Probiotic mixture VSL#3: An overview of basic and clinical studies in chronic diseases
Fang-Shu Cheng, Department of Dermatology, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province, China
Fang-Shu Cheng, Class 85 of 101k, China Medical University, Shenyang 110004, Liaoning Province, China
Dan Pan, Li-Xuan Sang, Department of Geriatrics, the First Affiliated Hospital, China Medical University, Shenyang 110001, Liaoning Province, China
Bing Chang, Min Jiang, Department of Gastroenterology, the First Affiliated Hospital, China Medical University, Shenyang 110001, Liaoning Province, China
Abstract
Probiotics are known as “live microorganisms” and have been proven to have a health effect on hosts at the proper dose. Recently, a kind of probiotic mixture including eight live bacterial strains, VSL#3, has attracted considerable attention for its combined effect. VSL#3 is the only probiotic considered as a kind of medical food; it mainly participates in the regulation of the intestinal barrier function, including improving tight junction protein function, balancing intestinal microbial composition, regulating immune-related cytokine expression and so on. The objective of this review is to discuss the treatment action and mechanism for the administration of VSL#3 in chronic diseases of animals and humans (including children). We found that VSL#3 has a therapeutic or preventive effect in various systemic diseases per a large number of studies,including digestive systemic diseases (gastrointestinal diseases and hepatic diseases), obesity and diabetes, allergic diseases, nervous systemic diseases,atherosclerosis, bone diseases, and female reproductive systemic diseases.
Key words: VSL#3; Intestinal barrier function; Chronic diseases; Intestinal microbial balance; Cytokines; Therapeutic use
INTRODUCTION
The internationally endorsed definition of probiotics is live microorganisms which,when administered in adequate amounts, confer a health benefit to the host[1]. VSL#3 is a commercial probiotic mixture consisting of eight bacterial strains: Four strains ofLactobacillus(Lactobacillus acidophilus,Lactobacillus plantarum,Lactobacillus casei, andLactobacillus delbrueckiisubspeciesbulgaricus), three strains ofBifidobacterium(Bifidobacterium breve, Bifidobacterium longum, andBifidobacterium infantis), and one strain ofStreptococcus(Streptococcus salivariussubspeciesthermophilus). Different kinds of bacterial strains exert different effects. The gene clusters ofS. thermophilusare forecasted to code most of the defense systems. The gene clusters ofBifidobacteriumare forecasted to code tight adherence pili in order to promote intestinal barrier integrity,and the genomes ofLactobacillusare predicted to encode signaling proteins[2]. VSL#3 has a protective effect on intestinal barrier function (IBF), which is one of the important functions for treating multiple chronic diseases. This article provides insight into the physiological characteristics of VSL#3 and its involvement in the treatment of chronic diseases. Furthermore, we review the results from a large number of basic and clinical studies about digestive systemic diseases and the use of VSL#3 for other systemic diseases (Figure 1), which are indicative of future directions for VSL#3-based therapy. VSL#3 is a kind of formula probiotic with sufficient evidence-based medical evidence in some digestive systemic diseases, but evidence is insufficient in many other systemic diseases. We need to observe whether VSL#3 is effective in these diseases in the future.
EFFECTS OF VSL#3 ON IBF
Several factors have been identified to be responsible for IBF: The mechanical barrier,biological barrier, chemical barrier, and immune barrier. The effects of VSL#3 on IBF are shown in Figure 2.
Effects of VSL#3 on mechanical barrier function
The mechanical barrier is mainly comprised of intestinal epithelial cells (IEC) and the protein networks between IEC, including desmosomes, adherent junctions, and tight junctions. Tight junctions maintain epithelial polarity and regulate selective paracellular ion solute transport[3,4]. Among all the components in the intestinal epithelium, VSL#3 mainly affects tight junctions. VSL#3 could lead to a recovery of tight junction protein injury with significantly increased occludin and zonula occludens-1 (ZO-1) and significantly decreased claudin-2 in the intestinal mucosa[5-7].The influence of VSL#3 on tight junction proteins is achieved by increasing the protein level and enzymatic activity of T-cell protein tyrosine phosphatase (TCPTP),which is the protein product of the tyrosine-protein phosphatase non-receptor type 2(PTPN2) gene, andPTPN2has a protective effect against inflammatory bowel disease(IBD)[5,8]. Then, VSL#3 mediates its beneficial effect by reducing interferon-γ (IFN-γ)signaling in a TCPTP-dependent manner and correcting the reduction in transepithelial electrical resistance (TER)[5]. VSL#3 probably increased TER by activating the mitogen-activated protein kinase p42/44 and p38 pathway in T84 cells[9]. Besides, the increase of phosphorylated-p38 (P-p38) and phosphorylated-extracellular signal-regulated kinase (P-ERK) signaling pathways by VSL#3 is also important in improving tight junction protein expression and IBF in rats[7].
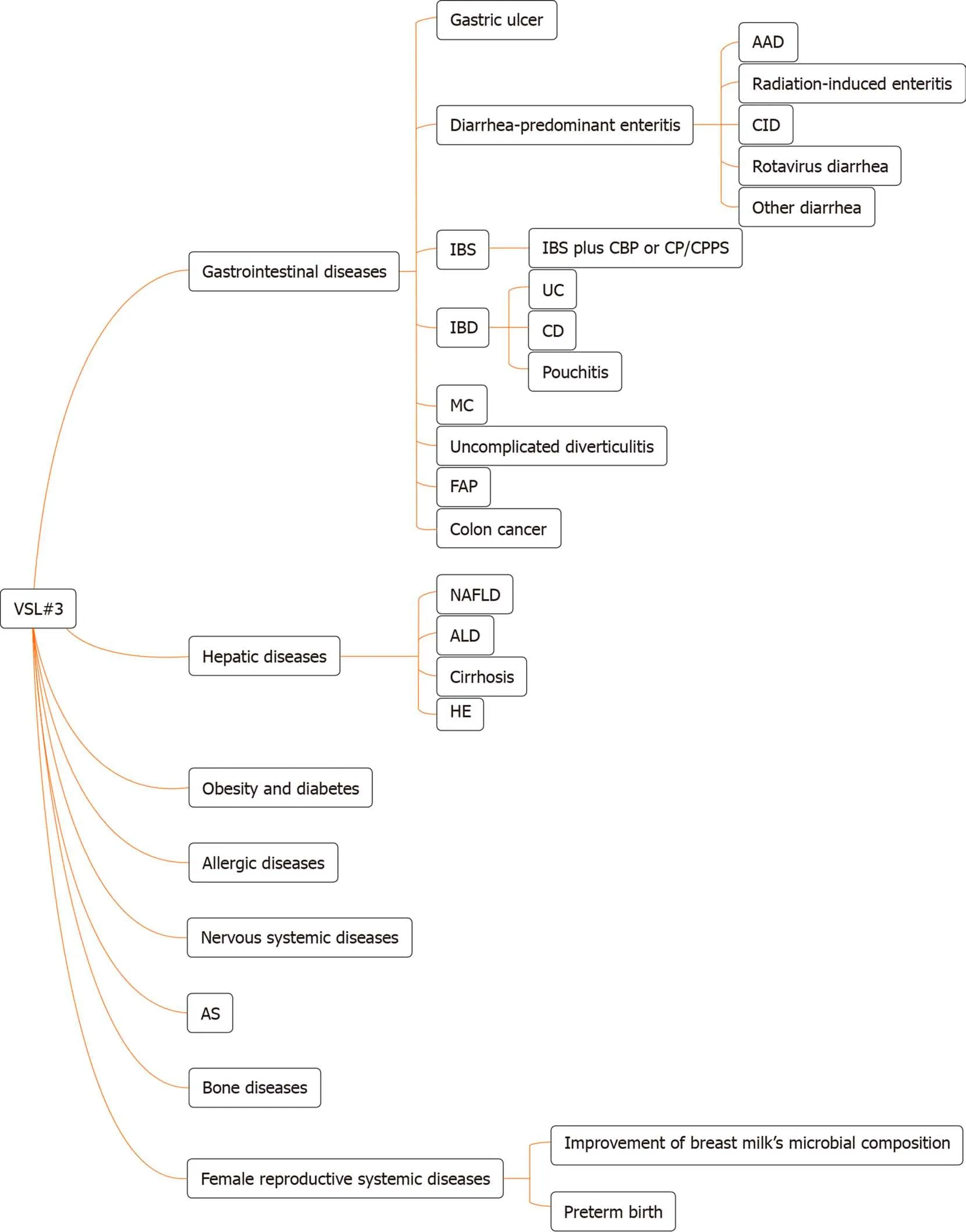
Figure 1 The types of disease for which VSL#3 can work. AAD: Antibiotic-associated diarrhea; CID: Chemotherapy-induced diarrhea; IBS: Irritable bowel syndrome; CBP: Chronic bacterial prostatitis; CP: Chronic prostatitis; CPPS: Chronic pelvic pain syndrome; IBD: Inflammatory bowel disease; UC: Ulcerative colitis;CD: Crohn’s disease; MC: Microscopic colitis; FAP: Familial adenomatous polyposis; NAFLD: Non-alcoholic fatty liver disease; ALD: Alcoholic liver disease; HE:Hepatic encephalopathy; AS: Atherosclerosis.
Effects of VSL#3 on biological barrier function
The biological barrier is formed by the resident intestinal microbiota co-inhabiting the intestinal cavity or colonizing the surface of the intestinal mucosa[3]. Most of the intestinal bacteria groups are members ofBacteroidetesandFirmicutesphyla[10].Moreover, large numbers of anaerobes grow on the surface of the intestinal mucosa,such asBifidobacteria, which can resist the invasion of foreign pathogens[3]. The production of short-chain fatty acids (SCFAs) by gut microbial fermentation reduces intestinal pH and plays an important role in maintaining intestinal biological barrier function[3,11]. VSL#3 has been reported to increase the richness and diversity of the bacteria in the intestinal mucosa, mainly by increasing the total concentration of anaerobes (includingLactobacillusandBifidobacteria) and reducing fungal diversity.However, the alteration in bacterial diversity was not caused by the colonization of bacterial strains in VSL#3 and had an independent effect[12]. The change ofBifidobacteriumconcentration was particularly obvious in individuals with a low endogenous bifidobacterial concentration, but no obvious changes were shown in individuals with a high endogenous bifidobacterial concentration[13]. In addition, the fecal concentrations ofEnterococci,Clostridia,Bacteroides, andColiformswere not altered significantly, which indicated that the beneficial effect of VSL#3 was not mediated by inhibiting the endogenous microbiota[14]. The positive effects of VSL#3 treatment were also mediated by SCFA acetate. Acetate significantly reduced colonic permeability by reducing colonic inflammation and promoting growth of the commensalLactobacillus[11].
Effects of VSL#3 on chemical barrier function
The chemical barrier includes bile, digestive fluid, lysozymes, mucopolysaccharides,other chemical substances, and antibacterial substances secreted by the intestinal tract[3]. Mucus secreted by goblet cells in the intestinal epithelium forms a first-line protective mucus layer which is able to prevent foreign pathogens from penetrating the barrier into deep tissue[15]. VSL#3 was able to increase basal luminal mucin content by 60% in Wistar rats, and it was suggested that VSL#3 regulated mucin gene expression and mucus secretion by increasingMUC2,MUC3, andMUC5ACgene expression in IEC. The changes inMUC2(3.5 fold) andMUC5AC(4.5 fold) were more prominent thanMUC3(three-fold)[9,16]. Besides, VSL#3 was reported to induce ileal bile acid deconjugation and fecal bile acid secretion. It exerted this effect by inhibiting the intestine-liver farnesoid X receptor-fibroblast growth factor 15 (FXR-FGF15) axis,which resulted in increased cholesterol-7α-hydroxylase (Cyp7a1) and sterol-12αhydroxylase (Cyp8b1) gene expression. Besides, fecal bile acid secretion was associated with increased bile salt hydrolase (BSH) transcription and enzymatic activity in VSL#3-treated mice[17].
Effects of VSL#3 on immune barrier function
The immune barrier comprises gut-associated lymphoid tissue (GALT) and disseminated immune cells, which are the effector sites of intestinal mucosal immunity[3]. GALT includes intraepithelial lymphocytes (IEL), lamina propria lymphocytes (LPL), and Peyer’s patches. Most IEL and LPL are CD103+and CD3+T cells, but the number of CD20+B cells is significantly small[18]. Furthermore, secreted immunoglobulin A (sIgA) is the main humoral immune component, and it is the first line of defense to prevent intestinal mucosal adhesion and colonization[3]. It has been shown that VSL#3 could normalize intestine epithelial barrier integrity and dampen inflammatory responses partly by decreasing the mucosal secretion of the proinflammatory cytokines tumor necrosis factor-α (TNF-α) and IFN-γ in interleukin(IL)-10 gene-deficient mice[19,20]. Similarly, VSL#3 exerted its anti-inflammatory cell protective effect by inhibiting proinflammatory nuclear factor-κB (NF-κB) pathway activation. For instance, VSL#3 decreased NF-κB stimulated endogenous immune response gene monocyte chemoattractant protein-1 (MCP-1), inhibited the degradation of NF-κB inhibitor IκB, and induced heat shock protein (HSP) at the transcriptional level in murine colonic epithelial cells. The mechanism of action was the early inhibition of the chymotrypsin-like activity of the proteasomeviasoluble factors produced by VSL#3[21]. Except for the pro-inflammatory cytokine pathway, the inhibition of proinflammatory chemokines in IEC is also important in maintaining intestinal immune barrier function. Studies demonstrated thatLactobacilluscontained in VSL#3 induced the inhibition of proinflammatory T-cell chemokine interferoninducible protein-10 (IP-10) secretion in IEC, probably associated with inducing IP-10 ubiquitination, secretingprtP-encoded lactocepin (protective bacterial protease), and destroying the vesicular pathway[22-24]. VSL#3 DNA was shown to inhibit epithelial IL-8 secretion in response toSalmonellaDNA and proinflammatory stimuli (such as TNF-α)[20].
However, one study showed an opposite mechanism of VSL#3. It demonstrated that a high dose of VSL#3 (5 × 1010CFU/d) locally stimulated epithelial innate immunity by increasing the production of TNF-α and activating the NF-κB pathway to restore IBF. The stimulation effect required the temporary colonization of VSL#3 in the intestinal lumen[25]. On the other hand, another study also indicated that VSL#3 exerted its beneficial effect by producing the proinflammatory cytokines IL-6 and IL-12 (p40) in bone marrow-derived macrophages isolated from Balb/c mice[26].
Dendrite cells (DC) are the key cells in the production of immune responses and the modulation of inflammatory signaling pathways. The stimulation of human DC with an appropriate dose of VSL#3 will induce the maturation of immature DC[27,28].However, VSL#3 (105organisms/mL) did not change the immature phenotype and costimulatory molecule expression of DC, and VSL#3 (107organisms/mL) was shown to increase the expression of CD40, CD80, CD86, and major histocompatibility complex (MHC) class II I-Ad during the last 3 d of culture in bone marrow-derived dendritic cells[29]. The effects of VSL#3 on DC maturation were dose- and timedependent, and reached the maximum at the 107dose after 18 h of co-culture[28].Besides, VSL#3 has been shown to decrease the expression of CD80 and increase the expression of CD83, but it had no effect on CD86[30]. In terms of cytokine expression,VSL#3 inhibited the lipopolysaccharide (LPS)-induced expression of chemokines(CXCL9, CXCL10, CCL2, CCL7, and CCL8) mainly by inhibiting STAT-1 phosphorylation under the stimulation of human monocyte-derived DC with LPS and VSL#3[27]. VSL#3 also decreased IL-12 (p40) production induced by LPS, and was a potent inducer of IL-10 produced by DC from blood and intestinal tissue[30].Additionally, one study showed that VSL#3 had an anti-inflammatory effect by decreasing the influx of innate immune cells (CD11b+) and adaptive immune cells(CD4+/CD8+) in the intestinal mucosa and reduced proinflammatory serum cytokines(IL-17, IL-1α, IL-1β, and granulocyte-macrophage colony-stimulating factor [GMCSF])[31]. Then, a study showed that VSL#3 down-regulated the signaling pathway of Toll-like receptors (TLR), mainly TLR4, and the related effector pathways such as T-cell and B-cell receptor signaling in IL-10 knockout (KO) mice. Meanwhile, VSL#3 upregulated the peroxisome proliferator-activated receptor α (PPARα) signaling pathway and lipid signaling genes, which had a potent antagonistic action on the NF-κB inflammatory process[32].
EFFECTS OF VSL#3 ON GASTROINTESTINAL DISEASES
Gastrointestinal diseases are a worldwide problem, and the imbalance of intestinal microbiota is one of the important factors in multiple gastrointestinal diseases. Thus,probiotics play a significant role in these diseases by modulating intestinal microbiota.Studies have indicated that VSL#3 provides benefits in gastric ulcer, diarrheapredominant enteritis, irritable bowel syndrome (IBS), ulcerative colitis (UC),pouchitis, and colitis in animals and humans.
Gastric ulcer
Gastric ulcer healing is a complex process, involving the filling of mucosal defects through the proliferation and migration of epithelial cells and connective tissue cells to restore the mucosal architecture[33]. A study demonstrated that VSL#3 is effective at high concentrations (1.2 × 1010bacteria) in enhancing acetic acid-induced gastric ulcer healing by stimulating the expression and production of angiogenesis promoting growth factors, mainly vascular endothelial growth factor, in rats[34]. However, there are no related trials in humans.
Diarrhea-predominant enteritis
The occurrence of diarrhea is closely related to the change of gastrointestinal (GI)motility as a result of enteritis. Different bacterial components in VSL#3 showed different effects on GI motility in guinea-pig isolated tissue.Lactobacillusin VSL#3 stimulated the contraction of ileum segment. All bacteria and non-protein cytoplasm components (not DNA) of VSL#3 were able to induce reverse proximal colon relaxation and then decrease fecal frequency to improve enteritis[35]. Table 1 shows the clinical and patient-reported characteristics of trials in various kinds of diarrheapredominant enteritis.
Antibiotic-associated diarrhea:Diarrhea is a common adverse event of antibiotic medication. The occurrence of diarrhea is probably due to disruption of the normal gastrointestinal microbiota. The effect of probiotics deserves discussion[36].Clostridium difficile-associated diarrhea (CDAD) is the most serious form of antibiotic-associated diarrhea (AAD). A trial suggested that VSL#3 could prevent the occurrence of AAD significantly in patients exposed to systemic antibiotics in an average-risk hospital. A shorter hospital stay was related to the reduction of AAD incidence, but it was not significant[37]. The effect of VSL#3 on CDAD remained unknown because no CDAD cases were included in this trial.
Radiation-induced enteritis:Enteritis and colitis characterized by diarrhea are severe complications of radiotherapy that may lead to the development of bacterial overgrowth in patients who have cancer[38]. Two trials have addressed the utility of adding VSL#3 to radiation-induced enteritis therapy. They demonstrated that VSL#3 was a great approach to prevent the occurrence and reduce the severity of radiationinduced enteritis with good tolerance[39,40]. One trial showed that more patients treated with placebo had radiation-induced enteritis (51.8%vs31.6%) and suffered grade 3 or 4 diarrhea (55.4% and 1.4%) compared with patients treated with VSL#3. The upregulation of the innate immune reaction against invasive microbiota is probably one of the important mechanisms underlying the therapeutic effect of VSL#3 in patients with radiation-induced enteritis[39]. Recommendations for probiotic use from the Yale/Harvard workshop showed that the recommended level for VSL#3 use in radiation-induced enteritis was a C rating[41].
Chemotherapy-induced diarrhea:Chemotherapy can cause diarrhea, which worsens the quality of life of cancer patients. An animal study demonstrated that VSL#3 with irinotecan was able to prevent severe diarrhea following chemotherapy and weight loss in female rats[42]. However, it is still uncertain whether VSL#3 is effective in clinical application.
Rotavirus diarrhea:Rotavirus is one of the main causes of severe gastroenteritis in children[43]. It is transmitted directly through the fecal-oral route, and the main symptom of rotavirus infection is diarrhea, which is usually accompanied by abdominal pain, vomiting, and fever. One randomized controlled trial (RCT)indicated that the administration of VSL#3 resulted in better overall recovery rates and reduced frequency of oral rehydration salts and intravenous fluid use in children with acute rotavirus diarrhea[44].
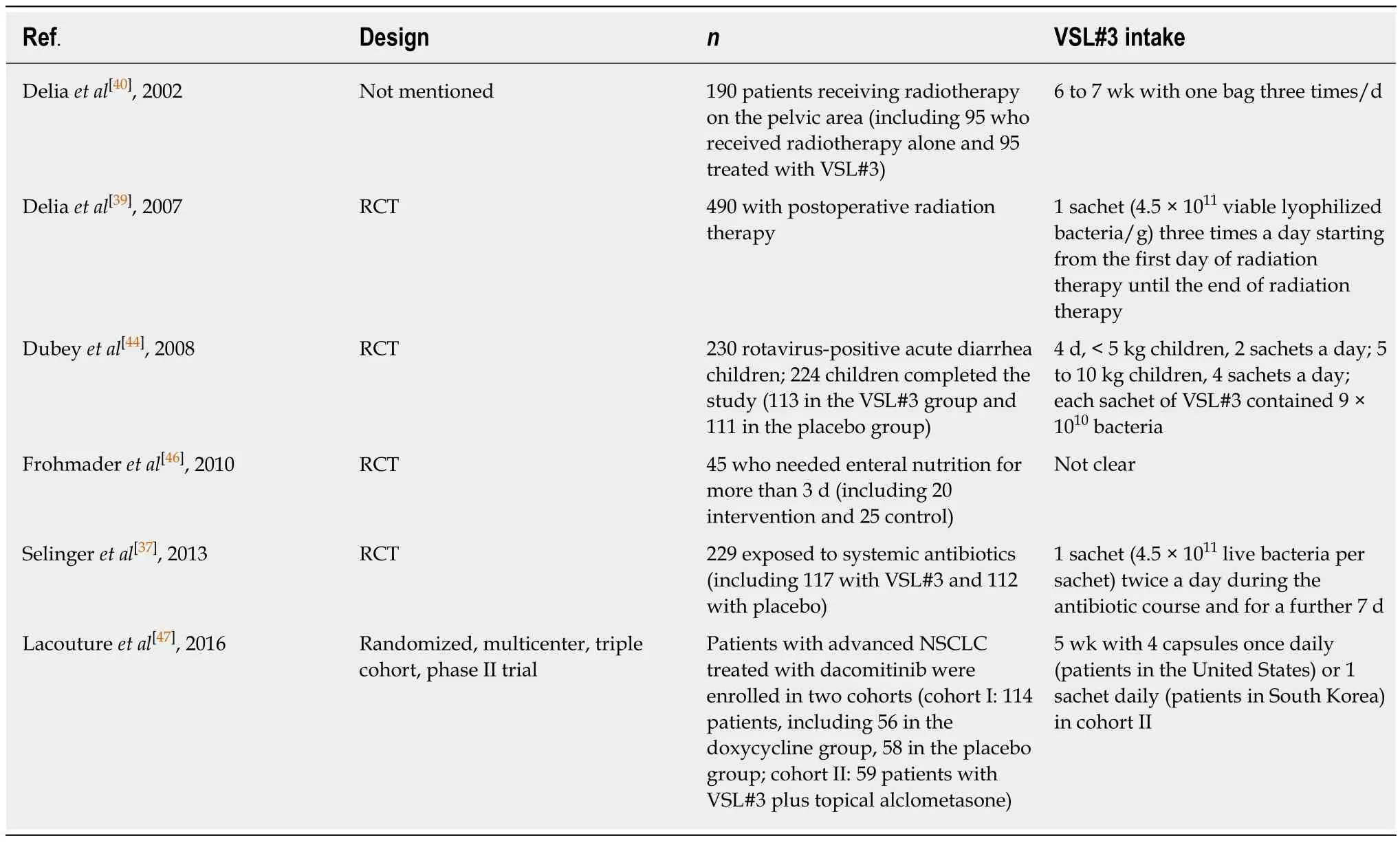
Table 1 Trials assessing the effect of VSL#3 in patients with diarrhea-predominant enteritis
Other diarrhea:Gastrointestinal complications are common complications in enterally-fed critically ill patients. They will prolong hospitalization and increase the mortality of patients[45]. A pilot trial indicated that VSL#3 had benefits in reducing the frequency of liquid and loose stool and stool weight, further minimizing diarrhea in enterally-fed critically ill patients[46].
A randomized phase II trial demonstrated that VSL#3 had no effect on the incidence of all-grade diarrhea adverse events and the quality of life of advanced nonsmall-cell lung cancer patients treated with dacomitinib, which is a tyrosine kinase inhibitor[47,48]. An improvement may have been induced by VSL#3 in tissue functions,but this requires confirmation.
IBS
IBS is a common chronic gastrointestinal functional disease characterized by sudden changes of two major symptoms: Constipation and diarrhea. The pathogenesis of IBS remains unclear, but an improvement of colon dysmotility and visceral hypersensitivity may play an important role in treating it[49]. One trial published the results of 25 patients with diarrhea-predominant IBS who consumed VSL#3 or placebo for 8 wk. It demonstrated that VSL#3 might relieve abdominal bloating,especially in patients with higher bloating scores, but VSL#3 had no significant effect on colonic transit and bowel function[50]. Another study showed that VSL#3 was able to dampen clinical symptoms by improving the mechanical distensions of the colonic wall in patients with diarrhea-predominant IBS[49]. In terms of bloating-predominant IBS, VSL#3 resulted in a reduction of flatulence scores during the first 4 wk (VSL#3 30.8 ± 2.5vsplacebo 40.1 ± 2.5) and retard of colonic transit compared with placebo,but no significant alteration in other individual symptoms was observed in patients administered with VSL#3[51]. In addition, another trial suggested that VSL#3 was able to improve IBS symptoms significantly by increasing salivary morning melatonin,particularly in males and individuals with normal circadian rhythm[52]. Furthermore,VSL#3 was superior to placebo in improving symptoms including abdominal pain and abdominal bloating in children with IBS[53]. But the mechanism has not been clearly studied. The recommended level for VSL#3 use in IBS was a B rating[41].
In a rat model of IBS, one study demonstrated that VSL#3 could reverse visceral hyperalgesia and allodynia and reset gene expression related to pain and inflammation (such as tryptophan hydroxylase 1 [TPH1])[54]. Also, VSL#3 treatment decreased visceral hypersensitivity probably by decreasing the number of mast cells in the colon and decreasing protease-activated receptor 2 and transient receptor potential vanilloid type 1 expression in dorsal root ganglia in an experimental rat model of IBS[55]. Furthermore, nitric oxide (NO) might participate in the beneficial effect of VSL#3 to some extent[56].
IBS plus chronic bacterial prostatitis or chronic prostatitis/chronic pelvic pain syndrome:In general, IBS has a large number of complications including gastrointestinal and non-gastrointestinal complications such as chronic pelvic pain[57].Moreover, patients with IBS and chronic bacterial prostatitis (CBP) had a significantly greater number of male accessory gland infections compared with patients with CBP alone[58]. Two trials considered the use of rifaximin followed by VSL#3 in patients with IBS and CBP or chronic prostatitis/chronic pelvic pain syndrome(CP/CPPS)[59,60]. The first trial showed that the therapy may improve urinary and gastrointestinal symptoms with significantly reducing total National Institute of Health Chronic Prostatitis Symptom Index scores in patients with diarrheapredominant IBS plus CP/CPPS[59]. Besides, the second trial demonstrated that longterm treatment with VSL#3 and rifaximin was effective in reducing the evolution of chronic prostatitis into more complicated infections of male accessory glands, such as chronic microbial prostate-vesiculitis or prostate-vesiculo-epididymitis (PVE), in infertile patients with IBS plus cured CBP[60]. All the information for the human trials above is shown in Table 2.
IBD
IBD consists of UC, Crohn’s disease (CD), and a kind of undefined enteritis. The etiology and pathogenesis of IBD are not quite clear at present. It is currently thought that possible factors include environmental factors, genetic susceptibility, infection factors, and a dysregulated immune system. An improper immune response to the intestinal microbiota probably contributes to the occurrence of IBD in genetically susceptible hosts. Dietary factors among environmental factors might influence immune function and could also be some of the risk factors for the occurrence of IBD[61,62]. One study indicated that milk and fried food were associated with an increased risk of IBD[63]. The treatment of IBD may be associated with multiple mechanisms, such as by altering the intestinal microbial composition to improve IBF.
UC:UC is a kind of chronic inflammatory colonic disease. In a systemic review,Eubacterium rectaleandAkkermansiawere decreased in all three studies, andEscherichia coli(E. coli) was increased in four of nine studies for patients with UC[64]. Traditionally,UC can be treated or maintained with immunomodulatory or anti-inflammatory drugs, such as 5-aminosalicylic acid (5-ASA)[65]. Also, VSL#3 has been reported to have a maintenance effect. One trial enrolled 20 patients with UC in remission who were treated with VSL#3 and identified that 15 patients were still in remission. The underlying mechanism is not completely understood, but it was shown that VSL#3 was able to induce a significant increase of protective bacterial strains (Streptococcus salivariussubspeciesthermophilus,Lactobacillus, andBifidobacteria) compared to their basal levels in feces of patients. Furthermore, VSL#3 could colonize the intestine and decrease fecal pH from the 20th day of treatment period and keep it stable[66].
Moreover, there are several trials (Table 3) demonstrating the therapeutic action of VSL#3 in adults with mild-to-moderate UC. In two trials, VSL#3 was both capable of reducing the UC disease activity index (DAI) scores and ameliorating clinical symptoms significantly compared with placebo[67,68]. One trial indicated that there were 42.9% of patients given VSL#3 who achieved remission and only 15.7% of patients given placebo who achieved remission[68]. Besides, VSL#3 and the traditional drugs seem to have a synergistic action. The mechanism is unclear, but it was possible that VSL#3 could increase the anti-inflammatory action of 5-ASA, which could reduce free radical production and then leukotriene and IL-1 production[67,69]. One trial also demonstrated that the combination therapy of low-dose balsalazide and VSL#3 was more effective than balsalazide or mesalazine alone in obtaining the remission of acute mild-to-moderate UC[70]. A longer treatment with VSL#3 may provide more of an improvement. It was shown that VSL#3 could improve more parameters (such as stool frequency) significantly in a 12-wk trial than in an 8-wk trial[67,68]. In addition, an open-label trial indicated that treatment with VSL#3 would induce remission (DAI ≤2) and response (decrease in DAI ≥ 3, but final score ≥ 3) in 77% of patients with active mild-to-moderate UC. Two bacterial components (S. salivariussubspeciesthermophilusandB. infantis) in VSL#3 played an important role in inducing the remission by reaching the diseased bowel site[71]. Then, the effect of alkaline sphingomyelinase was investigated in 15 UC patients treated with VSL#3 for 5 wk. The results clarified that VSL#3 up-regulated mucosal alkaline sphingomyelinase activity and improved UC[72].
VSL#3 also has a beneficial effect in children with UC. One RCT indicated that VSL#3 was effective in the maintenance of remission and reduction of recurrence inchildren with active UC[73]. Except for the role in maintaining remission, another pilot study demonstrated that therapy with VSL#3 resulted in a remission of the disease in children with mild-to-moderate acute UC[74]. In addition, the recommended level for VSL#3 use in maintaining the remission of UC was an A rating and the recommended level in inducing remission was a B rating[41].

Table 2 Trials assessing the effect of VSL#3 in patients with irritable bowel syndrome
Several studies have provided evidence about the effect of VSL#3 in dextran sulphate sodium (DSS)-induced colitis. One study demonstrated that VSL#3 (0.5 mL/d) decreased intestinal bacteria diversity related to tissue injury. It also locally produced conjugated linoleic acid which targets the myeloid cell peroxisome proliferator-activated receptor γ (PPARγ) in the colon to inhibit colitis[75]. Besides, one placebo-controlled study showed an obvious decrease in inflammation and a reduction of epithelial permeability upon administration of high-dose VSL#3 (15 mg/d for 7 d) to patients[76]. However, one study showed that VSL#3 (0.2 mL/d,containing 4 × 109CFU of freeze-dried VSL#3 for 14 d) was not able to heal DSS-induced murine colitis or reinforce the mucus barrier. It revealed that VSL#3 had a tendency to decrease histological inflammation but not change colon inflammatory characteristics. Furthermore, the increase ofBifidobacteriawas not enough to repair the intestinal barrier impairment[77]. The above results are not exactly consistent, and the negative result may be related to the sex of mice and the induced pattern of DSS colitis. The dose, mode of administration, and treatment course of VSL#3 also impact the result to some extent. In addition, the anti-inflammatory effect is still the main therapeutic mechanism of VSL#3 in DSS-induced rat colitis. Both heat-killed and live VSL#3 could reduce DSS-induced rat colitis by reducing IL-23, IL-6, STAT3 and phosphorylated-STAT3 (P-STAT3) expression in colonic tissue[78,79]. The expression of inflammatory-related mediators (inducible nitric oxide synthase [iNOS],cyclooxygenase-2 [COX-2], and NF-κB) was also inhibited by VSL#3[80]. In DSS-induced murine colitis, VSL#3 was shown to reduce the production of proinflammatory chemokine KC and macrophage inflammatory protein-2 (MIP-2)and up-regulate transforming growth factor-β (TGF-β), fibroblast growth factor-1, and vascular endothelial growth factor-A (VEGF-A) in Muc2-deficient mice[11].Furthermore, VSL#3 improved various parameters of DSS-induced colitis (such as myeloperoxidase [MPO]) in weanling rats[81]. TLR9 signaling and myeloid differentiation marker 88 (MyD88) also played an important role in mediating the anti-inflammatory effect of VSL#3[26].
CD:CD is a kind of patchy transmural inflammation affecting any part of the intestinal tract. The pathogenesis of CD is associated with the defect of mucosal innate immunity accompanied by the failure of clearing bacteria and bacterial debris, whichleads to an improper adaptive immune response to the intestinal commensal bacteria.The increase of T-helper 1 (Th1) cytokines by LPL was observed in human and murine CD[82,83]. In a systemic review,ChristensenellaceaeandCoriobacteriaceaein all three studies andFaecalibacterium prausnitziiin six of eleven studies were decreased compared with controls, andActinomyces,Veillonella, andE. coliwere increased in two studies for patients with CD[64]. Some patients with CD will require an operation, but surgery cannot cure it, and post-operative recurrence is common[84]. One RCT assessed the ability of VSL#3 to prevent human CD endoscopic recurrence after surgery. In this trial, 10% of patients in the early VSL#3 group (administration of VSL#3 for the whole 365 d) did not have severe lesions at the 90th day but developed severe recurrence at the 365th day compared with 26% of patients in the late VSL#3 group (administration of VSL#3 from the 90th day to the 365th day)[85]. This finding indicated that the exposure time of VSL#3 is closely associated with its therapeutic effect. However, for the two groups, the Crohn’s Disease Activity Index and Inflammatory Bowel Disease Questionnaire (IBDQ) scores were similar[85].
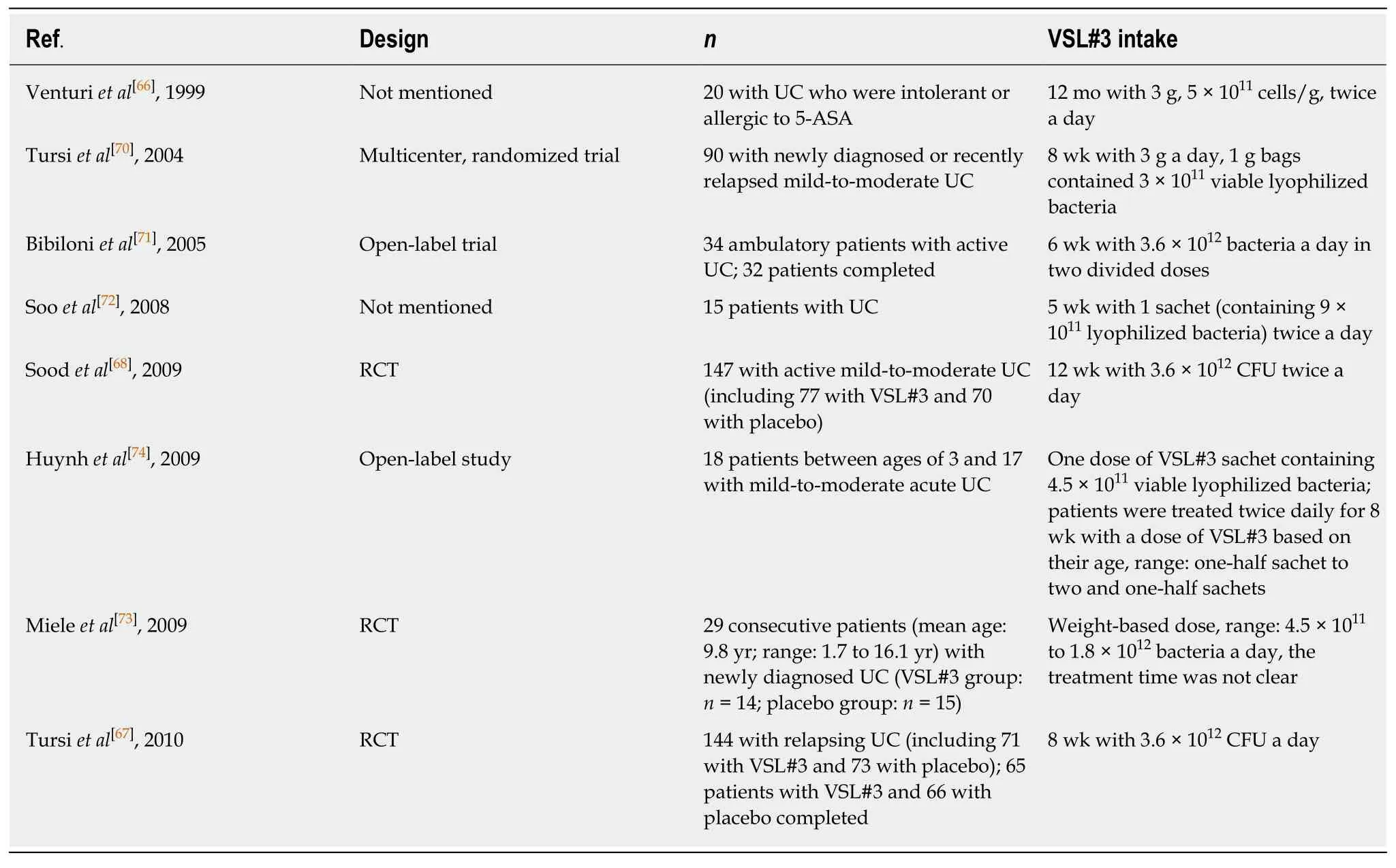
Table 3 Trials assessing the effect of VSL#3 in patients with ulcerative colitis
Moreover, several studies elucidated the protective effect of VSL#3 in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis from different mechanisms. For instance, VSL#3 was proven to reduce the up-regulation of gene clusters associated with mast cells in TNBS colitis by suppressing chemokine gene expression[31].Recently, a study demonstrated a different protective mechanism of VSL#3 mediated by the increase of IL-10 and IL-10-dependent TGF-β-bearing regulatory cells in a latent-associated protein form[86]. Then, one study showed thatBifidobacteriaand VSL#3 had similar effects in ameliorating intestinal inflammation by decreasing the concentrations of serum and fecal high mobility group box 1, and both probiotic treatment might decrease F4/80+levels in lamina propria mononuclear cells in TNBS-induced murine colitis[87]. Besides, it was shown that VSL#3 resulted in lower macroscopic and microscopic damage in the proximal colon and lower macrophage infiltration in rat acute TNBS-induced colitis[88].
Pouchitis:Pouchitis is a nonspecific inflammation of the ileal reservoir, which is known as a long-term complication after ileal pouch anal anastomosis (IPAA)surgery. It is characterized clinically by abdominal pain, bloating, and increased stool frequency[89]. Four clinical trials in Table 4 demonstrated that VSL#3 could prevent or maintain remission of chronic pouchitis[12,14,90,91]. It was reported that 10% of patients in the VSL#3 group had an onset of acute pouchitis compared with 40% of patients in the placebo group after IPAA for UC, and more patients treated with VSL#3 (17 patients, 85%) maintained antibiotic-induced remission of pouchitis compared with patients treated with placebo (one patient, 6%)[14,90]. The recommended level was an A rating[41]. In addition, an open-label trial clarified that high-dose VSL#3 (3.6 × 1012bacteria/d) was effective in treating mildly active pouchitis. In this trial, almost 70%of patients achieved complete remission after treatment with VSL#3[92]. The recommended level was a C rating[41]. It suggested that the recommended level of VSL#3 in inducing the remission of pouchitis was not as good as that in preventing and maintaining the remission of pouchitis. Also, VSL#3 could improve the quality of life of patients by improving IBDQ scores significantly[14,92]. The potential mechanism of VSL#3 may be mediated by improving IBF. Although VSL#3 was effective in the therapy of chronic pouchitis, an open-label trial indicated that most patients with antibiotic-dependent pouchitis were not capable of accessing the long-term therapy of VSL#3, mainly due to recurrent symptoms[93].
Microscopic colitis
Microscopic colitis is a chronic inflammatory disease of the intestine characterized by chronic diarrhea with a normal endoscopic appearance of the colon[94]. A randomized,open-label trial demonstrated that VSL#3 had a benefit in inducing and maintaining short-term clinical remission of active microscopic colitis compared with mesalamine.In this trial, 46% of patients treated with VSL#3 achieved clinical remission compared with 8% of patients treated with mesalamine with a significantly lowering stool weight, mucus, and diarrheal rate. Furthermore, a significant decrease of iNOS scores in the VSL#3 group suggested a decrease in the inflammatory status of the disease[95].The trial had limitations probably due to a small sample size.
Uncomplicated diverticulitis
Diverticulitis is an inflammation related to the diverticula of the colon. An open, pilot clinical trial demonstrated that a combination therapy of VSL#3 and balsalazide had a better effect than VSL#3 treatment alone in preventing relapse and maintaining the remission of uncomplicated diverticulitis[96].
Familial adenomatous polyposis
Familial adenomatous polyposis (FAP) is an inherited autosomal dominant disorder characterized by high proliferation of epithelial cells in the pouch mucosa[97]. A randomized, pilot trial demonstrated that sulindac therapy alone and treatment with VSL#3 and inulin tended to reduce cell proliferation and increase glutathione S-transferase (GST) enzyme activity in patients with FAP and IPAA, while the combination treatment with sulindac, inulin, and VSL#3 had no effect[98]. However,the trial had such a small number of patients enrolled that the conclusion might not be stable or reliable.
Colon cancer
Colitis is a chronic inflammatory colonic disease, and patients have a higher risk of suffering from colorectal cancer (CRC) compared with healthy people. It was reported that the risk of CRC had a 2.4-fold increase when UC existed in population-based cohorts[99]. Three studies showed the inhibitory or preventive effect of VSL#3 on azoxymethane (AOM)/DSS-induced murine colitis-associated carcinogenesis by comparing VSL#3 with other traditional drugs (metformin, balsalazide, and 5-ASA)[100-102]. The combination of metformin and VSL#3 was able to decrease inflammation and tumor progression by inhibiting macrophage infiltration, and the positive effect of VSL#3 against murine UC-associated carcinogenesis was achieved by adjusting the proportion of beneficial and harmful bacteria in feces and the intestinal mucosa[100,101]. One study showed that VSL#3 could retard the development of colonic inflammation to dysplasia and cancer, accompanied by an increase in antiangiogenic factor vitamin D receptor (VDR) in a rat model of colitis-associated CRC[103]. However, in terms of murine colitis-associated CRC, two studies showed contrasting results. The first study showed that VSL#3 had excellent anti-carcinogenic and anti-inflammatory activities by regulating mucosal CD4+T cell responses,involving increasing IL-17-expressing CD4+T cells in mesenteric lymph nodes.Interestingly, the study showed an increased concentration of colonic TNF-α in mice treated with VSL#3 compared to controls[104]. In contrast, the second study showed that VSL#3 was not able to protect against colitis-associated CRC. VSL#3 produced the effect of enhancing tumorigenesis probably by inducing the depletion of protective commensal bacteria. However, the effect was not related to inflammatory cytokines[105]. The different results may be associated with the type of mice and the induced pattern of murine CRC used in the two studies. The dose, mode of administration, and treatment course of VSL#3 may also have impacted the results. Inthe first study, wild-type C57BL/6 mice were treated with AOM (10 mg/kg) at the sixth week, followed by a 2.0% DSS treatment for a week, and IL-10-deficient 129/SvEv mice were treated with 5 × 107CFUHelicobacter typhloniusby using oral gavage. VSL#3 was administered daily by orogastric gavage using a ball tip gavage needle at a dose of 1.2 × 109bacteria/mouse/d[104]. In the second study, IL-10-deficient 129/SvEv mice were treated with specific pathogen free (SPF) bacteria for 7 wk, then mice received an injection of AOM (10 mg/kg) for 6 wk. The oral dose of VSL#3 was 109CFU per mouse/d[105].
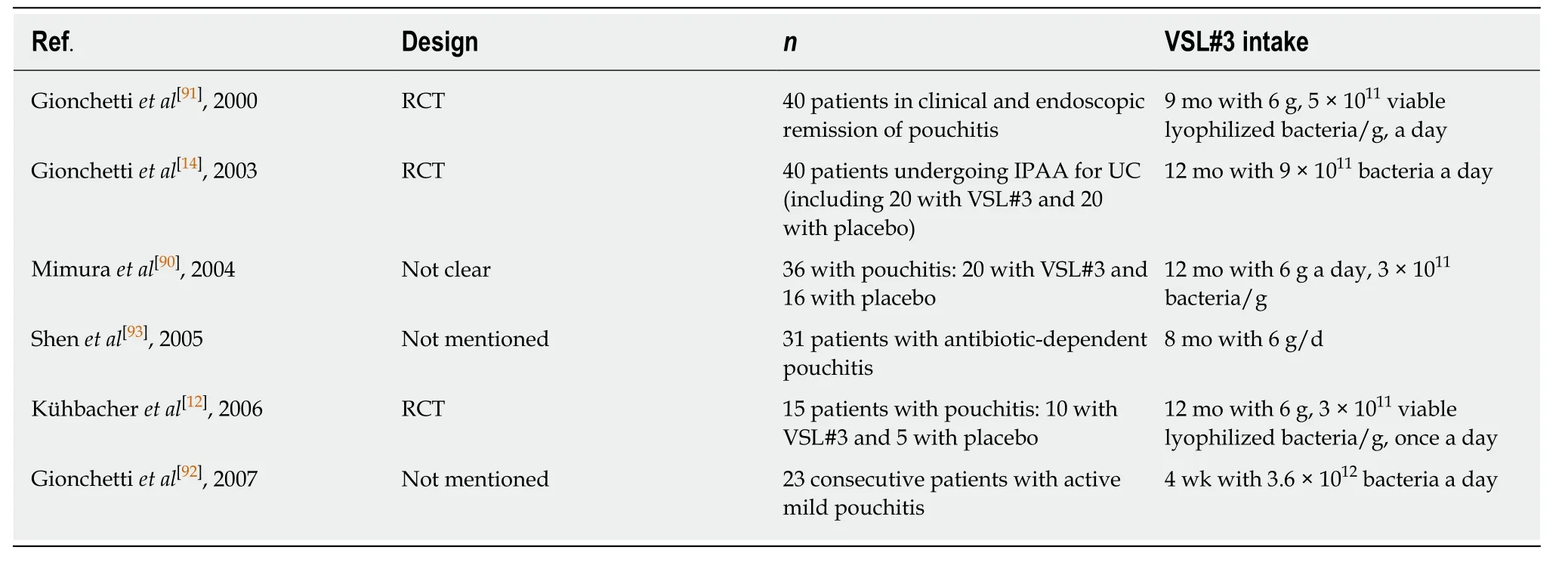
Table 4 Trials assessing the effect of VSL#3 in patients with pouchitis
EFFECTS OF VSL#3 ON HEPATIC DISEASES
There is a close relationship between the liver and intestine tract. The metabolism of bile acid is tightly connected with the intestinal microbiota. The probiotic mixture VSL#3 was shown to modulate intestinal microbiota, thus down-regulating the intestinal-liver FXR-FGF15 axis to induce ileal bile acid deconjugation, fecal bile acid excretion, and the synthesis of bile acid in the liver[17]. Hence, VSL#3 may have a potential for treatment of chronic liver diseases and their complications.
Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is an increasing problem worldwide, and is now one of the leading causes of chronic liver diseases in Western countries[106]. It encompasses a spectrum of histopathological changes from simple liver steatosis to steatohepatitis, advanced fibrosis, and cirrhosis, and NAFLD may be caused by intestinal dysbiosis, which increases intestinal permeability to bacterial products and harmful substances[107,108]. Intestinal dysbiosis can also cause intestinal dysmotility,inflammation, and other immune responses that may contribute to hepatic inflammation and fibrosis[108]. One study in Table 5 showed that VSL#3 could decrease liver damage in NAFLD and hepatitis C virus-related hepatitis patients[109]. In addition, pediatric NAFLD has also been proven to be responsive to VSL#3, as shown in Table 5. VSL#3 could decrease the severity of NAFLD, mainly by increasing the levels of glucagon-like peptide 1 (GLP-1) and activated GLP-1 (aGLP-1)[110,111]. It was shown that the increase of SCFA butyrate by VSL#3 was able to stimulate the secretion of GLP-1 from intestinal L-cells which were responsible for synthesizing the pre-propetide of GLP-1[110,112]. GLP-1 could stimulate the endocrine pancreas, thus improving insulin sensitivity in order to improve glucose and fat metabolism[110].Besides, it was suggested that the administration of VSL#3, by increasing GLP-1, was able to regulate the metabolic flux of muscle mitochondria, make valine be completely degraded, and finally produce succinyl-coenzyme A, the intermediate product of citric acid cycle, which is used for energy metabolism[111]. These improvements of energy metabolism provide benefits for the recovery of childhood NAFLD. The recommended level for VSL#3 use in NAFLD (including adults and children) was a C rating[41].
Although there are nearly no clinical trials clarifying the effect of VSL#3 on adult NAFLD, there are several studies demonstrating that VSL#3 was able to improve murine NAFLD. It acts by improving liver histology and liver insulin resistance, anddecreasing the level of serum alanine aminotransferase[113]. VSL#3 was proven to be able to improve hepatic insulin resistance by reducing the TNF-α-IκB kinase β-NF-κB pathway, the activity of TNF-regulated kinase Jun N-terminal kinase, and uncoupling protein-2[113-115]. TNF-regulated kinase Jun N-terminal kinase could promote insulin resistance by increasing the serine phosphorylation of insulin receptor substrate-1[116].The increase of hepatic natural kill T cell expression stimulated by lipids extracted from VSL#3 also contributed to the improvement of hepatic insulin resistance[117,118].The action mechanism of VSL#3 on insulin sensitivity is shown in Figure 3.
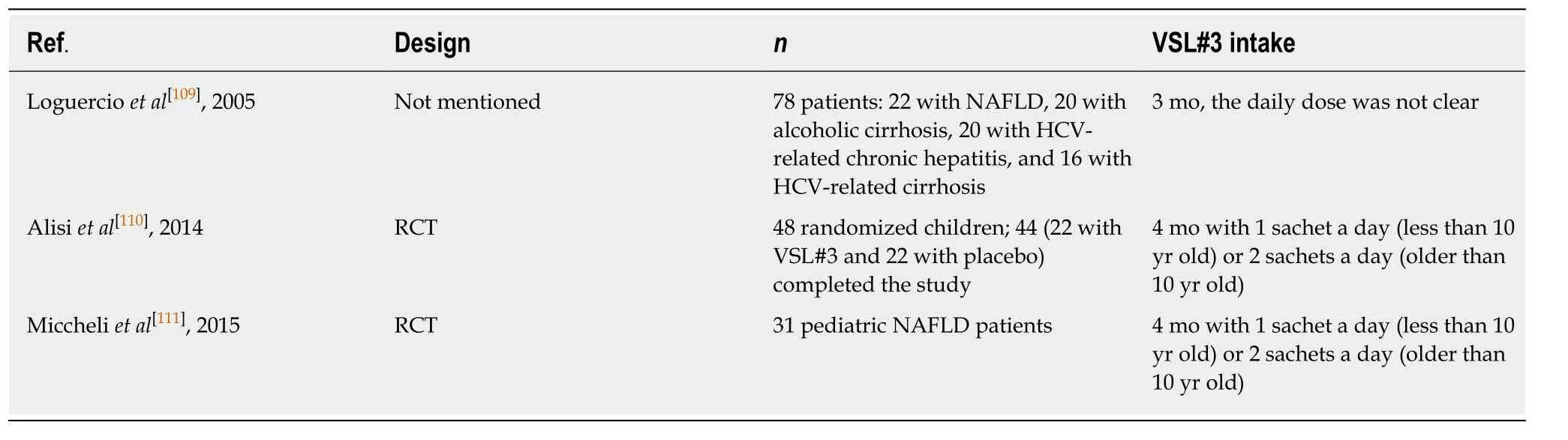
Table 5 Trials assessing the effect of VSL#3 in patients with non-alcoholic fatty liver disease
Non-alcoholic steatohepatitis (NASH) is the advanced stage of NAFLD, and metabolic syndrome increases the risk of having NASH[119]. VSL#3 was not able to prevent liver steatosis or inflammation but was able to reduce hepatic fibrosis and the accumulation of collagen and α-smooth muscle actin (α-SMA) in methionine cholinedeficient (MCD) diet-induced murine NASH[120]. Furthermore, VSL#3 significantly increased the expression of liver PPARα and PPARγ, which was inhibited by TNF-α,and decreased the expression of pro-collagen and matrix metalloproteinases compared to an MCD diet alone[120,121]. Besides, VSL#3 maintained MCD diet-induced expression of TGF-β but increased the expression of bone morphogenic protein and activin membrane-bound inhibitor (Bambi), having a negative regulatory effect on TGF-β-family signaling[120].
Alcoholic liver disease
Intestinal barrier dysfunction may be related to endotoxemia and eventually cause hepatic inflammation and the development of alcoholic liver disease (ALD)[122]. One trial indicated that VSL#3 could mitigate liver damage in patients with alcoholic liver cirrhosis by decreasing the plasma levels of malondialdehyde, 4-hydroxynonenal, and S-nitrosothiols. The plasma levels of cytokines (TNF-α, IL-6, and IL-10) also improved[109]. Besides, one study demonstrated that VSL#3 was as effective as glutamine in rats with experimental acute ALD, and the combination therapy was more effective. The action mechanism mainly proceeded by reducing intestinal permeability[122]. The recommended level for VSL#3 use in ALD was a C rating[41].
Cirrhosis
Impaired IBF, bacterial overgrowth, and disturbance of local immunity contribute to bacterial translocation (BT) in cirrhosis; BT is one of the major causes of many complications of cirrhosis[123,124]. Gram-negative enteric bacilli, such asE. coli, are relatively effective actors for BT to the mesenteric lymph nodes[125]. Also, some proinflammatory pathways (such as TNF-α) worsen liver dysfunction[126]. Two clinical trials, as shown in Table 6, showed that VSL#3 led to the amelioration of hepatic and systemic hemodynamic disturbances accompanied by decreased hepatic venous pressure gradient in order to improve the symptoms in patients with cirrhosis[127,128].One trial demonstrated that adjunctive VSL#3 significantly improved the response rate (percentage of patients with a decrease in hepatic venous pressure gradient from baseline of ≥ 20% or to ≤ 12 mmHg) compared with the control group (58%vs31%).VSL#3 could lead to a reduction in pro-inflammatory cytokine TNF-α plasma levels.However, no differences could be observed in the plasma level of IL-6 or NO[127].Another trial observed a significant decrease in plasma aldosterone levels induced by VSL#3, but the changes in the other measured parameters, including the stool microbiota, were not significant[129]. Apart from the above-mentioned plasma component changes, VSL#3 treatment induced an increase in the plasma levels of albumin and hemoglobin, which might lead to lower model for end-stage liver disease scores in patients with decompensated liver cirrhosis[130]. The effect of VSL#3 was also proven in a rat model of biliary cirrhosis with portal hypertension. VSL#3 prevented endothelial dysfunction by improving vascular oxidative stress (reducing BT) and the local angiotensin system[131].
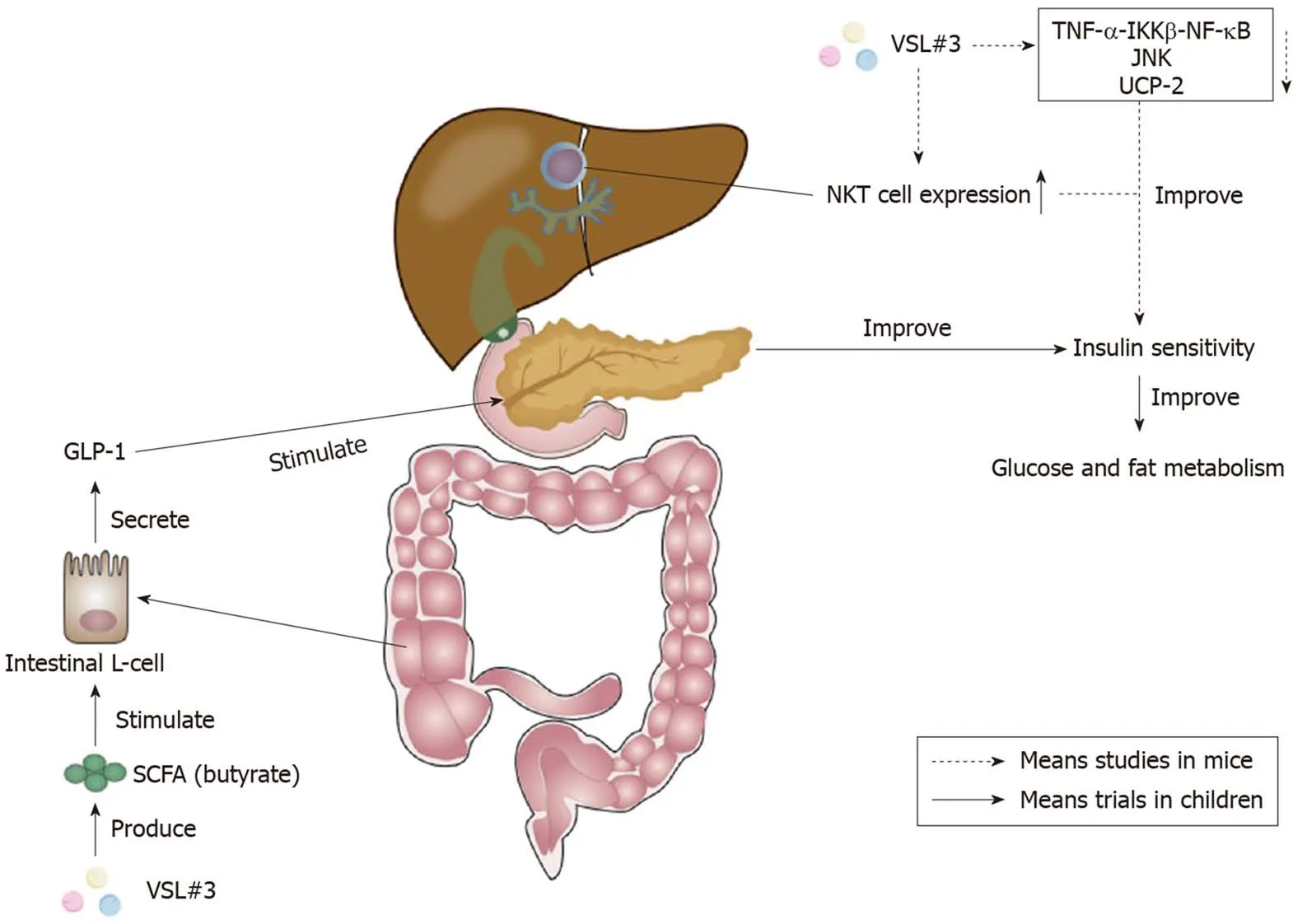
Figure 3 Effects of VSL#3 on insulin sensitivity. The increase of short-chain fatty acid (SCFA) butyrate caused by VSL#3 is able to stimulate the secretion of glucagon-like peptide 1 (GLP-1) from intestinal L-cells[112]. GLP-1 can stimulate the pancreas and ameliorate insulin sensitivity to improve glucose and fat metabolism[110]. Furthermore, VSL#3 can improve hepatic insulin resistance by reducing tumor necrosis factor-α (TNF-α)-IκB kinase β (IKKβ)-nuclear factor-κB (NF-κB) pathway, the activity of TNF-regulated kinase Jun N-terminal kinase (JNK), and uncoupling protein-2[113-115]. The increase of hepatic natural kill T (NKT) cells caused by VSL#3 also plays a significant role in the improvement of hepatic insulin sensitivity[117,118]. SCFA: Short-chain fatty acid; GLP-1: Glucagon-like peptide 1;TNF-α: Tumor necrosis factor-α; IKKβ: IκB kinase β; NF-κB: Nuclear factor-κB; JNK: Jun N-terminal kinase; UCP-2: Uncoupling protein-2; NKT: Natural kill T.
Hepatic encephalopathy
Patients with liver cirrhosis could cause systemic inflammation, which may be associated with hepatic encephalopathy (HE)[132]. HE, including minimal HE (MHE)and overt HE (OHE), is a common complication of cirrhosis with impaired quality of life. Sometimes MHE can develop into OHE. The development of HE could be related to alterations in the intestinal microbiota (higherVeillonellaceae,Alcaligeneceae, andEnterobacteriaceae), ammonia, endotoxemia, and inflammation (IL-2, IL-6, IL-13, and TNF-α)[133,134]. Two trials, as shown in Table 7, investigated the effect of VSL#3 compared with other traditional medicines in MHE. They demonstrated that VSL#3 had similar efficacy in improvement of MHE with lactulose and L-ornithine L-aspartate[135,136]. MHE improved in 62.5% of patients taking lactulose and 69.7% of patients taking VSL#3, and the amelioration of MHE was associated with decreased serum ammonia levels[135].
Also, there have been two trials, as shown in Table 7, investigating the preventive effect of VSL#3 against OHE[137,138]. An open-label RCT investigated the effect of VSL#3 in primary prophylaxis of OHE. Seven patients in the VSL#3 group (n= 86, 42 with MHE) and 14 in the control group (n= 74, 33 with MHE) developed OHE. This change is probably related to the significant decrease in the number of patients with MHE and the small intestinal bacterial overgrowth in the VSL#3 group[137]. The secondary prophylaxis efficacy of VSL#3 was investigated in another trial in patients with cirrhosis who have recovered from an episode of HE. There was a tendency to reduce the development of OHE in the VSL#3 group. Fewer patients hospitalized for HE were observed in the VSL#3 group compared with the placebo group (19.7%vs42.2%) over a 6-mo period. The results also indicated that VSL#3 improved systemic inflammatory response syndrome and model for end-stage liver disease scores, and decreased plasma indole, renin, aldosterone, and brain natriuretic peptide levels significantly[138]. The recommended level for VSL#3 use in HE was an A rating[41].
VSL#3 administration in chronic liver diseases still has its own limitations. Weneed further studies to clarify its therapeutic effect in portal hypertension and its complications.
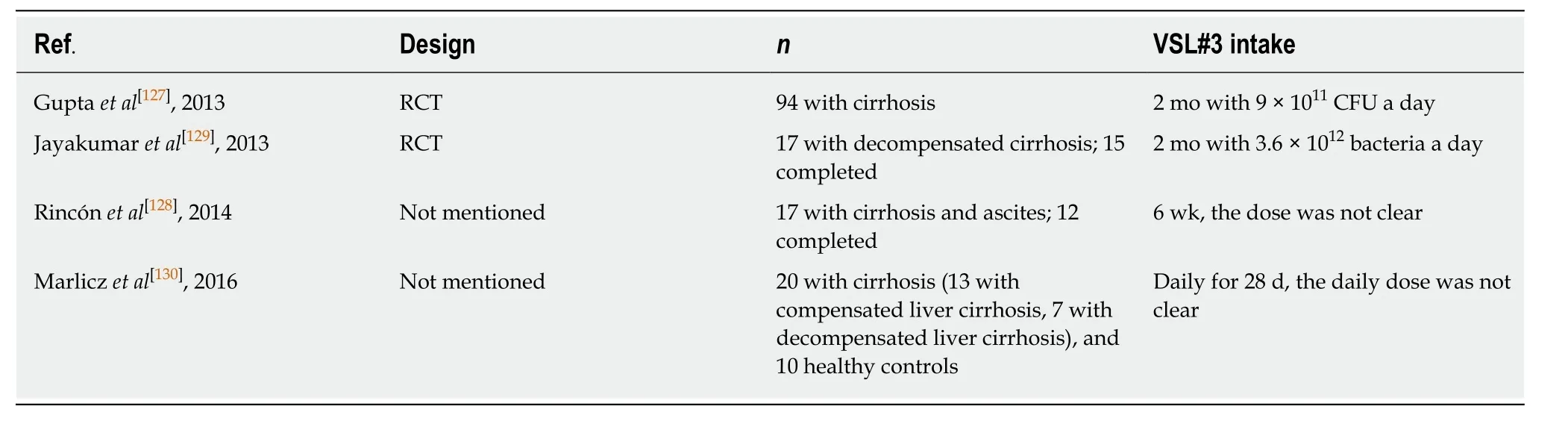
Table 6 Trials assessing the effect of VSL#3 in patients with cirrhosis
EFFECTS OF VSL#3 ON OBESITY AND DIABETES
VSL#3 has been shown to significantly decrease body and fat mass during a high-fat diet compared with placebo[139,140]. In overweight (body mass index [BMI] > 25) adults without diabetes, VSL#3 (one capsule every day before any meal or breakfast, 1.125 ×1011CFU/capsule) was able to significantly reduce total cholesterol, triglyceride, lowdensity lipoprotein, and very-low-density lipoprotein concentrations and increase high-density lipoprotein concentration[141].
At present, there are no definite clinical trials about the effect of VSL#3 on diabetes mellitus. However, the efficacy of VSL#3 on diabetes has been researched in obesity and non-obesity murine models. Increasing evidence supports the idea that impaired IBF promotes the access of infected pathogens to mucosal immune elements, which may eventually lead to diabetogenic immune responses and insulin resistance[142]. Two studies demonstrated that oral administration of VSL#3 was able to prevent spontaneous autoimmune diabetes in non-obese diabetic (NOD) mice[143,144]. The prevention of autoimmune diabetes was related to increased IL-10 production from the Peyer's patch, spleen, and pancreas and decreased β-cell destruction[143]. VSL#3 treatment increased the release of protolerogenic components of the inflammatory corpuscles, such as indoleamine 2,3-dioxygenase (IDO) and IL-33. These changes regulate intestinal immunity in VSL#3-treated NOD mice by promoting the differentiation of tolerant dendritic cells and reducing the differentiation of Th1 and T-helper 17 (Th17) cells in the intestinal mucosa[144]. Besides, IDO inhibited T cell proliferation by limiting the local tryptophan concentration directly, thus reducing Th1 and Th17 cell differentiation[145]. In addition, VSL#3 treatment increased protolerogenic species, and decreased the relative abundance of species, such asBacteroidetes S24-7, in NOD mice[144]. However, one study showed that VSL#3 could not delay diabetes probably because it colonized the intestine poorly and could not overcome the effect of diabetogenic microbiota[146]. The result contradicts previous studies and the reasons for this need to be further studied. Moreover, one study demonstrated that VSL#3 was able to prevent diabetes in high fat diet-induced obesity mice by inhibiting weight gain and insulin resistance. Increased transcript levels of free fatty acid receptor 3 (Ffar3) were observed in VSL#3-treated murine intestine. VSL#3 could protect against glucose intolerance and obesity in Lepob/obmice,and significantly increase proopiomelanocortin levels and decrease neuropeptide Y and agouti-related protein levels in the hypothalamus, which are related to energy homeostasis[112,147].
EFFECTS OF VSL#3 ON ALLERGIC DISEASES
It is known that VSL#3 has the capacity to regulate immune responses, so it has been considered to treat allergic diseases. There have not been definite conclusions about the efficacy of VSL#3 on allergic diseases in clinical trials, but a potential effect of VSL#3 has been shown in animal studies. Several studies demonstrated that the oral administration of VSL#3 had a beneficial effect in ameliorating anaphylactic symptoms in murine food allergy induced by shrimp tropomyosin (ST) or peanut byinhibiting T-helper 2 (Th2) immune reactions[148-150]. Meanwhile, the administration of VSL#3 had a regulating effect on allergen-induced Th2 responses by turning primary immune reactions to a T regulatory (Treg)/T-helper 0 (Th0)-type profile[28]. Th2-secreted cytokines IL-5 and IL-13 decreased and Treg/Th1 cytokines IL-10 and IFN-γ increased in the presence of VSL#3[149]. TGF-β, induced by VSL#3 supplementation,was capable of reducing the Th2 inflammation related to food anaphylaxis in a mouse model of peanut sensitization. It acted through the induction or maintenance of regulatory T cells expressing FOXP3[150]. The administration of VSL#3 (1.5 × 1010CFU in 250 µL of PBS daily) inhibited the β-lactoglobulin (BLG)-induced allergic reaction,mainly by increasing intestinal sIgA in BLG-sensitized mice[151].
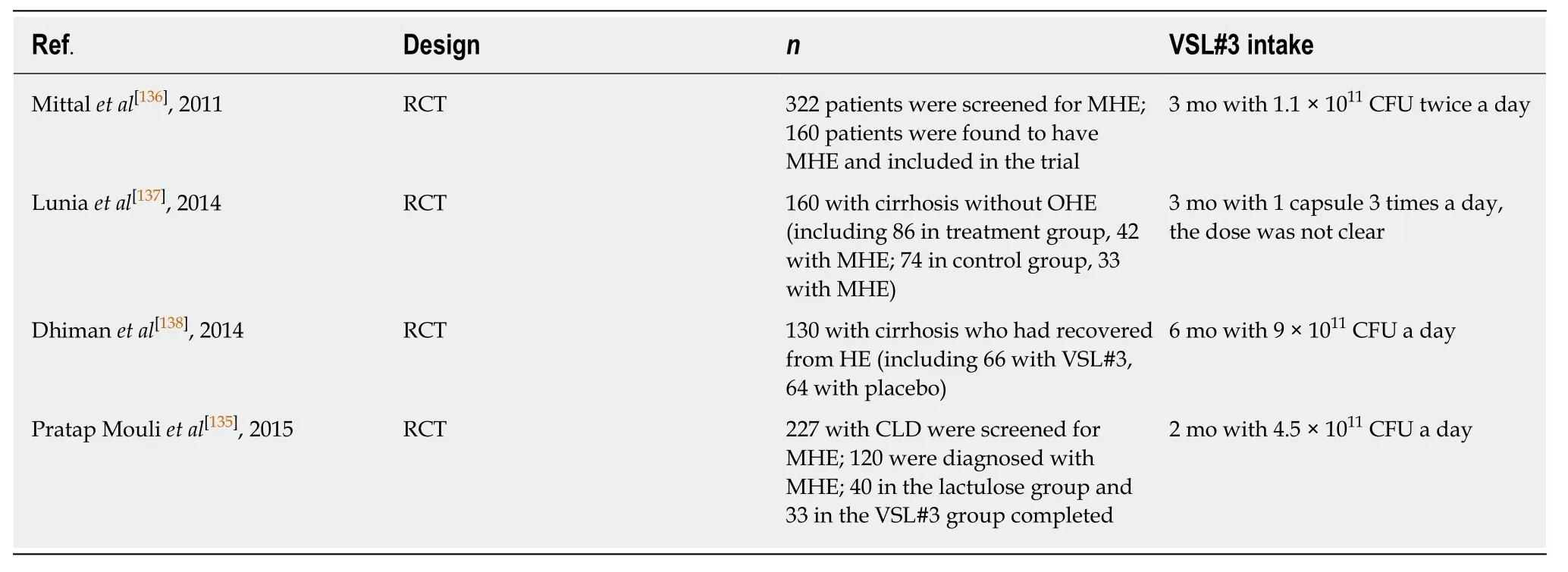
Table 7 Trials assessing the effect of VSL#3 in patients with hepatic encephalopathy
EFFECTS OF VSL#3 ON NERVOUS SYSTEMIC DISEASES
Two studies showed that VSL#3 was able to improve some types of nervous systemic disorders and related brain mechanisms through the gut-brain axis when dysbiosis caused by a cafeteria diet or the ageing process occurred in rats[152,153]. VSL#3 was proven to prevent diet-induced memory deficits on the place task but had no effect on anxiety-like behaviors on the elevated plus maze (EPM) in rats[152]. Furthermore, one study indicated that VSL#3 improved the age-related deficits in long-term potentiation in aged rats. The effect was accompanied by a decrease of microglial activation markers and an increase of brain-derived neurotrophic factor and synapsin.The effects above might be due to the increase in the amounts ofBacteroidetesandActinobacteriacaused by VSL#3[153]. One study showed that the effects of VSL#3 on the nervous system are independent of the changes in the intestinal microbiota in rats.VSL#3 could significantly improve sickness behaviors and brain dysfunction,probably by decreasing microglial activation and brain inflammatory monocyte infiltration in bile duct ligation mice with liver inflammation[154]. Furthermore, one study demonstrated that the post-injury treatment of VSL#3 improved locomotor recovery after murine spinal cord injury and activated neuroprotective mucosal immune cells[155].
EFFECTS OF VSL#3 ON ATHEROSCLEROSIS
Atherosclerosis (AS) is a chronic disease that might be associated with the bacteria from the oral cavity and even the gut[156]. Two studies assessed the ability of VSL#3 to protect against AS in ApoE−/−mice. They demonstrated that VSL#3 had a beneficial effect on AS and perhaps worked by significantly reducing the development of atherosclerotic or aortic plaques and biomarkers of vascular inflammation. Vascular cell adhesion molecules and intercellular adhesion molecules were shown to be reduced by VSL#3 in both two studies[157,158]. One study indicated that VSL#3 (2.78 ×1011CFU/d for 12 wk) was able to significantly lower gelatinase matrix metalloproteinase-9 , but another plasma atherosclerotic biomarker, total plasminogen activator inhibitor-1, was significantly elevated by the combination of telmisartan(positive control drug) and VSL#3[157]. The reason for this is not clear. The administration of VSL#3 (2 × 1010CFU/kg/d, 6 d per week for 12 wk) effectively decreased the percentage of CD36 positive cells among circulating macrophages[158].
EFFECTS OF VSL#3 ON BONE DISEASES
It has been reported that the oral administration of VSL#3 (twice a week with 1 × 109CFU) could protect against ovariectomy (OVX)-induced bone loss in sex steroiddeficient mice by increasing intestinal barrier integrity and inhibiting intestinal and bone marrow inflammation[159].
EFFECTS OF VSL#3 ON FEMALE REPRODUCTIVE SYSTEMIC DISEASES
Diseases of the female reproductive system include various kinds of diseases, such as gynecological inflammation, gynecological tumor, menstrual disorder, and infertility.In this review, we mainly investigated the effect of VSL#3 on breast milk’s microbial composition and preterm birth.
Improvement of breast milk’s microbial composition
Breast milk provides nutritious substances for infant growth and development, and it also provides several altered beneficial microbiota includingStaphylococci,Streptococci,Lactobacilli, andBifidobacteria[160,161]. These beneficial bacteria in maternal milk will transfer from mother to infant and colonize the infant intestine during lactation[161].One RCT demonstrated that oral VSL#3 was able to improve breast milk’s microbial composition with a significant increase of the levels ofBifidobacteriaandLactobacillicompared with placebo in mothers during the perinatal period. The improvement in breast milk’s microbial composition caused by VSL#3 was not associated with differences in the milk concentrations of oligosaccharides and lactoferrin, and the results indicated that the administered VSL#3 did not pass from the maternal intestine to mammary gland through an endogenous approach, but had a systemic effect[162].
Preterm birth
The balance of vaginal microbiota is important during pregnancy, and abnormal vaginal communities (such as bacterial vaginosis) may be associated with preterm delivery and perinatal complications[163]. One trial showed the potential implications of VSL#3 in preventing preterm birthviaan anti-inflammatory effect on vaginal immunity in healthy pregnant women. The consumption of VSL#3 led to a decrease of the pro-inflammatory chemokine Eotaxin. There were no significant alterations in the amount of the principal vaginal bacteria in women orally administered with VSL#3. However, VSL#3 played a potential role in counteracting the decrease inBifidobacteriaand the increase inAtopobium. VSL#3 was also capable of modulating theLactobacilluspopulation, which was associated with the decrease ofL. helveticusspecies[164]. One study indicated that VSL#3 was able to survive and stay stable in a continuous culture system simulating the vaginal environment and inhibit the vaginal vault pathogenGardnerella vaginalis[165].
SIDE EFFECTS AND LIMITS RELATED TO VSL#3
There are almost no serious side effects related to the use of VSL#3 in most diseases.Among these side effects, only very few are intolerable. A meta-analysis showed that 33 patients experienced mild side effects (mainly bloating) out of 353 UC patients treated with VSL#3[166]. Three patients experienced abdominal pain out of 60 patients with HE who received VSL#3[135]. One patient was reported to experience abdominal cramps, vomiting, and diarrhea out of 20 patients with pouchitis who received VSL#3[90]. Finally, of the 31 pouchitis patients treated with VSL#3, two experienced intolerable side effects: One experienced bloody bowel movements and the other experienced severe constipation, bloating, and gas[93].
The use of VSL#3 also has some limits. VSL#3 contains only eight bacterial strains,and the intestinal microecosystem of each patient is complex, so these strains may not be suitable for all patients. Achieving the one-to-one treatment of formula probiotics and individuals is difficult. The results mentioned above of VSL#3 in the treatment of various diseases are not exactly consistent, which may have been caused by different sources of VSL#3. One study indicated that proteomic analyses showed differences in protein abundances, identities, and origins of VSL#3 from different sites[167]. Therefore,we suggest that the protein abundances should be identified through proteomic analyses in the clinical and basic studies of VSL#3 in the future.
CONCLUSION
In the past, it has been reported that VSL#3 has a profound effect on digestive systemic disorders, especially gastrointestinal disorders. Apart from this, many studies demonstrated that VSL#3 has a beneficial effect on obesity and diabetes,allergic diseases, nervous systemic diseases, AS, bone diseases, and female reproductive systemic diseases. In most cases, the use of VSL#3 is safe and welltolerated, but more precise mechanisms and effects of VSL#3 still need to be studied.To achieve one-to-one individualized treatment, we need to prepare exclusive probiotics according to the real-time monitoring of patients' intestinal microbiota.However, the current detection technology is not accurate enough. The study of the intestinal microbiota is challenging since most cannot be cultivated[10]. Thus, the solutions to the above problems will hopefully provide a new direction for precise medicine in the future.
 World Journal of Clinical Cases2020年8期
World Journal of Clinical Cases2020年8期
- World Journal of Clinical Cases的其它文章
- Clinicopathological differences and correlations between right and left colon cancer
- Bedside score predicting retained common bile duct stone in acute biliary pancreatitis
- Stability and infectivity of coronaviruses in inanimate environments
- Status, challenges, and future prospects of stem cell therapy in pelvic floor disorders
- Unusual presentation of congenital radioulnar synostosis with osteoporosis, fragility fracture and nonunion: A case report and review of literature
- Predictive factors for central lymph node metastases in papillary thyroid microcarcinoma
