in training group and 0.70 in validation group.According to the measured probabilities for pCR,several groups were assigned.The high probability group demonstrated correct predictions for pCR in 100% for the training set and 67%for validation sets,respectively.
Jwaet al.(37)investigated clinical features correlated with ypN status after preoperative CRT in rectal cancer.Patient age,tumor differentiation,cN stage,ypT stage,lymphovascular invasion,and perineural invasion were significantly associated with LN status after CRT.These clinical values were used for development of the nomogram for prediction of ypN status in a training cohort(n=891).A separate cohort(n=258)was used for validation of the established nomogram.The nomogram showed good discrimination ability in training and validation cohorts.The calibration plot demonstrated good agreement between actual and predicted LN status after preoperative CRT.
Nomograms for prediction of treatment response have also been investigated in breast cancer(38-40).Keamet al.(40)combined clinical pathologic variables of 370 breast cancer patients that are associated with pCR after neoadjuvant chemotherapy into a prediction nomogram.Clinical variables included initial tumor size,estrogen receptor,human epidermal growth factor receptor 2 and Ki67.The nomogram based on these variables demonstrated good discrimination performance(AUC=0.830).The agreement between predicted and observed outcomes was good in the low probability area of calibration plot.However,in the high probability area,the predicted probability of pCR did not show a good agreement with the observed probability of pCR.This result may be from the relatively low pCR rate(32 of 370 patients,8.6%).
Our prediction nomogram demonstrated good performance of discrimination and calibration abilities in the training set.However,despite good discrimination ability in the validation set,the calibration plot did not show perfect agreement;there was good correlation in low and high probability of pCR but not in the middle probability area.If the tumor response is definitely good or bad(TRG 1 or TRG 4),the actual and predicted probability will be correlated well and calibration results is good.But,moderate tumor response(TRG 2 or 3)compared with definitely good or bad(TRG 1 or 4)response may be not correlated well and calibration results are poor.This indicates the need for a more reliable prediction model for those with intermediate scores.
Endoscopic morphometric change is easily accessible,and the RT-PCR approach is less time consuming and more reliable than IHC analysis.Our prediction model demonstrated high discrimination and calibration abilities in identifying patients who are likely to develop pCR and thus have a favorable predicted outcome and might be able to avoid major surgery.However,this was a monocentric study,before implementation of this prediction model in practice,it should be tested and validated on another large independent patient cohort from multi-centers.This study is planned as the next step in our work.
Conclusions
The present research demonstrated the benefit of the developed model in prediction of pCR after preoperative CRT in patients with rectal cancer.Our prediction nomogram encompassed clinical and biological factors including tumor location,endoscopic findings,and four biomarkers and achieved high sensitivity and sufficient accuracy in prediction of pCR.This finding suggests that cancer sensitivity to CRT should be evaluated as a complex system,rather than as separate processes.We used a combination of biomarkers and morphometric endoscopic findings that showed distinct patterns of expression and features between pCR and non-pCR and developed a prediction nomogram.Despite certain limitations,this study demonstrated the importance of a multidirectional approach and the utility of a prediction nomogram for identifying patients likely to show pCR and development of treatment strategies.
Acknowledgements
This study was supported by faculty research grant(No.6-2016-0060)from Yonsei University College of Medicine.
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2020年2期
Chinese Journal of Cancer Research2020年2期
- Chinese Journal of Cancer Research的其它文章
- Evaluation and reflection on claudin 18.2 targeting therapy in advanced gastric cancer
- Oncometabolic surgery:Emergence and legitimacy for investigation
- Data mining-based model and risk prediction of colorectal cancer by using secondary health data:A systematic review
- Phase 1/2 study of concurrent chemoradiotherapy with weekly irinotecan hydrochloride for advanced/recurrence uterine cancer:A multi-institutional study of Kansai Clinical Oncology Group
- p16/Ki-67 dual-stained cytology used for triage in cervical cancer opportunistic screening
- Analysis and external validation of a nomogram to predict peritoneal dissemination in gastric cancer
