Clinicopathological differences and correlations between right and left colon cancer
Ioannis Kalantzis, Konstantinos Gkoumas, Department of Gastroenterology, Korgialenio-Mpenakeio Hellenic Red Cross Hospital, Athens 11526, Greece
Ioannis Kalantzis, Konstantinos Miltiadou, Christos Kosmas, Nikolaos Ziras, Department of Oncology, Metaxa Anticancer Hospital, Piraeus 18537, Greece
Afroditi Nonni, Kitty Pavlakis, Harikleia Gakiopoulou, First Department of Pathology, National and Kapodistrian University of Athens, Medical School, Athens 11527, Greece
Eumorphia-Maria Delicha, Independent Biostatistical Consultant, ASTAT, Statistics in Clinical Research, Glyfada 16675, Greece
Konstantinos Miltiadou, Hepatogastroenterology Unit, Second Department of Internal Medicine, Attikon University General Hospital, Athens 12462, Greece
Abstract
BACKGROUND
The differences in histopathology and molecular biology between right colon cancer (RCC) and left colon cancer (LCC) were first reported in the literature by Bufill in 1990. Since then, a large number of studies have confirmed their differences in epidemiology, clinical presentation, comorbidities and biological behaviours, which may be related to the difference in prognosis and overall survival (OS) between the two groups.
AIM
To investigate statistically significant differences between Greek patients with LCC and RCC.
METHODS
The present observational study included 144 patients diagnosed with colon cancer of any stage who received chemotherapy in a Greek tertiary oncology hospital during a 2.5-year period. Clinical information, comorbidities,histopathologic characteristics and molecular biomarkers were collected from the patients’ medical records retrospectively, while administered chemotherapy regimens, targeted agents, progression-free survival (PFS) periods with first- and second-line chemotherapy and OS were recorded retroactively and prospectively. Data analysis was performed with the SPSS statistical package.
RESULTS
Eighty-six males and 58 females participated in the study. One hundred (69.4%)patients had a primary lesion in the left colon, and 44 (30.6%) patients had a primary lesion in the right colon. Patients with RCC were more likely to display anaemia than patients with LCC [odds ratio (OR) = 3.09], while LCC patients were more likely to develop rectal bleeding (OR = 3.37) and a feeling of incomplete evacuation (OR = 2.78) than RCC patients. Considering comorbidities,RCC patients were more likely to suffer from diabetes (OR = 3.31) and coronary artery disease (P = 0.056) than LCC patients. The mucinous differentiation rate was higher in the right-sided group than in the left-sided group (OR = 4.49), as was the number of infiltrated lymph nodes (P = 0.039), while the percentage of high-grade differentiation was higher in the group of patients with left-sided colon cancer than in RCC patients (OR = 2.78). RAS wild-type patients who received anti-epidermal growth factor receptor (EGFR): Treatment experienced greater benefit (PFS: 16.5 mo) than those who received anti-vascular endothelial growth factor treatment (PFS: 13.7 mo) (P = 0.05), while among RAS wild-type patients who received anti-EGFR treatment, LCC patients experienced greater benefit (PFS: 15.8 mo) than the RCC subgroup (PFS: 5.5 mo) in the first-line chemotherapy setting (P = 0.034). BRAF-mutant patients had shorter PFS (9.3 mo)than BRAF wild-type patients (14.5 mo) (P = 0.033). RCC patients showed a shorter tumour recurrence period (7.7 mo) than those with LCC (14.5 mo) (P <0.001), as well as shorter (OS) (58.4 mo for RCC patients; 82.4 mo for LCC patients) (P = 0.018).
CONCLUSION
RCC patients present more comorbidities, worse histological and molecular characteristics and a consequently higher probability of tumour recurrence, poor response to targeted therapy and shorter OS than LCC patients.
Key words: Colorectal neoplasm; Epidermal growth factor; Vascular endothelial growth factor; Histology; Molecular biology; Metabolic syndrome
INTRODUCTION
According to the World Health Organization, 13% of all deaths are due to neoplastic diseases. It is estimated that by 2025, there will be an increase in incidence of cancer,with 19.3 million new cases per year[1]. Colorectal cancer is one of the major causes of morbidity and mortality in North America, Europe and in regions with similar lifestyle and dietary habits. Globally, it is estimated that colon cancer is the third most common malignancy in men (12.8%), after lung and prostate cancer, and the second most common in women (13.1%), after breast cancer, with a mortality rate equal to the incidence rate[2]. Despite progress in prevention and treatment, there are alarming data indicating that the rate of new cases in patients under the age of 50 is increasing and that the incidences of colon cancer and rectal cancer will increase by 90% and 124.2%, respectively, at ages from 20 to 34 years by 2030[3]. The incidence of right colon cancer (RCC) is also different from that of left colon cancer (LCC), with the latter presenting a higher rate (51%vs42% in the United States)[4]. However, over the last five decades, there has been an increase in the incidence of RCC, which is probably due to genetic and environmental factors as well as to better diagnostic methods[5].
Bufill[6]first mentioned the epidemiological, histopathological, biological and molecular differences between the right and left colon in 1990. According to his study,developmental and biological differences between the proximal and distal colon may reflect different susceptibilities to neoplastic transformation, and these differences may explain the different pathogenetic mechanisms between the diseases[6].
During embryogenesis, the right colon (the caecum and the ascending and proximal two-thirds of the transverse colon) is derived from the midgut, while the left colon (the distal one-third of the transverse colon, the descending and sigmoid colon,and the rectum) is derived from the hindgut[7]. The arterial supply of these two segments is different; as the right colon is supplied by the superior mesenteric artery,while the left colon is supplied by the inferior mesenteric artery, and the microbial populations and exposure to various toxic substances and bile acids are also different between them[8,9]. Additionally, right colon tumours tend to be more frequent in females than in males and at older ages than at younger ages and tend to present at more advanced stages than left colon tumours; in addition, right colon tumours tend to be large exophytic tumours with poor differentiation, a mucinous histology and associated infiltrated lymph nodes. They usually metastasize in the peritoneal cavity,and their main symptom is anaemia. In contrast, left colon tumours appear mainly in males and at younger ages and occupy a larger diameter of the colon lumen than right colon tumours, resulting in the occurrence of obstructive incidents and changes in bowel habits, while the most common metastasis sites are the liver and lungs[7,10].Molecular carcinogenesis pathways also appear to be different among them, with left colon tumours mainly originating from the chromosomal instability pathway, while microsatellite instability (MSI) is mostly detected in right colon tumours[10]. In addition, differences are also observed in the colorectal carcinogenesis-associated RAS-RAF-MAPK signalling pathway between the two segments of the colon, with right colon tumours exhibiting higher rates of mutantKRASandBRAFgenes than left colon tumours[11-13]. The effect of targeted regimens is also of interest, as different rates of overall survival are observed depending on the RAS mutation status and the administered antibody, as well as in relation to the location of the primary tumour[14].
All the above findings are likely to explain the different rates of overall survival(OS) between the two subgroups, as RCC patients experience shorter OS than LCC patients[15]. The purpose of this study is to investigate statistically significant differences among Greek patients with RCC and LCC based on epidemiological,clinical, histological and molecular characteristics as well as differences between them in terms of disease progression time periods in the first- and second-line treatment setting and OS, taking into account the administered targeted treatment.
MATERIALS AND METHODS
Study population and data collection
The current study includes a combination of retrospective and prospective observations of patients with colon cancer, regardless of stage. During a 2.5-year period we addressed an invitation to participate in our study to all colon cancer patients who received chemotherapy regimens in a Greek Oncology Centre. Of those patients, all patients who fulfilled the inclusion criteria were included in the study.Inclusion criteria were the presence of histologically confirmed colon cancer, the patient's signed informed consent and the presence of updated medical records.
After approval by the Institutional Review Board of the Hospital, epidemiological data, clinical presentation and concomitant illnesses at the onset of the disease, as well as the patients’ performance status (PS), stage of the disease, primary tumour location(PTL), surgical operations and metastatic tumour locations, were collected from each patient's medical record. Patients’ histological reports were analysed with respect to major histological features (histological type, differentiation, number of removed and infiltrated lymph nodes, necroses, vascular and lymphatic emboli and perineural infiltration). The molecular biomarkers included in the study were theKRAS,NRAS(exons 2, 3 and 4) andBRAF(V600E mutation) genes, as well as genes responsible for microsatellite instability (MLH1,MSH2,MSH6andPMS2).
A PTL of the right segment of the colon was defined as the presence of a tumour in the appendix, caecum, or ascending or transverse colon, while the left-sided group included tumours in the descending or sigmoid colon and rectum, as in most published studies. The time periods and chemotherapy regimens used from the time of histological diagnosis to the date of death or the end of follow-up were both retrospectively and prospectively recorded.
Statistical analysis
χ2statistics were employedto investigate the distributional properties of categorical variables (sex, symptoms, comorbidities, histology, and genes) in terms of left or right colon location (left or right). To determine statistical significance, the magnitude of association between the tumour location and the variable of interest was presented by an odds ratio (OR) and the 95% confidence interval (CI). An independent samplesttest was employed to investigate the distributional properties of continuous variables with respect to tumour location (left or right). The time to event analysis was diseasespecific and was carried out in different subgroups based on the clinical staging at diagnosis: (1) Time to recurrence (for patients with initial stage I, II and III disease)was defined as the time from diagnosis to progression to stage IV. If progression to stage IV was not observed, the data were censored at the last date of patient followup. (2) Time to first progression (PFS 1, for all patients with final stage IV disease) was defined as the time from first-line chemotherapy administration to progression. No censoring was applied since all patients included in the analysis progressed. (3) Time to second progression (PFS 2, for all patients with final stage IV disease after first-line chemotherapy) was defined as the time from second-line chemotherapy until progression occurred. No censoring was applied since all patients included in the analysis progressed. And (4) Time to disease-specific survival (for all patients with final stage IV disease after second-line chemotherapy) was defined as the time from diagnosis to death. If death was not observed, the data on OS were censored at the last date the patient was known to be alive.
The time to event distribution curves between variables of interest were tested by log-rank statistics and were graphically illustrated by Kaplan-Meier curves. Cox proportional analysis was employed to test the independent prognostic factors in the time to event analysis. All tests were 2-sided, and the level of statistical significance was set to α = 0.05. For the purpose of the multivariate analysis no missing values were imputed.The statistical review of the study was performed by a biomedical statistician.
RESULTS
Epidemiological data
A total of 144 patients were included in the study, of which 139 were of Greek origin(96.5%). One hundred patients had a left colon PTL (69.4%), and 44 patients had a right colon PTL (30.6%) (Figure 1); 86 patients were male (59.7%), and 56 patients were female (40.3%), with an average age of 65.4 years at the date of diagnosis (left colon: 65.4 years; right colon: 65.5 years) (Table 1). Male sex was more frequent in LCC patients (n= 64.64%) than female sex (n= 36.36%), while the percentages of men(n= 22.50%) and women (n= 22.50%) were equal in RCC patients. The average body mass index (BMI) was 28.3, without differences between the right (29) and left (28)colon groups (P= 0.525) (Table 1).
Clinical presentation
Changes in bowel habits (diarrhoea/constipation: 39.2%), rectal bleeding (38.5%),abdominal pain (28%), a feeling of incomplete evacuation (21.7%), weight loss (18.9%),weakness (18.9%) and anaemia (18.9%) were the most commonly observed symptoms in all patients. No symptoms were reported by 11.2% of patients. In RCC patients,anaemia was recorded as the predominant symptom and was seen at a higher rate in RCC patients (31.8%) than in LCC patients (13.1%) (P= 0.008, OR = 3.09, 95%CI: 1.30-7.30); in contrast, rectal bleeding was the most frequent symptom in patients with LCC (46.5%) and was seen in 20.5% of patients with RCC (P= 0.003, OR = 3.37, 95%CI:1.47-7.69). A feeling of incomplete evacuation was more frequent in LCC patients(26.3%) than in RCC patients (11.45%) (P= 0.05, OR = 2.78, 95%CI: 1-7.69) (Table 2).
Only 13.4% of the patients were diagnosed by means of a preventive screening,while the proportion of patients in the LCC group who underwent medical evaluation due to lower gastrointestinal symptoms was greater (32.6%) than that in the RCC group (17.2%) (P= 0.052, marginal significance). The majority of the patients (78.9%)were fully active without restrictions (PS: 0), without significant differences between RCC and LCC patients.
Comorbidities
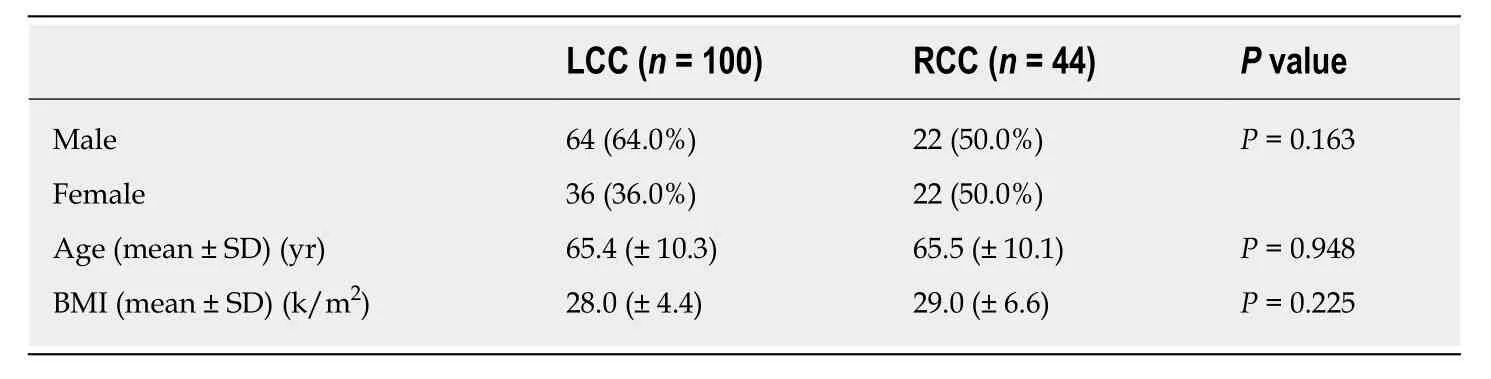
Table 1 Characteristics of right and left colon cancer patients
Concomitant illnesses were recorded from the patients’ medical records. Hypertension (41.7%), diabetes mellitus (16%), dyslipidaemia (14.6%) and coronary heart disease (12.5%) were the most commonly reported comorbidities. Statistically significant differences were identified regarding coronary heart disease, as RCC patients manifested a higher rate (20.5%) of coronary heart disease than LCC patients(9%) (P= 0.056, marginal significance), as well as diabetes mellitus, since RCC patients suffered a higher rate (13.6%) than LCC (8.2%) (P= 0.016) (Table 2).
Staging
At the time of diagnosis, 19.4% (n= 28) of the patients were classified as having stage I or II disease, 27.1% (n= 39) of patients had stage III disease, and 53.5% (n= 77) of patients had stage IV disease (Table 3). The stage of the tumour in the majority of patients was T3 (68.3%), followed by T4 (20.6%) and T1-T2 (11.1%) tumours (Table 3),and there were no differences between the two subgroups. The average number of removed lymph nodes was 15.7, with right colon tumour patients having a higher average number of removed lymph nodes (18.2) than left colon tumour patients (14.4)(P= 0.054, marginal significance). The percentage of infiltrated lymph nodes was higher in the N1group of RCC patients (52.3%) than in LCC patients (32%), who, in turn, showed a higher percentage of non-infiltrated lymph nodes (N0: 28%) than RCC patients (N0: 20.5%) (P= 0.039) (Table 3).
The liver was the most common location of metastasis for both colon segments(67%), followed by the lungs (31.3%) and peritoneum (11.6%). A histological diagnosis was obtained from the primary tumour biopsy in 47.2% of the patients and from surgical specimens of the primary tumour in 49.3% of patients, while the remaining 3.5% of patients were diagnosed from a biopsy of a metastatic lesion. The majority of patients underwent surgical resection of the primary tumour (79.9%), with the remaining 20.1% of patients receiving only palliative chemotherapy.
Histology
The predominant histological type was adenocarcinoma (91.7%,n= 132), followed by mucinous carcinoma (7.6%,n= 11). RCC patients demonstrated a higher rate of mucinous histology (15.9%) than LCC patients (4%) (P= 0.046, OR = 4.49, 95%CI: 1.24-16.25). In terms of histological differentiation, left colon tumours showed a higher rate(79.1%) of moderate/high differentiation than right colon tumours (53.7%) (P= 0.005,OR = 2.78, 95%CI: 1.26-6.13) (Table 4). No differences were observed between the two subgroups regarding necrosis, emboli and perineural infiltration (Table 4).
Molecular biology
Four gene groups were analysed in the majority of the histological specimens regardless of stage (KRAS:n= 134,NRAS:n= 113,BRAF:n= 92, and MSI-related genes:n= 85) (Table 5). The majority of patients had the wild-typeKRASgene (57.5%)compared to the mutant gene (42.5%), as was also observed withNRAS(wild-type:96.5%; mutant: 3.5%) andBRAF(wild-type: 90.2%; mutant: 9.8%) genes. The vast majority of patients presented microsatellite-stable tumours (MSS) (94.1%), and a low rate of MSI was observed (5.9%).
A statistically significant difference regarding theKRASgene was found between right and left colon tumours, as more LCC patients (63.8%) than RCC patients (42.5%)wereKRASwild type (OR = 2.39, 95%CI = 5.01-1.12,P= 0.036). In turn, more RCC patients (57.5%) than LCC patients (36.2%) wereKRASmutant (Table 5).
Adjuvant and perioperative therapy
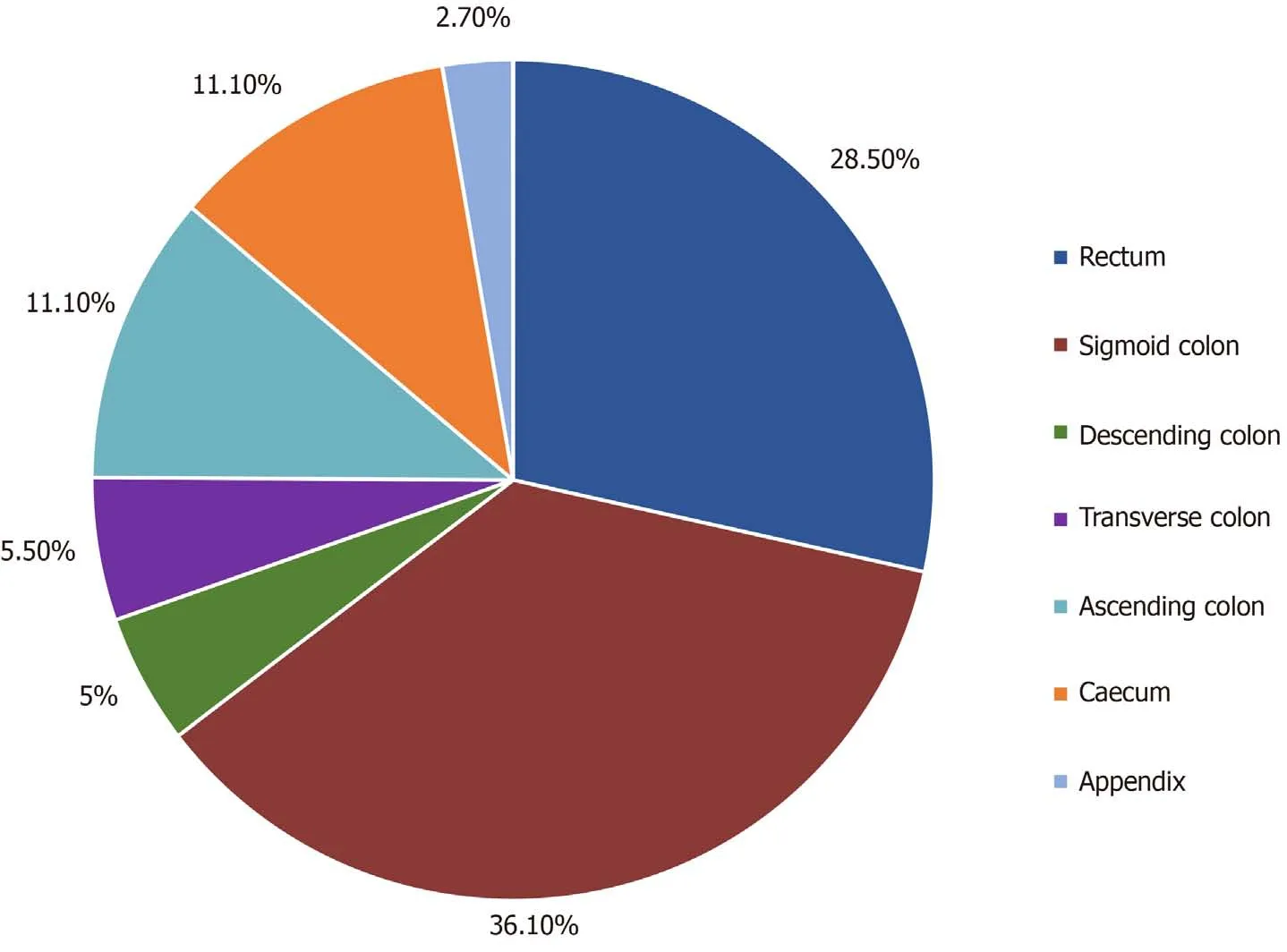
Figure 1 Distribution of primary tumour location in colon cancer patients.
Patients with stage II or III disease received adjuvant chemotherapy after surgical removal of the primary tumour. Seventy-five percent of the patients received XELOX and 25% of patients received FOLFOX as adjuvant therapy (Table 6). Patients with rectal colon cancer received perioperative chemoradiotherapy, according to their stage. Patients with stage II disease received combined chemoradiotherapy (RT-capecitabine) before surgery, while patients with stage III disease received chemoradiotherapy (RT-capecitabine) before surgery and chemotherapy with the XELOX regimen after surgery (Table 6). Patients with stage I disease did not receive adjuvant therapy.
Disease recurrence
A total of 39 (58.2%) out of the 67 patients with disease initially staged as I, II or III exhibited disease relapse. One patient was diagnosed with lung cancer at the same time, and another patient died from other causes after the completion of adjuvant therapy and was not included in the analysis. The median time to relapse was 42.9 mo. The median follow-up time for relapse was 47.7 mo (2.6-114.6 mo).
A statistically marginal significance was found between the two major histological types, as patients with mucinous carcinoma relapsed earlier (17 mo) than patients with adenocarcinoma (44.5 mo) (P= 0.068). Investigating the time to relapse based onRASstatus revealed thatRAS-mutant patients exhibit a shorter recurrence time period(29.9 mo) thanRAS-wild-type patients (53.7 mo) (P= 0.013) (Figure 2). The above result was also confirmed for the LCC group, since the time to relapse was shorter inRAS-mutant patients (35.6 mo) than inRAS-wild-type patients (53.7 mo) (P= 0.026)(Figure 3).
When examining the correlation between theBRAFgene and the presence of emboli in patients with stage I-III disease, marginal statistical significance was observed, withBRAF-mutant patients demonstrating a higher rate of emboli thanBRAF-wild-type patients (P= 0.053). The analysis of MSI and histology in patients with stage I-III disease showed that MSI-H tumours were found at a greater percentage in T4 than in T1-T3 tumours, and T1-T3 tumours in turn were more likely than T4 tumours to be MSS (P= 0.018). Low histological differentiation was more common in MSI-H tumours than in MSS tumours, which were more likely to have high/moderate histological differentiation (P= 0.028).
In multivariate analysis, independent predictive factors for recurrence in patients with stage I-III disease wereRASmutation status, asRAS-mutant patients had a higher risk of relapse thanRASwild-type patients [P= 0.002, hazard ratio (HR) =3.731, 95%CI = 1635-8513], and the presence of (vascular and lymphatic) emboli (P=0.025, HR = 3.221, 95%CI = 1161-8938) (Table 7). Including theBRAFgene in multivariate analysis, independent predictive factors for recurrence wereRASmutant status (P= 0.007, HR = 3.815, 95%CI = 1.434-10.152) and (marginally) the presence of emboli (P= 0.051, HR = 3.733, 95%CI = 1-13.985), despite the increase in the number of cases entered into the model.
First line chemotherapy
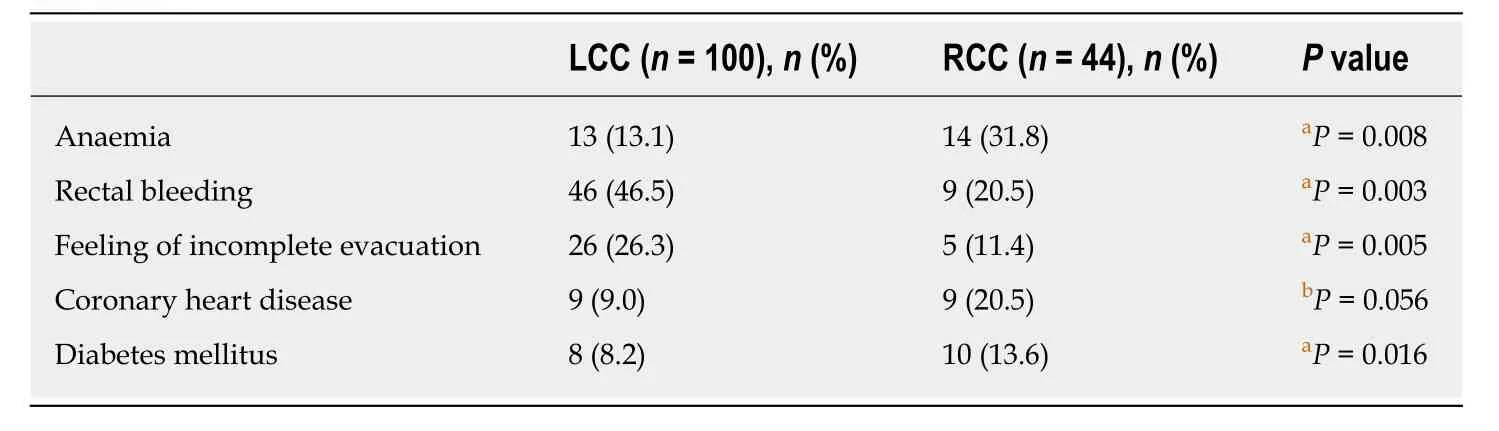
Table 2 Clinical presentation and comorbidities with statistically significant differences between left and right colon cancer patients
Patients with stage IV disease (n= 77) along with relapsed patients with stage I-III disease (n= 39) received first-line chemotherapy. One patient with stage IV disease was diagnosed simultaneously with breast cancer and was not included in the study(total: 115 patients). Among chemotherapy regimens, 69.5% of patients received FOLFOX and 29.6% of patients received FOLFIRI, while one patient with a neuroendocrine neoplasm received cisplatin-etoposide (0.9%) (Table 8). Regarding the antibodies administered to patients with stage IV disease, 69.3% of patients received bevacizumab, and 30.7% of patients received panitumumab in combination with the above chemotherapy regimens, based onRASmutation status (Table 9). The median time to disease progression was 12.3 (1.1-62.5) mo.
Among patients with initial stage I-III disease, a significant difference was observed between LCC and RCC, as the time to progression in LCC patients was 14.5 mo compared to 7.7 mo in RCC patients (P< 0.001) (Figure 4). No differences were observed between final stage IV LCC (12.2 mo) and RCC patients (12.5 mo) (P= 0.165)(Table 9).
Similarly, no difference in time to disease progression was found in patients with final stage IV disease based on RAS status (RASwild type: 13.8 mo;RASmutant: 11.5 mo), but in the subgroup of patients with initial stage IV disease, a statistically significant difference was revealed betweenRAS-wild-type (16.5 mo) andRAS-mutant patients (11.5 mo) (P= 0.018) (Figure 5). In patients with final stage IV disease,no differences were found between those who received bevacizumab (12 mo) and those who received panitumumab (14.5 mo) (P= 0.660) nor with regard to location of the primary tumour or the administered antibody (P= 0.177) (Table 9).
However, a significant difference was observed in the disease progression time period amongRAS-wild-type patients with final stage IV disease who received panitumumab in terms of PTL, as RCC patients demonstrated a shorter PFS (5.5 mo)than LCC patients (15.8 mo) (P= 0.034) (Figure 6) (Table 9). Statistically significant differences in the time to disease progression were also found among patients with initial stage IV disease according toRASmutation status and the administered antibody, asRAS-mutant patients who received bevacizumab experienced the earliest disease progression (11.5 mo), followed byRAS-wild-type patients who received bevacizumab (13.7 mo), while a longer time to disease progression was found in the group ofRAS-wild-type patients who received panitumumab (16.5 mo) (P= 0.05).
BRAF analysis was performed in 65.2% of patients with final stage IV disease (n=75 patients). Ninety-two percent (n= 69) exhibited wild-type and 8% (n= 6) exhibited mutantBRAFgenes.BRAF-mutant patients demonstrated a shorter disease progression time (9.3 mo) thanBRAF-wild-type patients (14.5 mo) (P= 0.033) (Figure 7), a result that remained significant after adjustment for PTL (P= 0.046). In multivariate analysis, the only independent factor for disease progression wasBRAFmutation, with patients withBRAFmutation having a greater risk for disease progression during first-line chemotherapy (P= 0.040, HR = 2.454, 95%CI = 1.044-5.772) (Table 10).
Second-line chemotherapy
Ninety-eight patients with final stage IV disease received second-line chemotherapy.In total, 72.4% of patients received FOLFIRI, and 27.6% received FOLFOX (Table 11).Regarding the antibodies administered during second-line chemotherapy, 34.5% of patients received bevacizumab, 26.8% received panitumumab and 38.1% received aflibercept, depending on theRASmutation status (Table 12). One patient with neuroendocrine carcinoma was not included in the study since he/she received FOLFIRI without a targeted agent. The median time to disease progression was 8.6(0.7-30.4) mo.
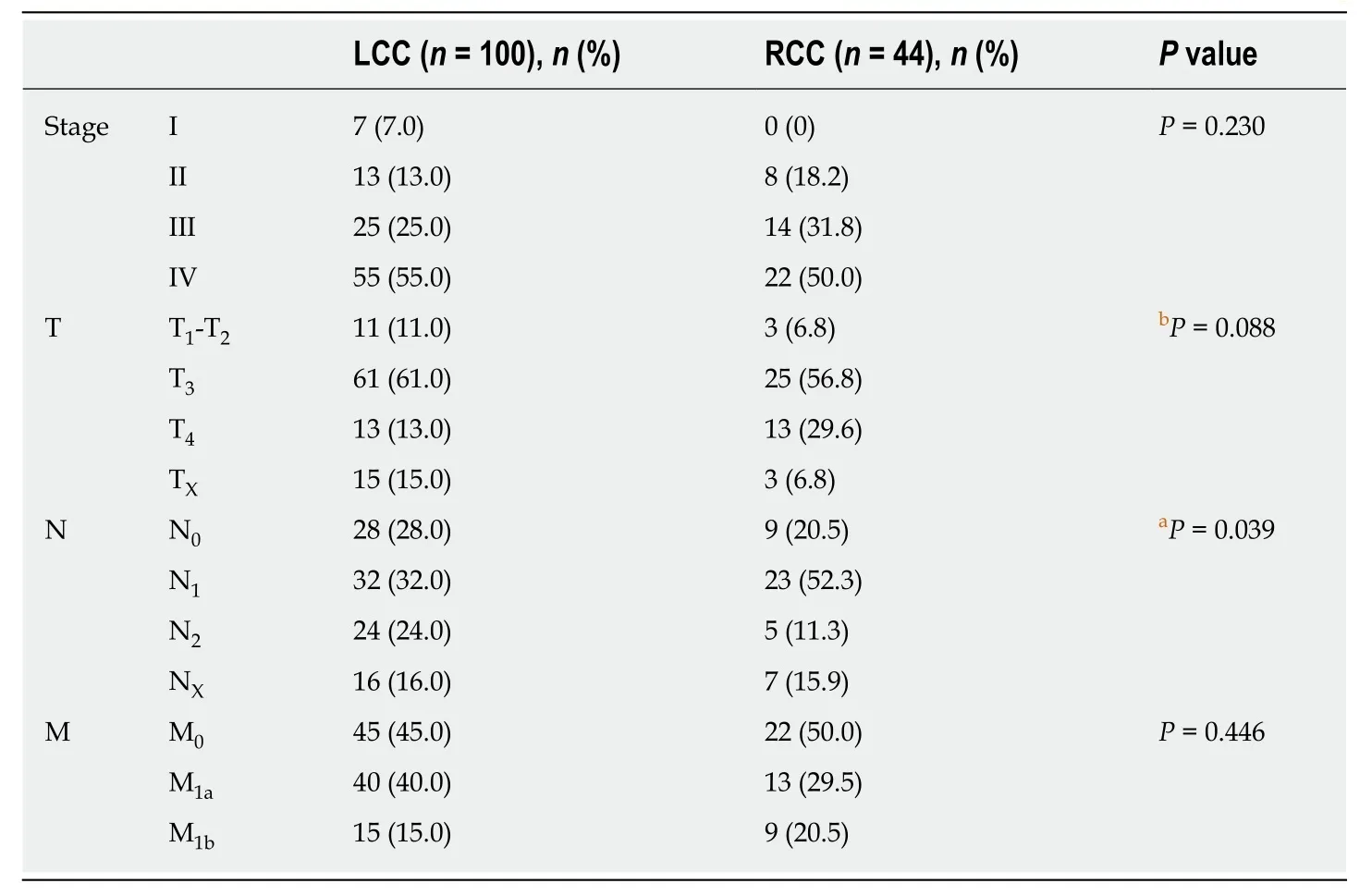
Table 3 Disease stage, tumour size (T), infiltrated lymph nodes (N) and metastatic load (M) in right and left colon cancer patients at the time of diagnosis according to AJCC 8th edition, 2017
No differences were found betweenRAS-wild-type (8.6 mo) andRAS-mutant patients (8.2 mo) (P= 0.334) or betweenBRAF-mutant (7.7 mo) andBRAF-wild-type patients (7.2 mo) (P= 0.571). Additionally, no differences were observed between LCC(8.2 mo) and RCC (8.6 mo) patients (P= 0.532), nor between patients administered different antibodies (bevacizumab: 9 mo, panitumumab: 9.7 mo, and aflibercept: 7.6 mo;P= 0.328), or between patients with different PTL (P= 0.193) (Table 12).
Survival
Eighty-eight out of 144 patients died (61.1%), while three patients were not included in colon cancer-related survival analysis. The median survival time was 53.8 mo. The median follow-up time for survival was 70.5 mo (1.4-185.2 mo).
A significant difference was revealed between patients with initial stage I-III disease (OS: 76.8 mo) and those with initial stage IV disease (OS: 44 mo) (P= 0.001).Regarding histological parameters, a survival-related statistically significant difference was found between patients with low differentiation (OS: 38 mo) and those with high/moderate differentiation (OS: 59.6 mo) (P= 0.002) and between patients with the presence (OS: 49.6 mo) and those with an absence (OS: 64.9 mo) of necroses(P= 0.075, marginal significance).
No differences in OS were observed between patients with final stage IV disease in terms of PTL (LCC: 54.7 mo; RCC: 52 mo) (P= 0.316), and no differences were observed between patients with initial stage IV disease in terms of PTL (LCC: 44 mo;RCC: 46.3) (P= 0.787). However, a significant difference in OS was found between LCC patients (82.4 mo) and RCC patients (58.4 mo) (P= 0.018) among patients with initial stage I-III disease (Figure 8).
No differences in OS related to patient genetic profile were found. More specifically, the OS ofRAS-wild-type patients was 54.8 mo, while that ofRAS-mutant patients was 49.4 mo (P= 0.287); the OS ofBRAF-mutant patients was 76.8 mo, while that ofBRAF-wild-type patients was 59.2 mo (P= 0.349). No differences were found between MSS and MSI-H patients (53.8 mo) (P= 0.648). Regarding administered antibodies, there were no differences in OS between patients treated with bevacizumab (49.4 mo) or panitumumab (54.6 mo) in the first-line chemotherapy setting (P= 0.780), nor between those receiving bevacizumab (54.8 mo), panitumumab(47.6 mo) and aflibercept (48.8 mo) in the second-line chemotherapy setting (P=0.846). The same conclusion was made for all possible combinations of administered antibodies in first- and second-line therapy settings (P= 0.693) (Table 13).
In multivariate analysis, independent factors for survival wereKRASmutation status, asKRAS-mutant patients had worse prognosis thanKRAS-wild-type patients(HR = 2.13, 95%CI = 1.162-4.605,P= 0.017), and the stage of disease at the time of diagnosis, with patients with initial stage IV disease demonstrating shorter OS thanpatients with other stages of disease at diagnosis (HR = 4.036, 95%CI = 1.922-8.475,P< 0.0001) (Table 14).
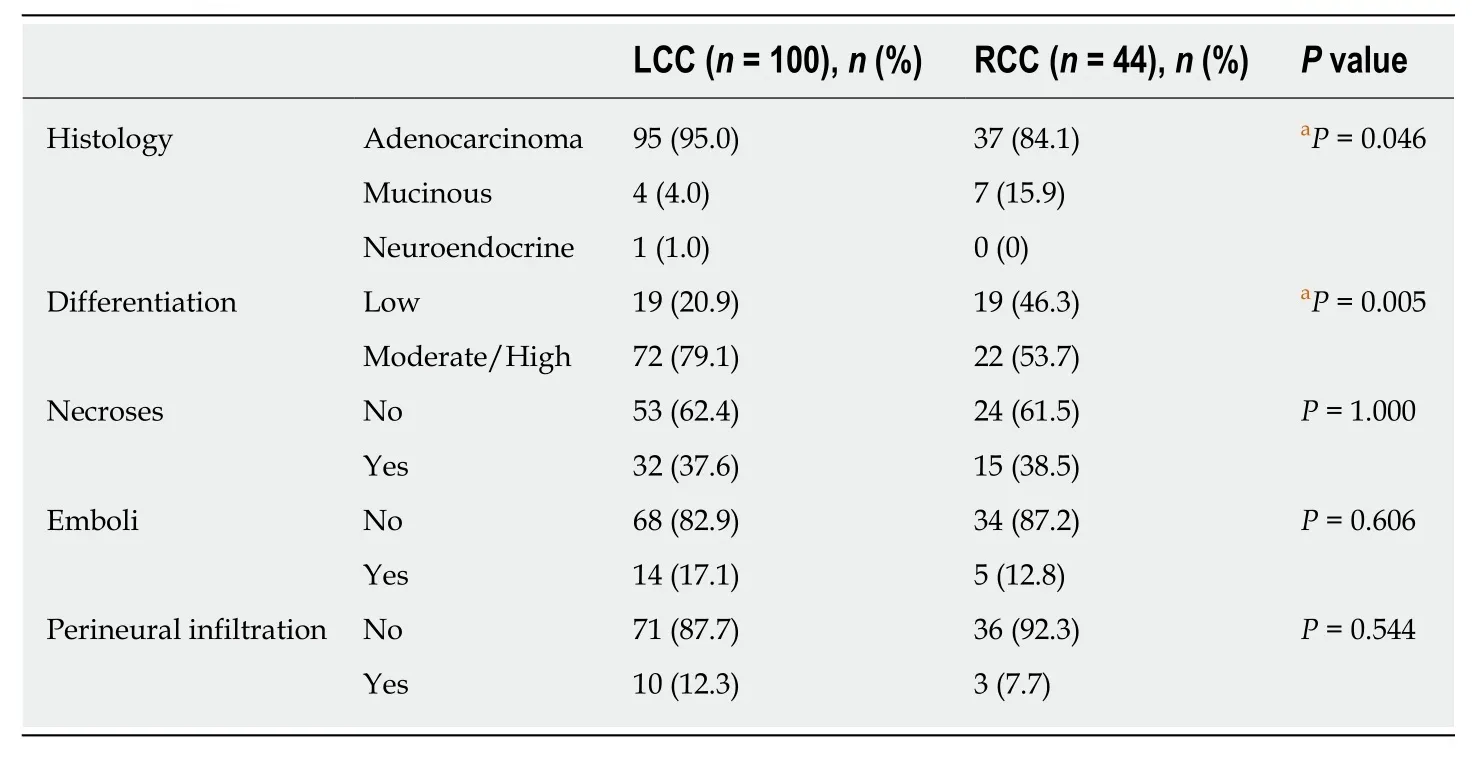
Table 4 Histology, differentiation, and presence of necrosis, emboli (vascular and lymphatic)and perineural infiltration in patients with right and left colon cancer according to the World Health Organization 2019 classification
DISCUSSION
Early studies of the diversity between the two types of colon at the histological and molecular levels led several years later to the hypothesis that they are two different disease entities, the characteristics of which should be taken into account in the choice of individualized treatment[6,10,16]. The RCC rate has increased in recent decades, with older women being affected more than younger women; however, this was not confirmed in our study, even for the over 65 age group (P= 0.131)[5,14].
In contrast, in addition to the differences noted in the existing literature reports,differences were found at the histopathological level, with right colon tumours presenting a higher rate of mucinous histology (OR = 4.49) and a greater number of removed and infiltrated lymph nodes than left colon tumours, while left colon tumours had higher rates of high/moderate differentiation than right colon tumours(OR = 2.78)[8,13]. The impact of histological differences between the two subgroups manifested as a shorter time to relapse in stage I-III patients with mucinous differentiation (17 mo) than in those with adenocarcinoma (44.5 mo), while the presence of emboli was also identified as an independent factor for recurrence (HR:3.221)[16,17].
The identified statistically significant differences in clinical presentation between RCC (anaemia OR = 3.09) and LCC patients (rectal bleeding OR = 3.37, feeling of incomplete evacuation OR = 2.78) imply a more obscure disease onset in RCC patients, which may contribute to delayed diagnosis and advanced stage at diagnosis in these patients[18]. In line with the above findings, the number of patients with LCC seeking medical care due to symptoms of the lower digestive system was twice as high as the number of RCC patients. Taking into account the flat morphology of serrated adenomas mainly found in the right colon, as well as the percentage of incomplete colonoscopies, the increased risk of diagnosis failure in the right colon becomes more apparent[9,14].
In the few studies related to comorbidities, RCC patients appeared to have higher rates of concomitant illnesses than LCC patients, which may be partially justified by the higher average age of the right subgroup[14,19]. The increased percentage of RCC patients suffering from coronary artery disease (P= 0.056) and diabetes mellitus (P=0.016) in our study probably reflects a correlation between Western lifestyle, metabolic syndrome and colorectal cancer and needs further study[20,21].
Disease stage at the time of diagnosis was confirmed as an important factor for survival in both univariate (initial stage I-III: 76.8 mo; initial stage IV: 44 mo,P=0.001) and multivariate analyses, with patients with initial stage IV disease having a worse prognosis than patients with other initial stages of disease (HR = 4.036,P<0.0001)[8].
Despite the fact that the prognostic value ofRASmutation status is disputed in theliterature, in our study, it seemed to play an important role in disease recurrence in patients with initial stage I-III disease (RASmutant: 29.9 mo;RASwild type: 53.7 mo,P= 0.013), in PFS in the first-line chemotherapy setting among patients with initial stage IV disease (RASmutant: 11.5 mo;RASwild type: 16.5 mo,P= 0.018), and in OS,withRAS-mutant patients demonstrating a worse prognosis thanRAS-wild-type patients (HR = 2.13,P= 0.017)[10,22-24].
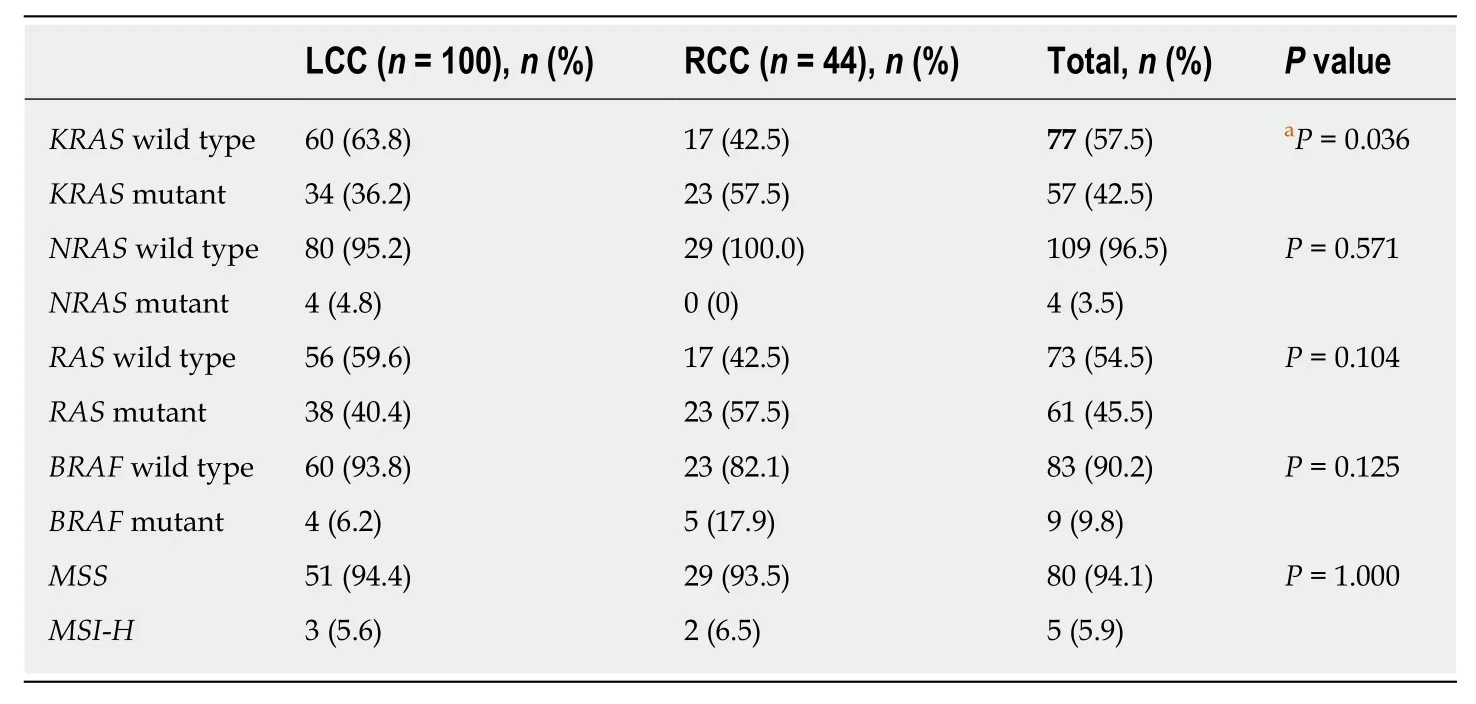
Table 5 KRAS, NRAS, BRAF and MSI-related genes in right and left colon cancer patients
On the other hand, the prognostic value of theBRAFgene was confirmed, as among patients with final stage IV disease, those patients withBRAFmutations demonstrated a shorter PFS time period (9.3 mo) in the first-line chemotherapy setting thanBRAF-wild-type patients (14.5 mo) (P= 0.033)[25].
The percentage of MSI-H tumours (5.9%) was lower than that reported in the literature, without differences between LCC (5.6%) and RCC (6.5%) (P= 1.000),probably due to the relatively few results regarding MSI in our study[26]. The presence of low histological differentiation and large tumour size in patients with MSI-H stage I-III disease in our study reveals the poor histological features of this subgroup and is consistent with already published studies[27].
PTL was found to play an important role in disease recurrence, as among patients with initial stage I-III disease, there was a significant time difference between LCC(14.5 mo) and RCC (7.7 mo) (P< 0.001); a similar difference was seen in OS, with patients with initial stage I-III LCC demonstrating longer OS (82.4 mo) than RCC patients (58.4 mo) (P= 0.018)[8,15,28,29].
A recent meta-analysis concluded that EGFR inhibitors provided a clear clinical benefit toRAS-wild-type patients[30]. In line with the above, the predictive value ofRASmutation status was confirmed in this study, as patients with initial stage IVRAS-wild-type disease who received panitumumab exhibited a better response in first-line chemotherapy (16.5 mo) than patients who received bevacizumab (13.7 mo)(P= 0.05)[31]. The predictive value ofRASmutation status was also enhanced by the results regarding PTL, as among patients with final stage IVRAS-wild-type disease who received panitumumab, LCC patients exhibited a better response (15.8 mo) in the first-line chemotherapy setting than RCC patients (5.5 mo) (P= 0.034)[32-36].
There are limited data with respect to the response of colon tumours to bevacizumab according to PTL. The study of Boisenet al[37]showed that patients with tumours of the sigmoid colon and rectum who received first-line chemotherapy with bevacizumab experienced a longer PFS than patients with tumours in the caecum and ascending colon, a result that was not confirmed in this study (LCC: 11.6 mo, RCC:12.5 mo)[37,38]. According to Arnoldet al[34], administration of bevacizumab in RCC leads to a better response in first-line chemotherapy than administration of other antibodies, a result that was also not confirmed in the present study (bevacizumab:12.5 mo; panitumumab: 5.5 mo,P= 0.111), and there was no difference in OS between the two antibodies (bevacizumab: 49.4 mo; panitumumab: 54.6 mo,P= 0.780)[15,34].Regarding the use of bevacizumab after first-line chemotherapy, previous studies support that continuation of VEGF inhibition in the second-line chemotherapy setting provides a very mild but significant benefit to survival, regardless of KRAS status[39,40].In our study, there was no benefit to survival associated with continuing the administration of bevacizumab after first-line chemotherapy, and none of the possible combinations of administered antibodies in the first- and second-line chemotherapy settings were associated with any significant differences (P= 0.693) (Table 13).
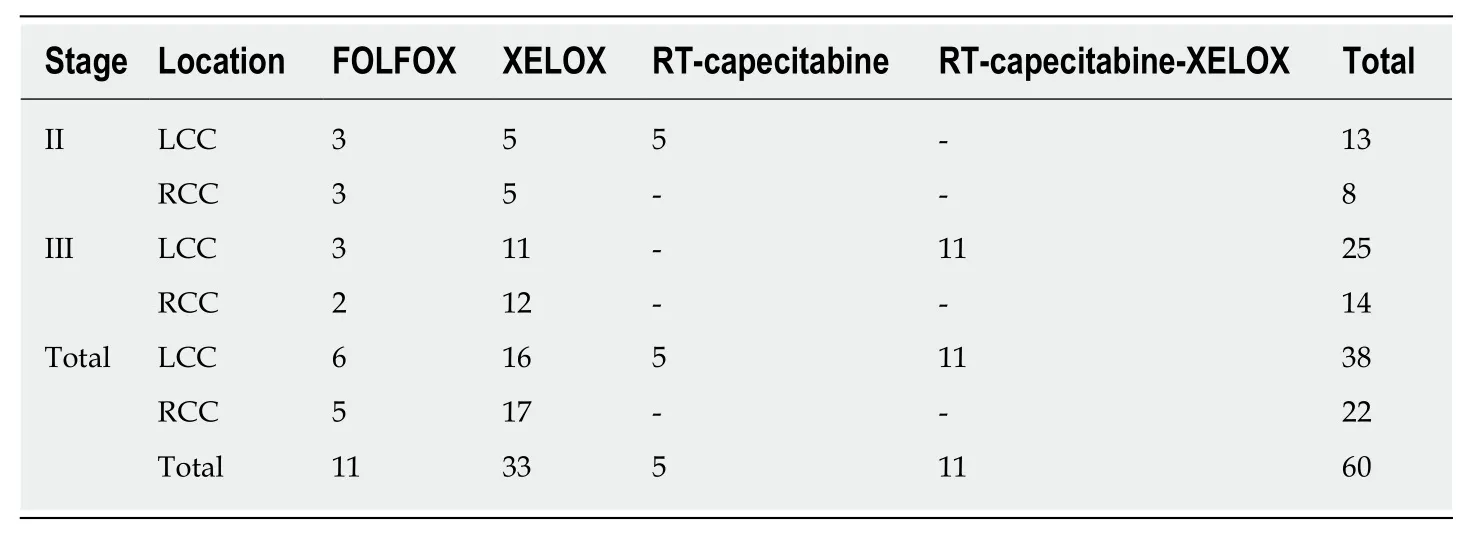
Table 6 Adjuvant and perioperative therapy across disease stages and locations
Limitations of our study include the relatively small total number of patients, the absence of molecular biomarkers and surgical specimens for all patients and the partially retrospective data collection.
In conclusion, left and RCC are two different disease entities within the same organ with differences at the histopathological, molecular and embryological level, as well as differences in terms of which molecular pathways of carcinogenesis are involved,and blood supply and exposure to microbial populations. The silent clinical presentation of RCC, combined with the older age at diagnosis and the presence of comorbidities, as well as the poor response to chemotherapeutic regimens and targeting agents, makes diagnosis and treatment a challenge to clinicians. The use of already known biomarkers is considered necessary for defining a personalized treatment plan, and PTL should be taken into account in therapeutic decision making.Further understanding of the biology of colon cancer can lead to the development of new effective therapeutic options or to the optimization of the existing ones.
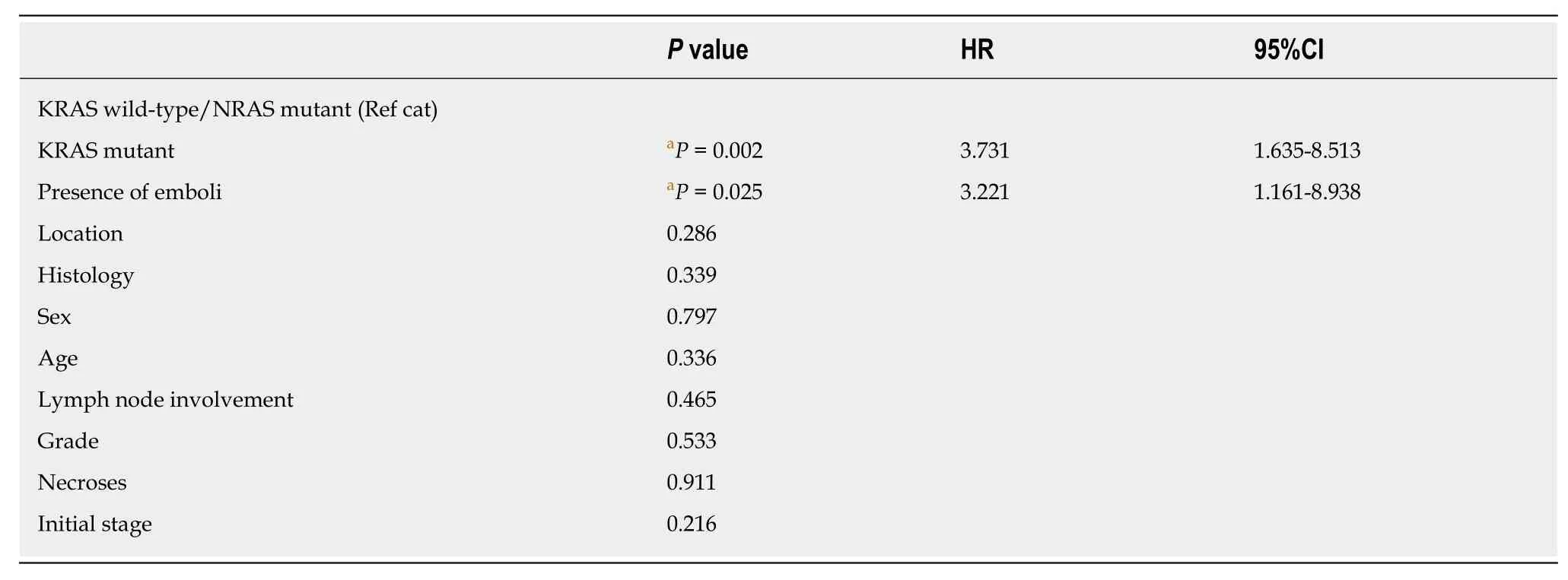
Table 7 Cox regression analysis of disease recurrence
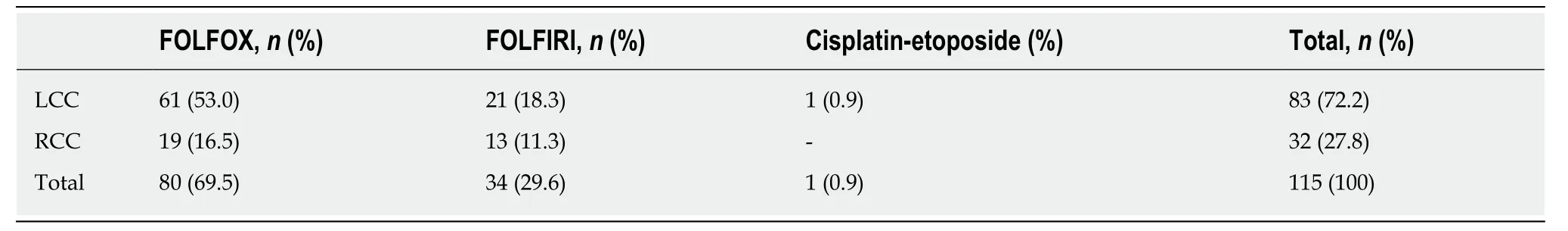
Table 8 Administered chemotherapy regimens in the first-line setting according to primary tumour location
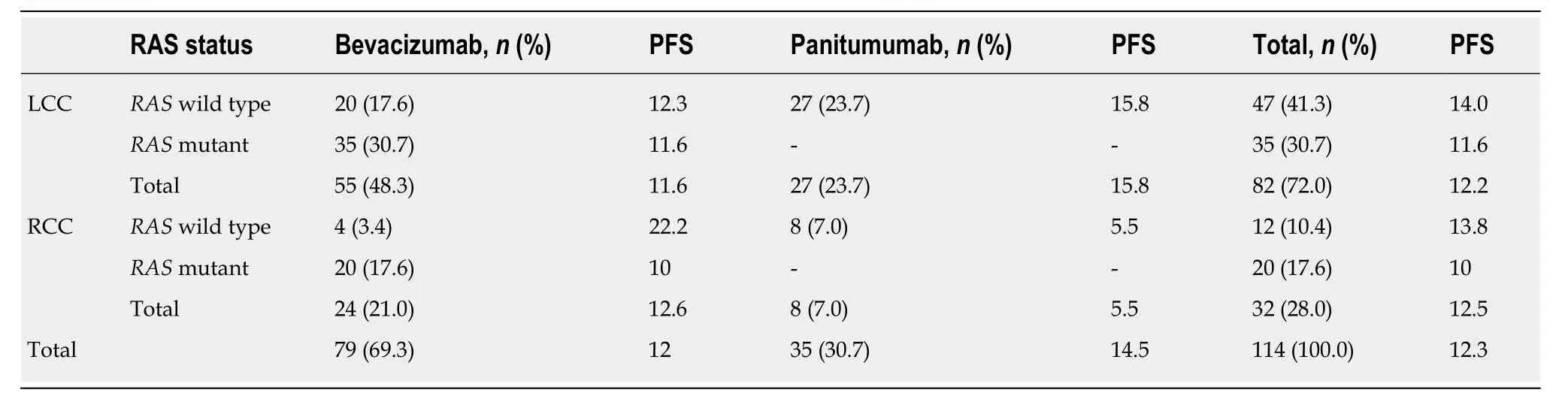
Table 9 Administered antibodies in the first-line setting according to RAS mutation status and progression-free survival time

Table 10 Cox regression analysis of first-line chemotherapy
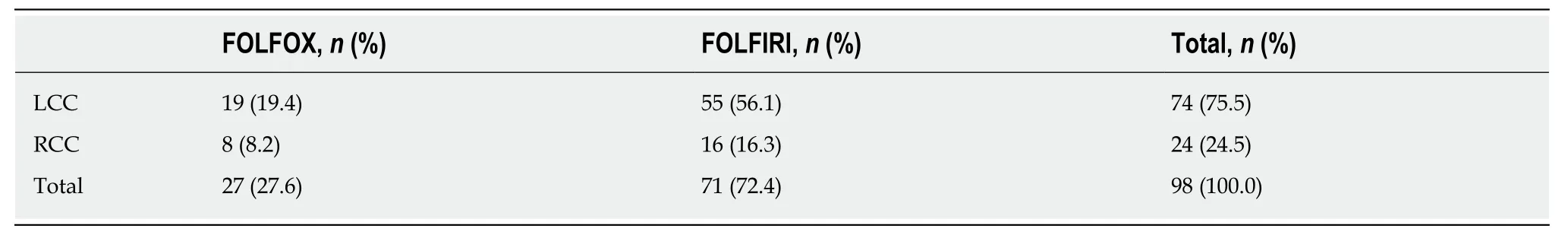
Table 11 Administered chemotherapy regimens in the second-line setting according to primary tumour location
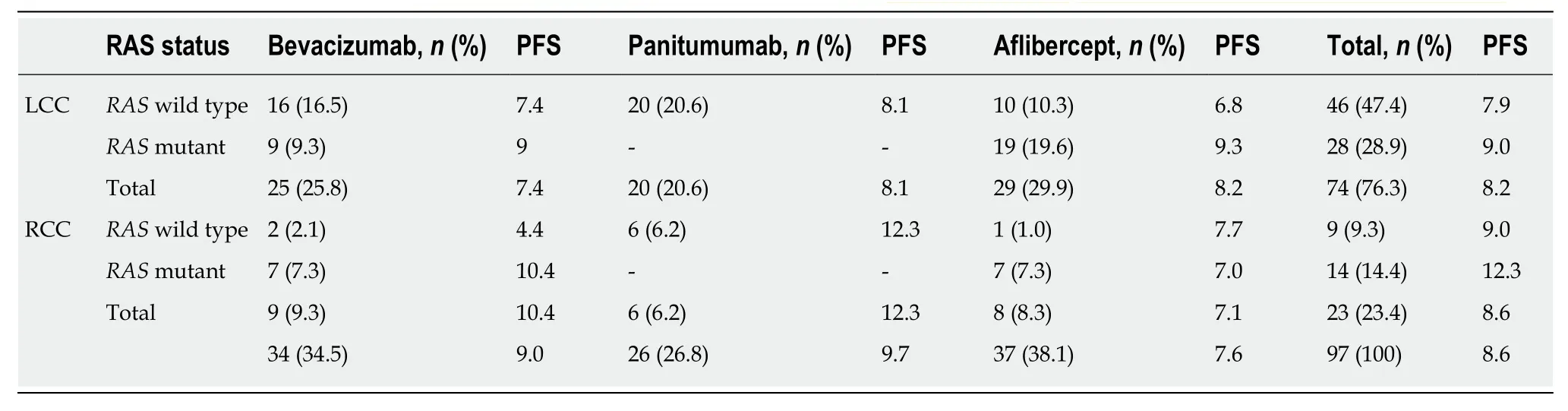
Table 12 Administered antibodies in the second-line setting according to RAS mutation status and time to progression-free survival
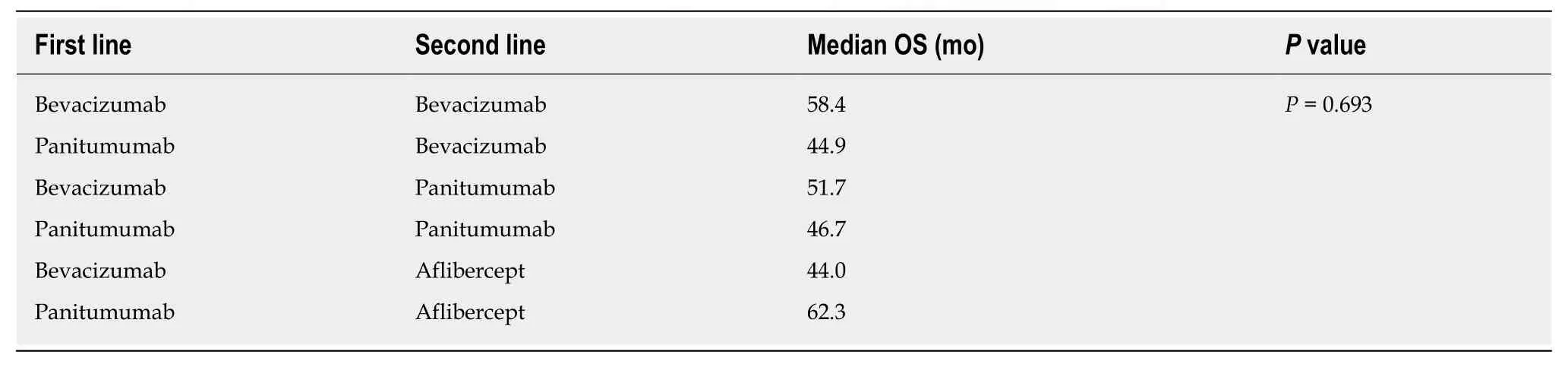
Table 13 Overall survival depending on the combination of administered antibodies in first- and second-line chemotherapy
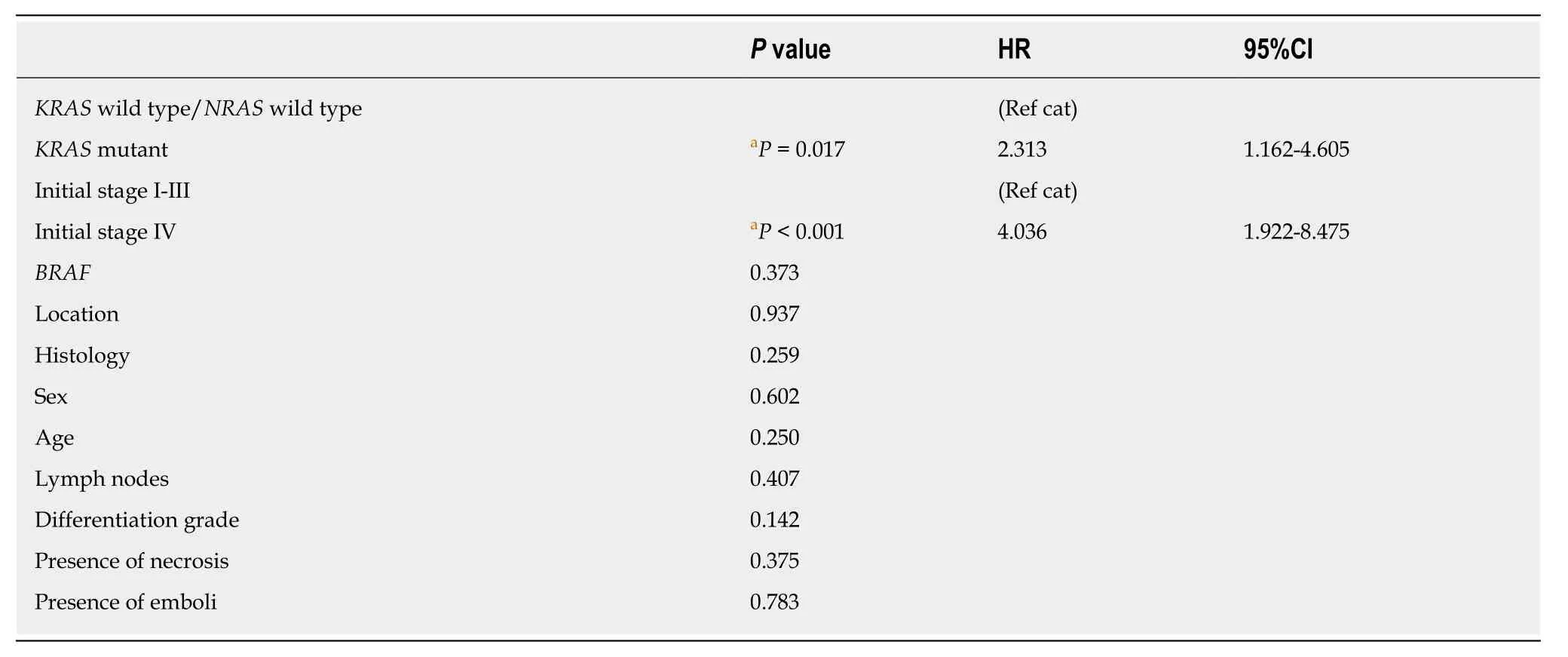
Table 14 Cox regression analysis of overall survival
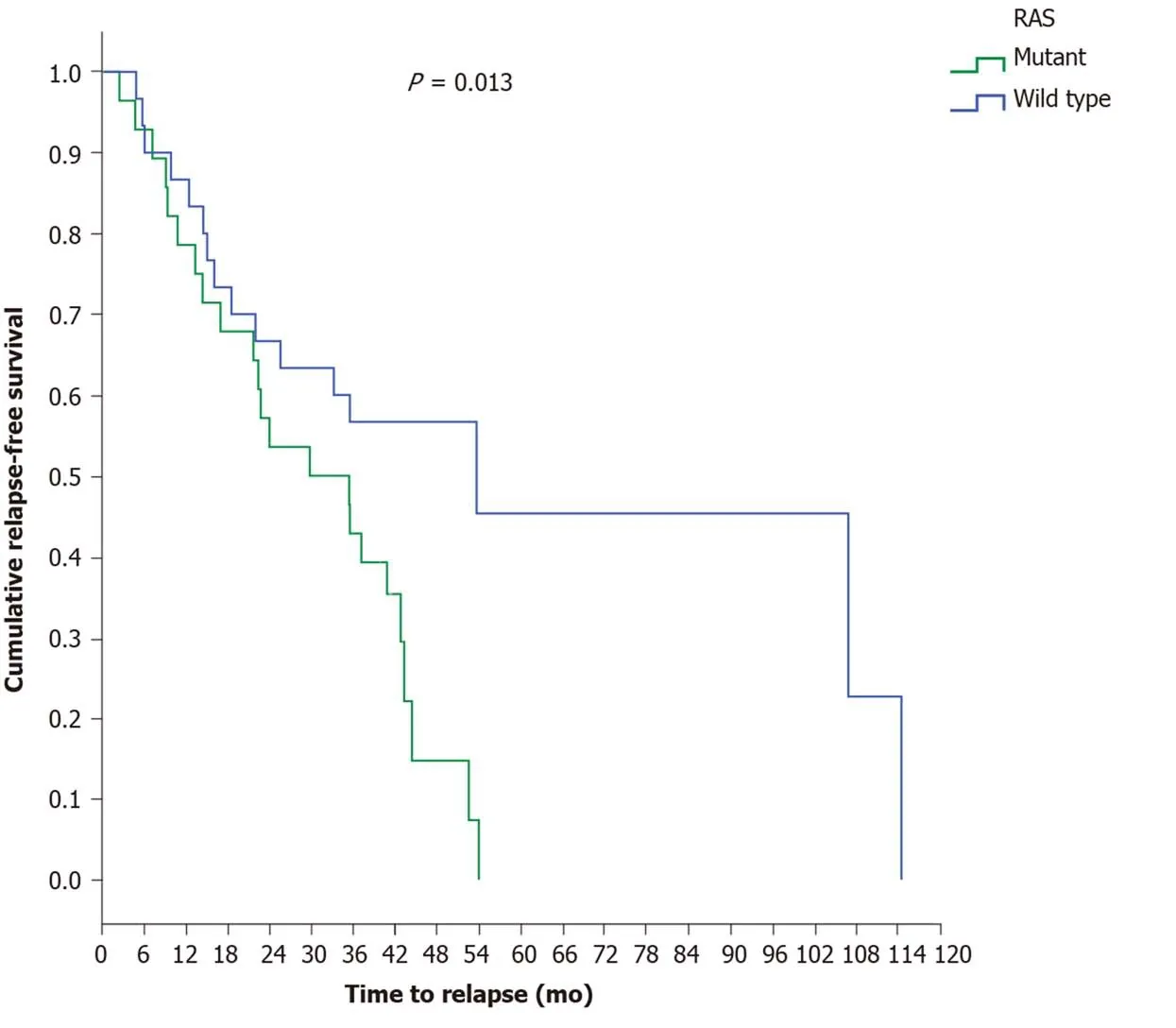
Figure 2 Disease recurrence in patients with stage Ι, ΙΙ and ΙΙΙ disease according to RAS mutation status.
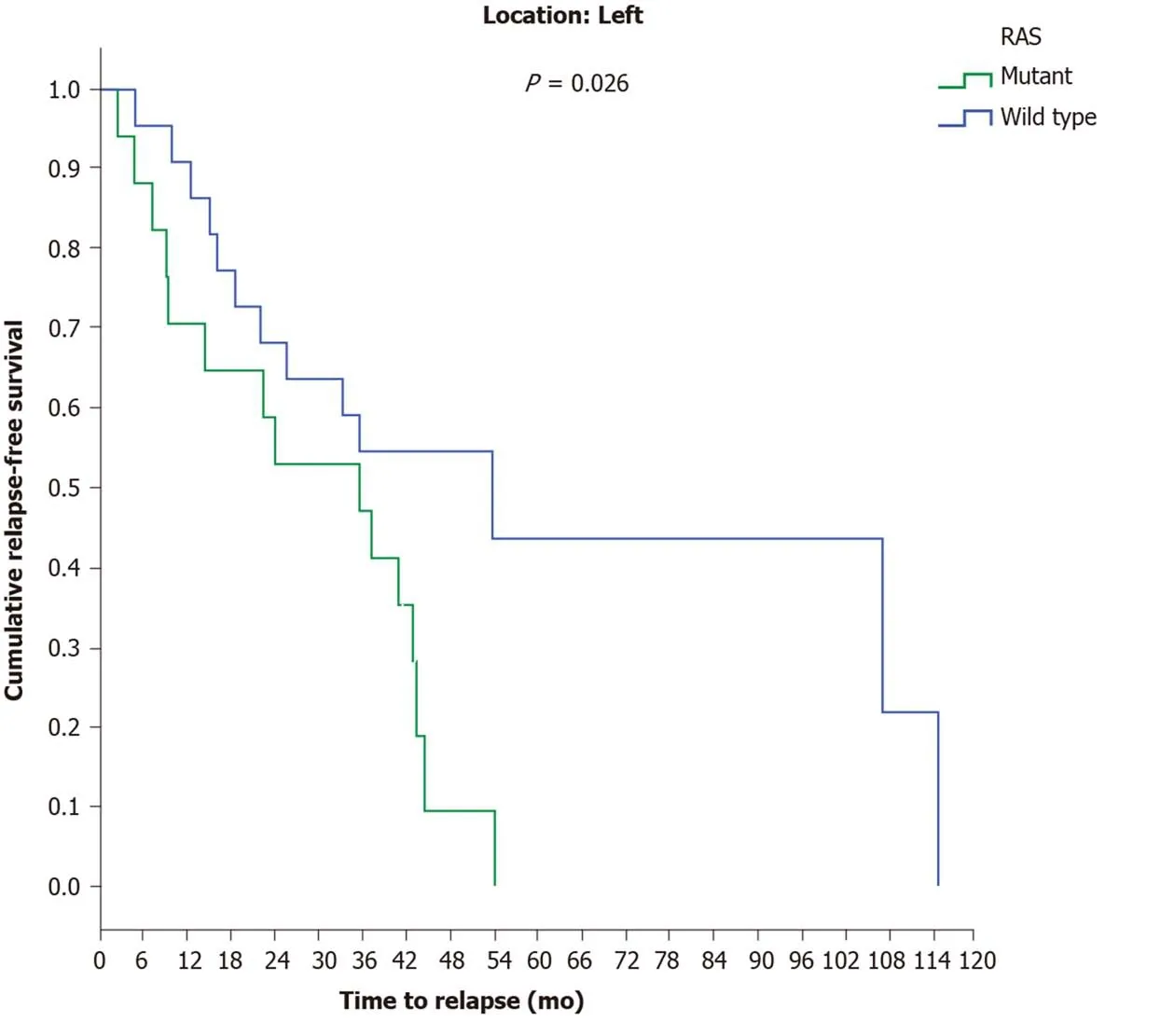
Figure 3 Disease recurrence in left colon cancer patients with stage Ι, ΙΙ and ΙΙΙ disease according to RAS mutation status.
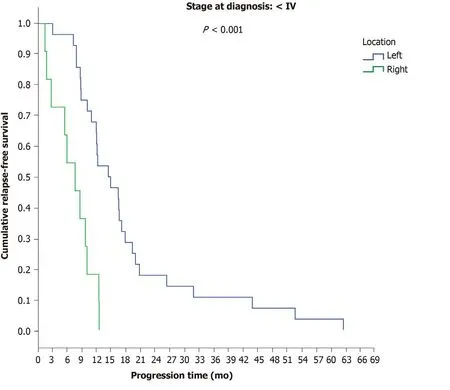
Figure 4 Progression-free survival in the first-line chemotherapy setting in patients with initial stage Ι, II and IΙΙ disease according to location.
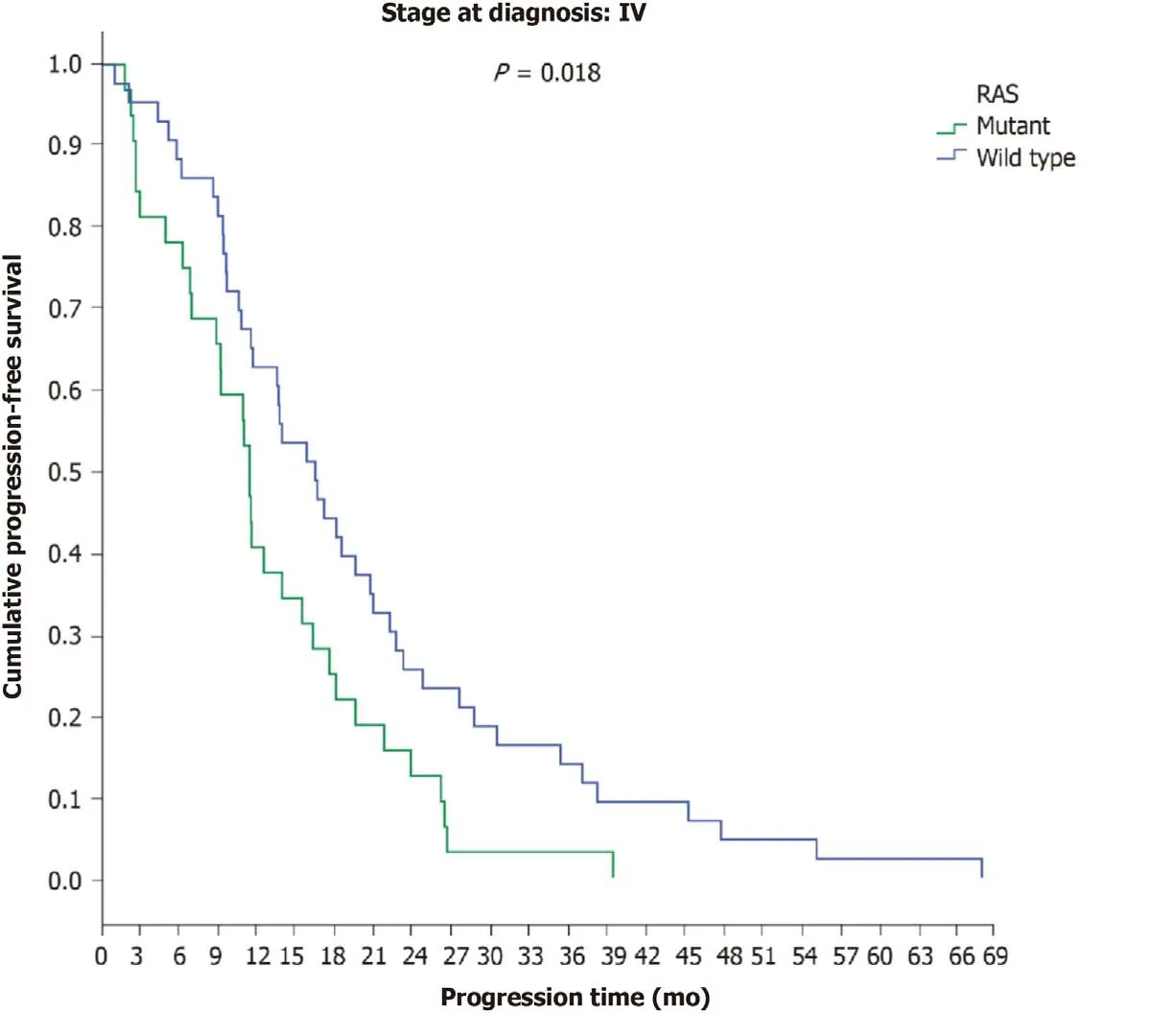
Figure 5 Progression-free survival in the first-line chemotherapy setting in patients with initial stage IV disease according to RAS mutation status.
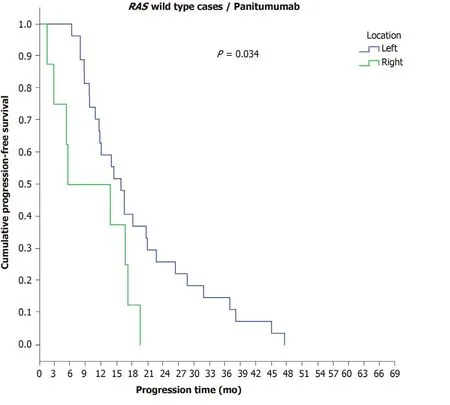
Figure 6 Progression-free survival in the first-line chemotherapy setting in patients with final stage IV RAS-wild-type disease receiving panitumumab according to primary tumour location.
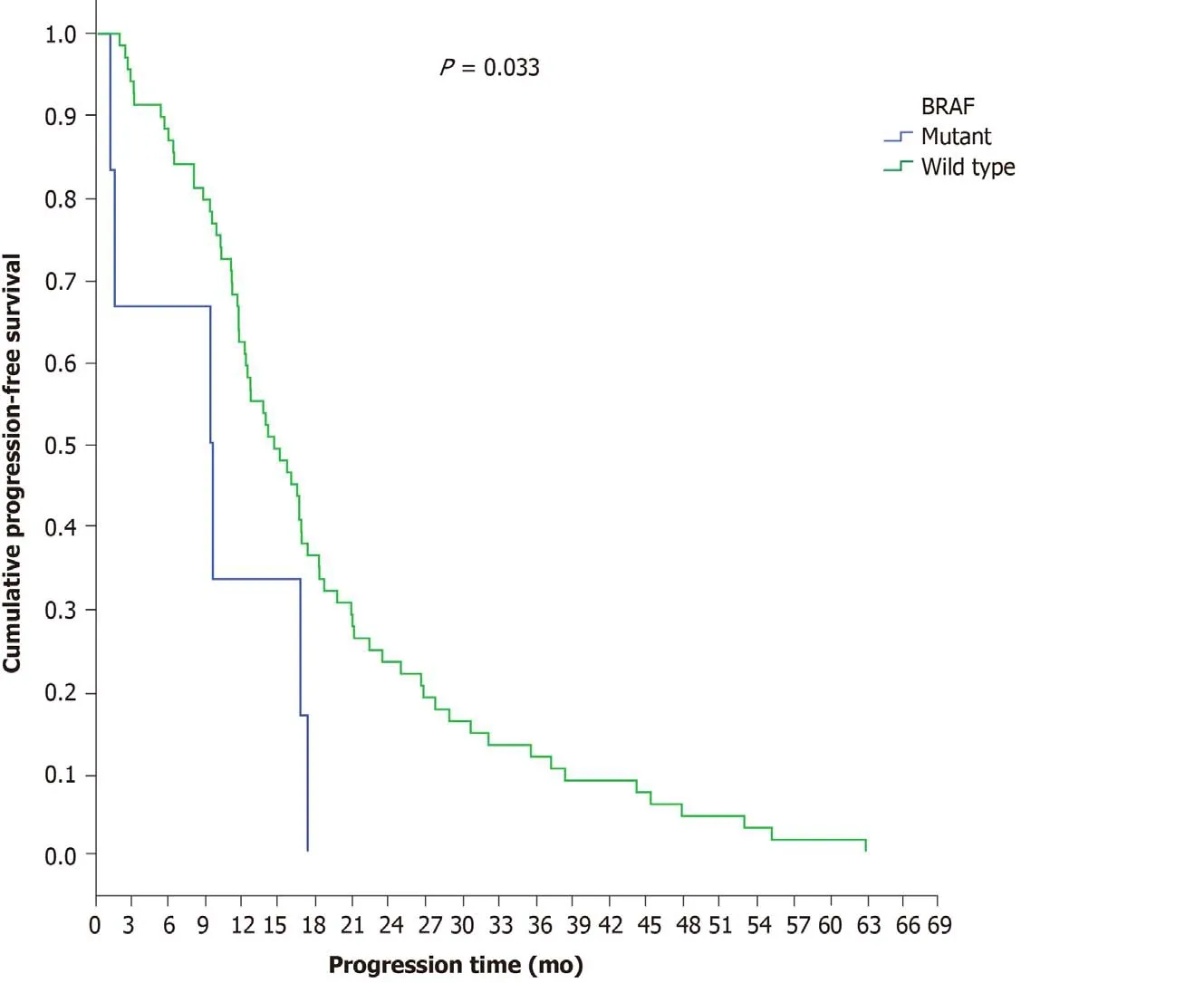
Figure 7 Progression-free survival in the first-line chemotherapy setting according to BRAF mutation status.
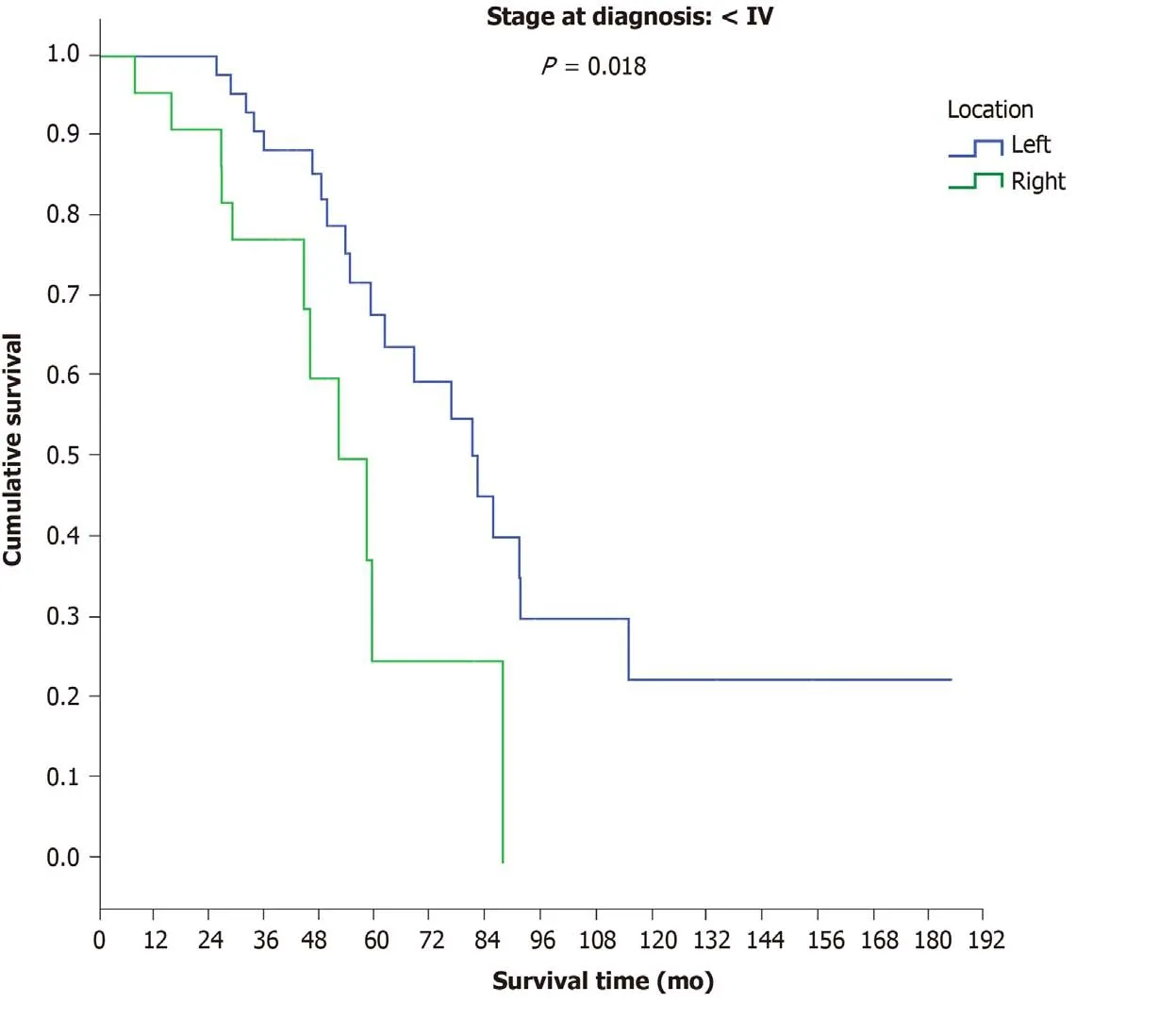
Figure 8 Overall survival among patients with initial stage I, II and ΙΙΙ disease according to primary tumour location.
ARTICLE HIGHLIGHTS
Research background
Left and right colon cancer present differences at histopathological, molecular and embryological level, blood supply and exposure to microbial populations. Different pathogenic pathways may contribute to the difference in disease behaviour and overall survival between them.
Research motivation
The absence of data regarding differences between right and left colon cancer in the Greek population was the study’s main concern. The outcomes may assist clinicians to define a therapeutic treatment plan based on molecular biology and primary tumour location.
Research objectives
The aim of this study was to investigate significant differences among Greek patients with right and left colon cancer based on epidemiological, clinical, histological and molecular characteristics as well as differences between them in terms of disease progression and overall survival as response to targeted therapy.
Research methods
A total of 144 patients with colon cancer of any stage were enrolled in this observational study.Data were collected retrospectively and prospectively during a 2.5-year period. Comparative analysis between and left and right colon cancer patients was performed. Multivariate Cox regression analysis was used to determine the independent predictive factors for progression free survival and disease specific survival.
Research results
Right colon cancer patients presented more comorbidities, worse histological and molecular characteristics as well as an insidious disease onset. Shorter overall survival, higher tumour relapse rate and poor response to targeted regimens of right colon cancer patients dictates different clinical, diagnostic and therapeutic approaches.
Research conclusions
We investigated the differences between left and colon cancer in terms of clinical presentation,histopathology and molecular biology in Greek colon cancer patients. Right colon cancer patients presented a higher rate of mucinous differentiation, infiltrated lymph nodes andKRASmutation,as well as more silent clinical presentation of the disease and a higher rate of coronary artery disease and diabetes. TheKRASgene revealed its predictive value sinceRAS-wild-type colon cancer patients who received panitumumab exhibited a better response than patients who received bevacizumab, a result that was also enhanced by the results regarding primary tumour location, as among patients withRAS-wild-type disease who received panitumumab, left colon cancer patients exhibited a better response than right colon cancer patients. On the other hand,the prognostic value of theBRAFgene was confirmed, asBRAFmutant patients demonstrated a shorter progression-free survival time period thanBRAF-wild-type patients. Disease stage at the time of diagnosis was confirmed as an important factor for survival in both univariate and multivariate analyses, while primary tumour location was found to play an important role in disease recurrence, as well as in overall survival among patients with initial stage I-III disease.Despite the relatively small size of this study and its partial retrospective nature we suggest that primary tumor location and molecular biomarkers should be taken in into account in therapeutic decision making.
Research perspectives
A personalized treatment plan should be based on histological and molecular characteristics of the tumor in association with primary tumor location. Large randomized control trials are needed to evaluate tumor response rate based on new emerging molecular biomarkers.
ACKNOWLEDGEMENTS
The authors would like to thank the employees of the Department of Patients Medical Records of Metaxa Anticancer Hospital for their time and contribution in identifying and providing the medical records of all patients who participated in the study.
 World Journal of Clinical Cases2020年8期
World Journal of Clinical Cases2020年8期
- World Journal of Clinical Cases的其它文章
- Bedside score predicting retained common bile duct stone in acute biliary pancreatitis
- Stability and infectivity of coronaviruses in inanimate environments
- Status, challenges, and future prospects of stem cell therapy in pelvic floor disorders
- Unusual presentation of congenital radioulnar synostosis with osteoporosis, fragility fracture and nonunion: A case report and review of literature
- Predictive factors for central lymph node metastases in papillary thyroid microcarcinoma
- Suicide attempt using potassium tablets for congenital chloride diarrhea: A case report
