Mucosa-associated lymphoid tissue lymphoma simulating Crohn’s disease: A case report
Ieva Stundiene, Vaidota Maksimaityte, Valentina Liakina, Jonas Valantinas, Centre of Hepatology, Gastroenterology and Dietetics, Faculty of Medicine, Vilnius University, Vilnius 03101, Lithuania
Valentina Liakina, Department of Chemistry and Bioengineering, Faculty of Fundamental Science, Vilnius Gediminas Technical University, Vilnius 10223, Lithuania
Valentina Liakina, Clinic of Gastroenterology, Nephrourology and Surgery, Faculty of Medicine, Vilnius University, Vilnius 03101, Lithuania
Abstract
BACKGROUND
Differential diagnosis between extranodal marginal zone lymphoma of mucosaassociated lymphoid tissue and inflammatory bowel disease is mainly based on histopathologic evaluation of intestinal biopsies, although there is no single definitive diagnostic investigation and that circumstance can lead to misdiagnosis in particular cases. Herein we present a rare, ulcerative form of marginal zone lymphoma which mimics the Crohn’s disease (CD) of upper digestive tract.
CASE SUMMARY
A 50-year-old man was presented with recurrent episodes of malaise and melena also weight loss. Enteroscopy of the small bowel demonstrated an ulcer in the jejunum. Microscopically, biopsies showed lymphoplasmacytic infiltrate.Diagnosis of CD was made. Primary treatment consisted of prednisone and azathioprine and was followed by azathioprine 100 mg per day with good clinical response in the following 2 years until relapse. At this time the results of endoscopic biopsies derived from proximal wall of stomach revealed Helicobacter pylori-negative marginal zone lymphoma of the gastric fundus.Immunophenotyping confirmed atypical CD20-positive cell population. Based on these biopsies, marginal zone lymphoma of mucosa-associated lymphoid tissue was diagnosed. Unfortunately, the contact with the patient was lost until one year later he was hospitalized with nausea, vomiting and severe pain because of gastrointestinal perforation. Four months later after laparotomy, the patient was treated with a course of chemotherapy. Complete remission was observed following 6 cycles of treatment.
CONCLUSION
This case report highlights the clinical relevance of knowledge and awareness of marginal zone lymphoma simulating CD.
Key words: Crohn disease; Mucosa-associated lymphoid tissue lymphoma; Rare diagnosis; Gastrointestinal diseases; Thiopurines; Case report
INTRODUCTION
Isaacson and Wright[1]first identified extranodal marginal zone lymphoma of mucosaassociated lymphoid tissue (MALT) lymphoma in 1983. It is a type of non-Hodgkin's lymphoma and it is defined as an extranodal lymphoma composed of morphologically heterogeneous small B-cells, including marginal (centrocyte-like) cells, a cell resembling monocytoid cells, small lymphocytes, scattered immunoblasts, and centroblast-like cells[1]. MALT lymphomas commonly follow dysregulation of the immune system triggered by sustained interference with chronic infections or autoimmune disorders.
Like every other gastrointestinal lymphoma, MALT lymphoma can be clinically confused with inflammatory bowel disease (IBD). Both of them can cause similar gastrointestinal symptoms such as abdominal pain, diarrhea, exhaustion, weight loss and malnutrition and lack of specificity in the clinical characteristics. On the other hand, they have other differences, including localization of the disease. Crohn’s disease (CD) can involve any segment of gastrointestinal tract from mouth to anus,especially the terminal ileum and ileocecal region[2]. Rarely, CD may affect the stomach or duodenum, sometimes-even esophagus[3]. Low-grade lymphomas can develop in every organ or cavity of the body that is lined by mucosa, although MALT is most often diagnosed in gastrointestinal organs of all non-Hodkin lymphomas, 5%-10%[4-6]. Even though, the primary gastric lymphoma still is a rare disease[7].
While endoscopy is the first choice for detecting lesions endoscopic findings are very important in differential diagnosis. Ulcers in CD patients tend to be longitudinal and stretched across several intestinal folds and ulcers in patients with lymphoma are usually characterized as irregular ulcers[8].
These two pathologies require specific treatment and provide different prognosis to the patient. The differentiation between these two pathologies sometimes is only based on histopathologic evaluation of intestinal biopsies.
The situation is further complicated because there is no single definitive diagnostic investigation or laboratory test to exist for the diagnosis of IBD[9]. All of the above leave the gastroenterologists at risk of making misdiagnosis of such patients.
While only a few cases of MALT lymphoma mimicking a CD have been reported,the goal of this case report is to promote the more attentive attitude of gastroenterologists to the rare cases of lymphomas. In connection with this, we want to encourage practitioners not to miss an unusual MALT lymphoma presentation like herein described case of 52-year-old patient with a previous diagnosis of small intestine CD who was later diagnosed for stomach and jejunum MALT lymphoma.
CASE PRESENTATION
Chief complaints
A 50-year-old Caucasian man was pre sented to our hospital with episode of malaise and melena.
History of present illness
He had reportedly lost 6 kg of weight during one year and had episodic abdominal cramping pain.
History of past illness
He had gastric ulcer disease diagnosed several years before.
Personal and family history
He had no other symptoms and no family history of gastrointestinal diseases or cancer (Figure 1).
Physical examination
Upon physical examination, no abnormal findings were found.
Laboratory examinations
Complete blood count results were as follows: Red blood cells, 3.14 × 1012/L [(4-5.5) ×1012/L]; hemoglobin, 87 g/L [(120-160) g/L]; white blood cells, 6.56 × 109/L [(4-10)×109/L]; platelets, 151 × 109/L [(100-300) × 109/L]. Biochemical examination revealed CRP 0.2 mg/L [(0-5) mg/L]. Serum albumin was not measured at this time.
Imagining examinations
Upper gastrointestinal endoscopy revealed ulceration of mucosa in antrum. There were a few 5 mm superficial ulcers in the antrum (Figure 2). There were no specific findings in colonoscopy. Enteroscopy of the small bowel demonstrated a 1.5 cm ulcer in the jejunum (Figure 3).
Histological findings
Microscopically, stomach biopsies showed lymphoplasmacytic infiltrate with neutrophils and eosinophils, also a detritus with leukocytes and granulation tissue of one of the ulcers where biopsy was taken from. Biopsies were negative forHelicobacter pylori (H.pylori) infection.
Microscopically, jejunum biopsies revealed lymphoplasmacytic infiltrate, a common finding in chronic CD, granulation tissue and crypt distortion suggesting chronic inflammation (Figure 4). Histopathology suggested the diagnosis of CD. Since there were no histological evidences of possible lymphoma, imunophenotyping was not performed at this time.
Primary diagnosis, treatment, and further diagnostic work-up
Diagnosis of CD was made. Primary treatment consisted of prednisone and azathioprine and was followed by azathioprine 100 mg per day.
Two years later, he had another melena episode and was hospitalized again.Laboratory testing indicated anemia - hemoglobin, 98 g/L (120-160 g/L). Lactate dehydrogenase, ferritin, electrolytes, glucose, renal function tests were all normal.CRP level was high 162.4 mg/L (0-5 mg/L). The patient was subjected to endoscopic esophageal gastroduodenoscopy. It revealed atrophic mucosal tissue interposed between normal appearing mucosa on the posterior wall of the stomach. Biopsy samples were acquired. The lumen along with mucosa of esophagus and duodenum were normal. Abdominal ultrasonography displayed a segmental ileum wall thickening. Enlarged lymph nodes (11 mm) were found in the lower part of the abdomen. No liver pathology was showed. Small bowel magnetic resonance imaging(MRI) enterography revealed local circumferential infiltration of the proximal part of the ileum. In addition, a mesenteric nodal mass was presented.
However, despite all the clinical details, the diagnosis of MALT lymphoma was hesitated. With the clinical picture and radiology findings, still not knowing results of the biopsy, it was decided to increase the dosage of azathioprine from 100 mg to 150 mg daily and discharge the patient from the hospital. CRP was 11.4 mg/L on the day patient was released from the hospital.
One week later, results of endoscopic biopsies derived from proximal wall of stomach revealedH. pylorinegative MALT lymphoma of the gastric fundus.Immunophenotyping of biopsies confirmed a B-cell population with the surface proteins listed in Table 1[10]. Based on these biopsies, MALT lymphoma was diagnosed.
Unfortunately, the contact with the patient was lost and he failed to get any treatment due to MALT lymphoma diagnosis at this time.
One year later he was hospitalized with nausea, vomiting and severe pain in the upper part of the abdomen. Physical examination revealed peritonitis. Computed tomography (CT) scan showed ileus of the small intestine and extraluminal air within the peritoneal cavity which led to gastrointestinal perforation diagnosis. The patient was scheduled for laparotomy. Intra-operatively, perforation was observed at mesenteric border of a jejunal segment 110 cm from the ligament of Treitz (Figure 5).During laparotomy, 10 centimeters of jejunum with mesentery was resected and abdominal drainage was established (Figure 6). A histopathology examination was performed of a resected brownish tissue fragment, measuring 1.8 cm × 0.7 cm × 0.6 cm.
FINAL DIAGNOSIS
Histology of the postoperative jejunum disclosed jejunal MALT lymphoma, same immunophenotype as the gastric (Figure 7).
TREATMENT
Four months later, according to the pathological findings, the patient was treated with a course of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy.
OUTCOME AND FOLLOW-UP
Complete remission was observed following 6 cycles of treatment, as visualized by CT scan.
DISCUSSION
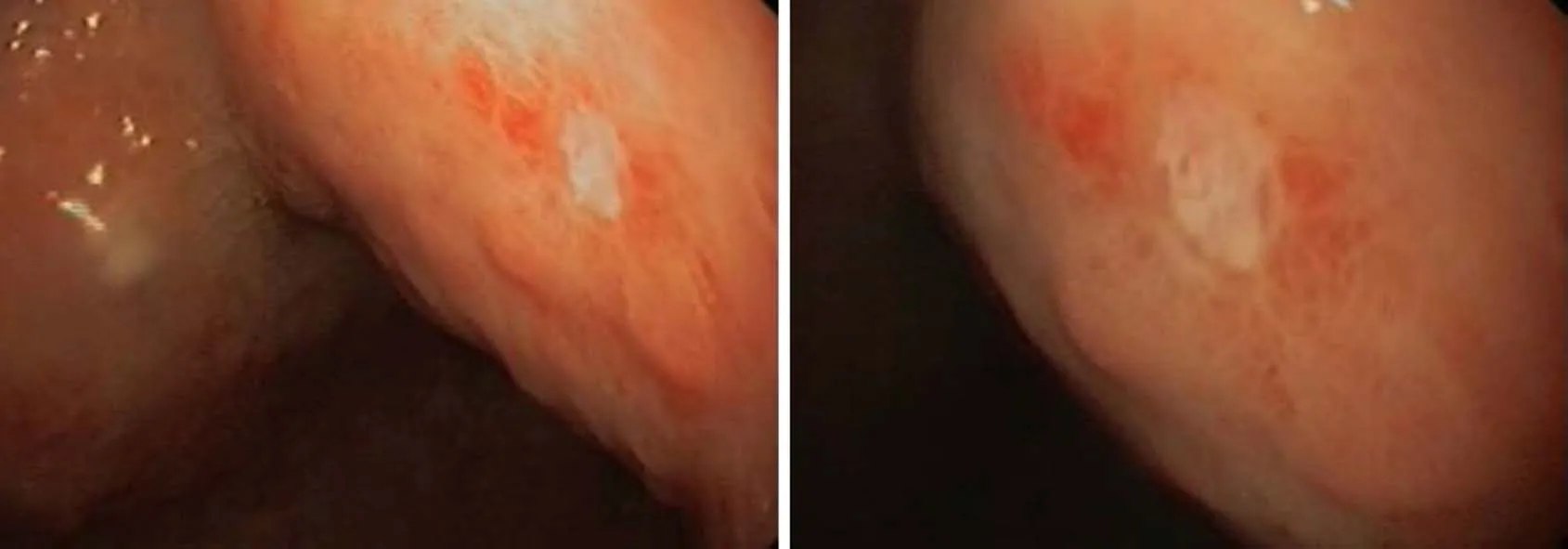
Figure 2 Superficial ulcer in stomach found during upper gastrointestinal endoscopy.
MALT lymphoma has the highest incidence between 50 and 60 years of age, but its prevalence has been shown to be significantly increased in patients over 40 years old.MALT lymphoma has no specific B-cells immunophenotype or antigenic profile and those circumstances complicate its diagnosis[7]. Gastric MALT lymphoma is most commonly found in the antrum but can also be present in any part of the organ[7].
CD is a chronic inflammatory disorder of the bowel and can occur from mouth to anus in any area of the gastrointestinal tract[11]. While there is no single definitive diagnostic test to confirm the diagnosis of CD, multiple examinations should be performed and should neither be based nor excluded on any one result or test. It is also very important to check a patient’s history of diarrhea, abdominal pain and weight loss. The most widely used diagnostic investigation these days is endoscopy with biopsies[12].
In the presented case, the patient was diagnosed with small bowel CD that occurs in 30% of all CD cases[13]. It is often hard to ensure the right diagnosis of small bowel due to the fact that it is beyond the reach of the gastroscope and colonoscope.Enteroscopy is the main diagnostic test that can provide biopsies and show pathology of the small intestine.
Our patient’s enteroscopy result revealed a 1.5 cm ulcer in the jejunum. Ulceration of small bowel is a symptomatic sign and suggest CD diagnosis but can also be congruent with other diseases, including carcinoma, Henoch-Schonlein purpura,lymphoma and a few others[14]. Primary gastrointestinal lymphomas endoscopically appear as a mass in 49% and only 4% appear as an ulcer. Most of the diffuse large B-cell lymphomas (86%) are mass-like and only 2% are ulcers[15]. MALT lymphoma ulcerative lesions are indistinguishable from CD lesions and this makes the differentiation between these two diseases even harder. The biopsy of our patient revealed lymphoplasmacytic infiltrate, which is a common finding in CD but also can be found in NH lymphomas.
Abdominal ultrasonography showed thickened mucosa and enlarged surrounding lymph nodes in a segment of the small intestine. Bowel wall thickening is also a common finding in both pathologies as well as enlarged lymph nodes. The MRI enterography revealed circumferential infiltration of the proximal part of the ileum.For CD, MRI has a sensitivity of 84% and a specificity of 100%[16]but in this particular report, findings were indistinguishable from lymphoma which made even harder to provide a diagnosis of MALT.
There are few cases of lymphoma mimicking CD in the literature. Kooet al[15]determined a small bowel diffuse large B-cell lymphoma mimicking CD. Capsule endoscopy in this case showed ulcers and lesions in the small bowel[15]. Erkanet al[17]reported the case of the terminal ileum Burkitt’s lymphoma simulating CD, where mesalasin was used to treat the patient. Lymphoma was only diagnosed by hystopathologic examination after patient’s death[17]. McCulloughet al[18]described the case of mantle zone lymphoma presented as a diffuse ileocolitis imitating IBD.
It is still under debate if the CD itself increases lymphoma risk compared with the general population. While some studies show that IBD produces a predisposition for the development of lymphoma[19,20], the majority of confirmations support the fact that it does not increase the risk of lymphoma across-the-board[21]. A large study by Lewiset al[22]included nearly 7000 patients from United Kingdom with the diagnosis of CD.The results showed that the risk of lymphoma in these patients is not statistically elevated in a significant manner compared to the control population[22].
Since MALT lymphomas are inherent of inflammation and relying upon a recent understanding of the immune cells participation in the keeping of intestinal homeostasis one can conceive that constant pressure of undesirable representatives of microbiome which attack epithelia can cause a failure to accomplish its regeneration process. This assumption is confirmed by cases of successfulH. pylori-associated MALT lymphoma treatment with antibiotics[23].
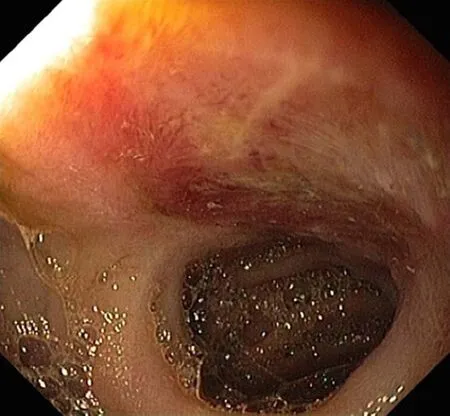
Figure 3 Ulcer in jejunum found during enteroscopy.
Taking into account persistent residence of activated immune cells endeavoring to stimulate regeneration of intestinal epithelium and misshapen epithelial-microbiota crosstalk in CD patients, a possibility is available for distortion of immune cell proliferation process in particular portion of such patients. A detailed study of peculiarities of immune cells functions in CD patients can provide clues for the elucidation of B-cell proliferation control deprivation and a better understanding of B-cell lymphoma pathology including MALT lymphomas.
It is necessary to mention the possibility of immunosuppression-related lymphomas in IBD. It is known that immunosuppressive therapy does increase the risk of non-Hodgkin lymphoma[24]. Our patient has been receiving a small dosage azathioprine therapy before being diagnosed with MALT lymphoma. As the analysis of more than 700000 Americans suffering from IBD revealed, treatment with thiopurines is associated with a 4-fold increase of lymphoma[25]. Moreover, the risk of lymphoma rises gradually for successive years of therapy while discontinuing thiopurine therapy reduces the risk in IBD patients. Khanet al[26]stated that the incidence rate of lymphoma during the first year, second year, third year, fourth year,and after 4 years of treatment with thiopurines was 0.9, 1.6, 5, and 8.9 cases per 1000 person-year, respectively[26]. A recent meta-analysis showed an overall standard incidence ratio for lymphoma of 4.92 in IBD patients receiving thiopurines either. It is also noted that risk level after 1 year of exposure became significant[27]. In the presented case, the patient was on azathioprine treatment for 2 years before being diagnosed with MALT lymphoma. Moreover, azathioprine dosage during time increased from 100 mg per day to 150 mg per day.
CONCLUSION
In our case, abdominal ultrasound, small bowel MRI and enteroscopy showed MALT lymphoma findings mimicking CD. However, the patient was misdiagnosed and received the wrong treatment. Although there are few cases of lymphoma mimicking CD in the literature it is still a very uncommon case. In addition, this case report highlights the clinical relevance of knowledge and awareness of MALT lymphoma simulating CD.
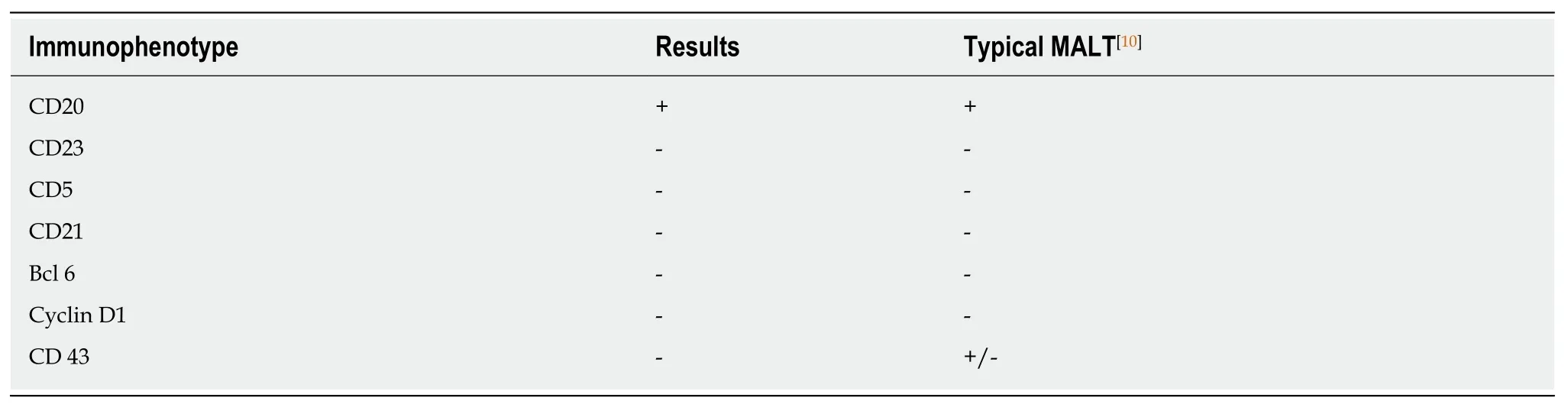
Table 1 Biopsies immunophenotyping
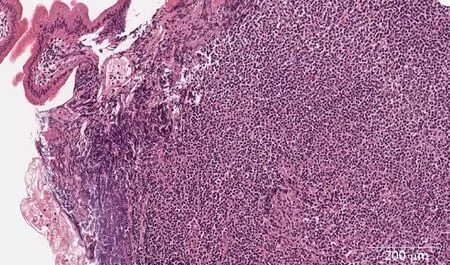
Figure 4 Lymphoplasmacytic infiltrate of jejunum mucosa with eosinophils and neutrophils (× 400).
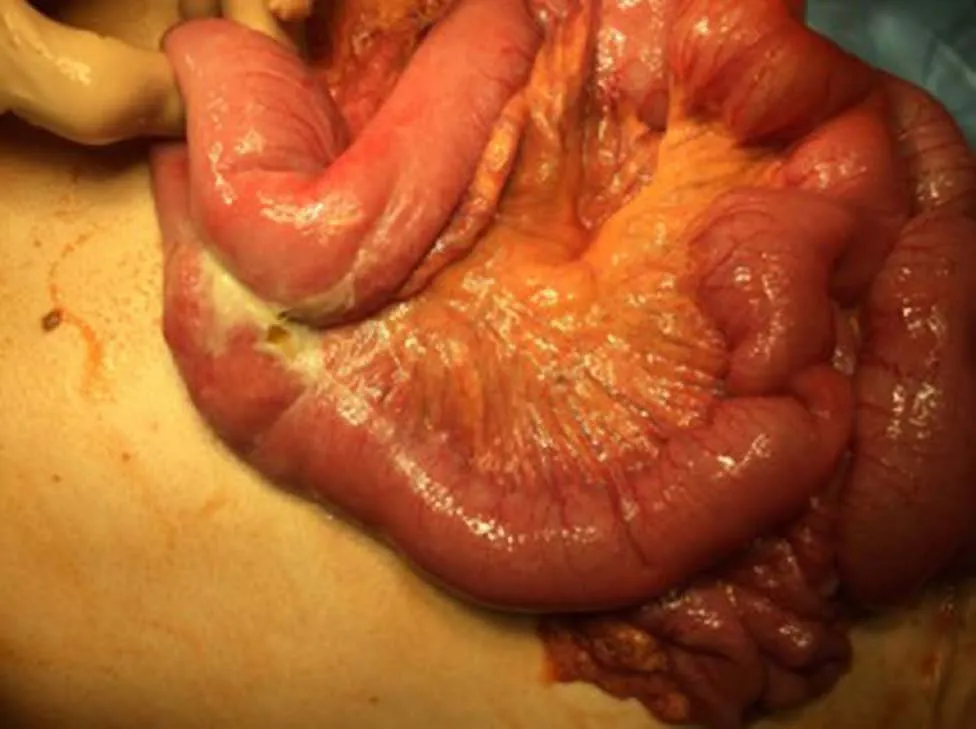
Figure 5 Intra-operative picture of jejunal perforation.
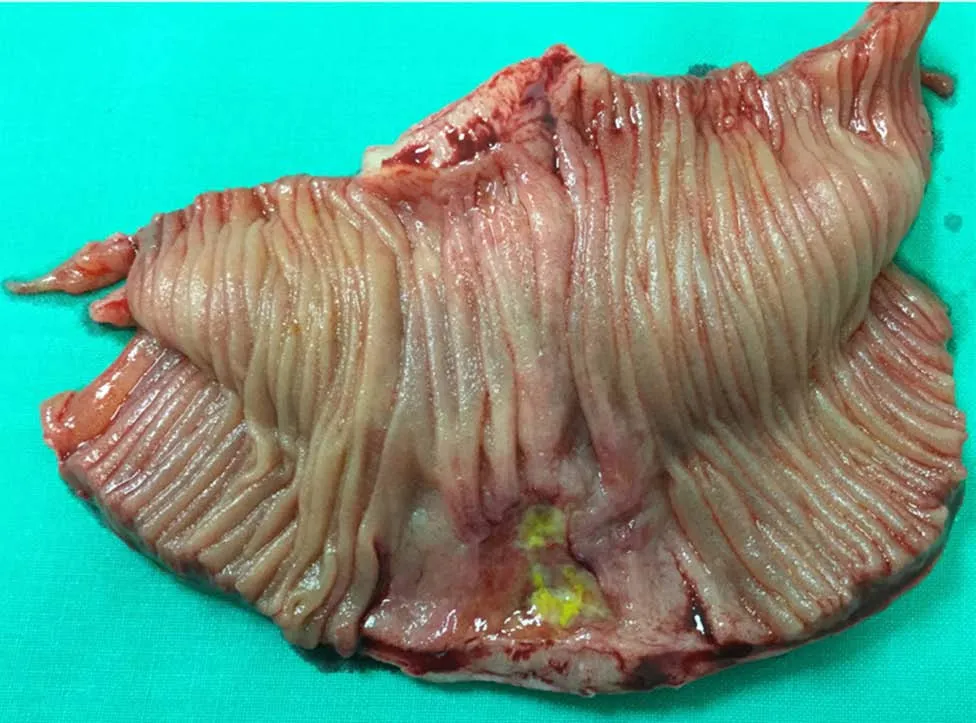
Figure 6 The resected segment of jejunum and mesentery.
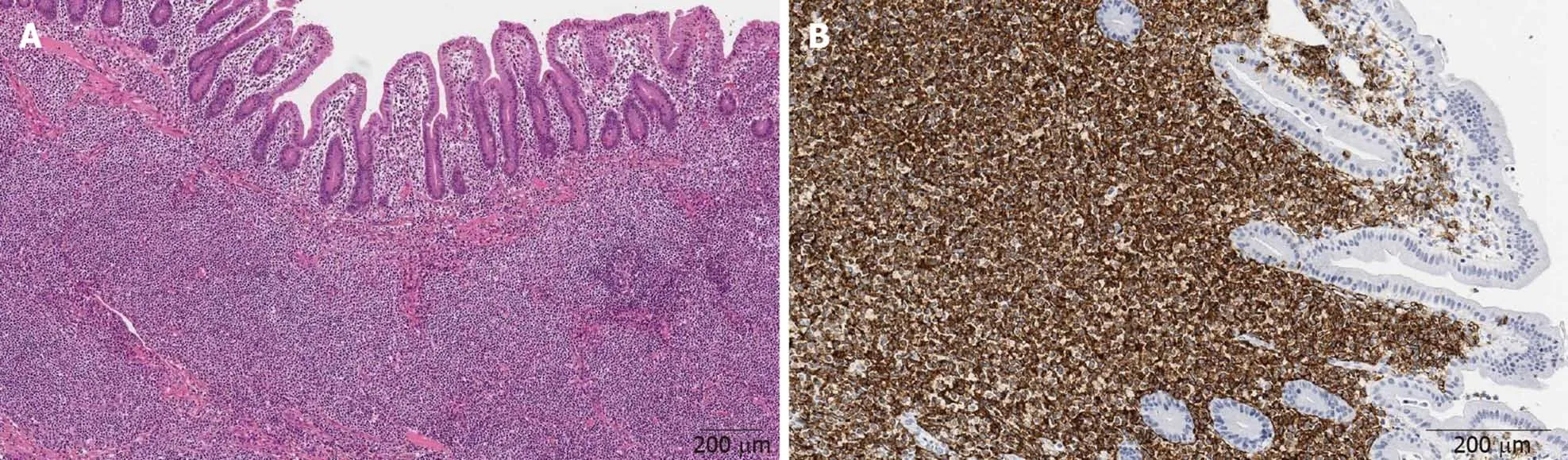
Figure 7 Histology of the postoperative jejunum disclosed jejunal mucosa-associated lymphoid tissue lymphoma. A: Dense population of atypical lymphoid cells in jejunum mucosa (× 200); B: CD20 immunohistochemical staining (× 400).
ACKNOWLEDGEMENTS
We express gratitude to our workmate gastroenterologist Tomas Jucaitis for the attentive endoscopic procedures performed and to the National Center of Pathology pathologist Dmitrij Seinin for comprehensive biopsy analysis.
 World Journal of Clinical Cases2020年8期
World Journal of Clinical Cases2020年8期
- World Journal of Clinical Cases的其它文章
- Clinicopathological differences and correlations between right and left colon cancer
- Bedside score predicting retained common bile duct stone in acute biliary pancreatitis
- Stability and infectivity of coronaviruses in inanimate environments
- Status, challenges, and future prospects of stem cell therapy in pelvic floor disorders
- Unusual presentation of congenital radioulnar synostosis with osteoporosis, fragility fracture and nonunion: A case report and review of literature
- Predictive factors for central lymph node metastases in papillary thyroid microcarcinoma
