Determination of Bisoprolol Fumarate by Fluorescence Quenching Method
Yu LIN, Xiaomei LAN
Guangxi University of Chinese Medicine, Nanning 530001, China
Abstract [Objectives] To establish a new method for indirect determination of bisoprolol fumarate based on fluorescence quenching technology. [Methods] In ammonia water and ammonium chloride buffer solution at pH=9.2, when λexcitation=277 nm and λemission=596 nm, with the increase of CCu2+, the fluorescence signal intensity of bisoprolol fumarate weakened, and the difference between the fluorescence intensity of bisoprolol fumarate itself and the fluorescence intensity of the test solution after the quencher Cu2+ was added (ΔF) and Cbisoprolol fumarate showed a good linear relationship. [Results] In the range of 15.39-76.93 μg/mL, ΔF=146.7 Cbisoprolol fumarate+482.1, r=0.998 8, and the detection limit is 0.139 1 μg/mL. [Conclusions] The fluorescence quenching method has been applied to the determination of actual samples with a recovery rate of 99.9% and an RSD of 2.7%. The results are satisfactory.
Key words Bisoprolol fumarate, Complexing, Fluorescence quenching method, Content determination
1 Introduction
Bisoprolol fumarate, as a highly selective β1adrenergic blocker, has the advantages of fast absorption, long half-life, low first-pass effect, balanced liver and kidney metabolism, and small individual pharmacokinetic differences. It is mainly used for treatment of hypertension, angina pectoris, arrhythmia, and heart failure. At present, bisoprolol fumarate is mainly determined by following methods: spectrophotometry[1-3], fluorescence spectrometry[4-5], electrochemical method[6], high performance liquid chromatography (HPLC)[7-9], liquid chromatography-mass spectrometry (LC-MS)[10-12]and so on.
In the molecular structure of bisoprolol fumarate, the nitrogen atom contains an lone paired electrons and can coordinate with a metal ion to form a complex, which can reduce the fluorescence intensity of bisoprolol fumarate and produce a quenching effect. Therefore, using this feature, we establish an indirect fluorescence quenching method for the determination of bisoprolol fumarate, in the hope of providing a new idea for the detection of bisoprolol fumarate.
2 Materials
RF-6000 fluorescence spectrophotometer (Shimadzu Enterprise Management (China) Co., Ltd.), UV-1780 UV-visible spectrophotometer (Shimadzu Enterprise Management (China) Co., Ltd.), 99.7% bisoprolol fumarate standard substance (National Institutes for Food and Drug Control), CuSO4·5H2O (AR, Chengdu Jinshan Chemical Reagent Co., Ltd.), ammonia (AR, Sinopharm Chemical Reagent Co., Ltd.), ammonium chloride (AR , Sinopharm Chemical Reagent Co., Ltd.)
3 Methods and results
3.1PreparationofthebasicstocksolutionBisoprolol fumarate standard solution: precisely weighed 0.076 9 g of bisoprolol fumarate standard substance, dissolved in distilled water and transferred to a 100-mL volumetric flask to fix the volume to the desired value, shook up to obtain 1.00×10-3mol/L solution, diluted to 5 times for use.
Bisoprolol fumarate test solution: precisely weighed two pieces of bisoprolol fumarate standard substance, pulverized and mixed evenly, then precisely weighed 0.076 7 g powder of bisoprolol fumarate standard substance, dissolved in distilled water and transferred to a 100-mL volumetric flask to fix the volume to the desired value, shook up and filtered, discarded the initial filtrate, took the subsequent filtrate as the test solution, and diluted to 5 times for use.
Cu2+stock solution: precisely weighed 0.249 7 g of CuSO4·5H2O and placed in a small beaker, dissolved and transferred to a 100-mL volumetric flask to fix the volume to the desired value, prepared 1.00×10-2mol/L of Cu2+solution, diluted to 10 times for use.
3.2ExperimentalmethodIn a 10 mL colorimetric tube, added an appropriate amount of 2.00×10-4mol/L bisoprolol fumarate standard solution, ammonia water and ammonium chloride buffer solution with pH=9.2, and 1.00×10-3mol/L of Cu2+solution, fixed the volume with distilled water, and reacted at room temperature for 30 min. Taking the reagent blank as a reference, the fluorescence intensityFof the test solution was measured whenλexcitationwas 277 nm andλemissionwas 596 nm. Pipetted an equal amount of 2.00×10-4mol/L of bisoprolol fumarate standard solution, ammonia water and ammonium chloride buffer solution at pH=9.2 into another 10 mL colorimetric tube, fixed the volume and shook up, reacted at room temperature for 30 min, measured the fluorescence intensityF1under the same conditions, and calculatedΔF=F1-F.
3.3FluorescencespectrumIn ammonia water and ammonium chloride buffer solution with pH=9.2, whenλexcitationwas 277 nm, bisoprolol fumarate generated an emission spectrum at 596 nm. Under the same experimental conditions, Cu2+was added to the same amount of bisoprolol fumarate and ammonia buffer solution, whenλemissionwas 596 nm, the fluorescence peak position of the system was not changed, but the signal weakened, resulting in quenching (Fig.1). In this experiment, we selectedλexcitationat 277 nm andλemissionat 596 nm.
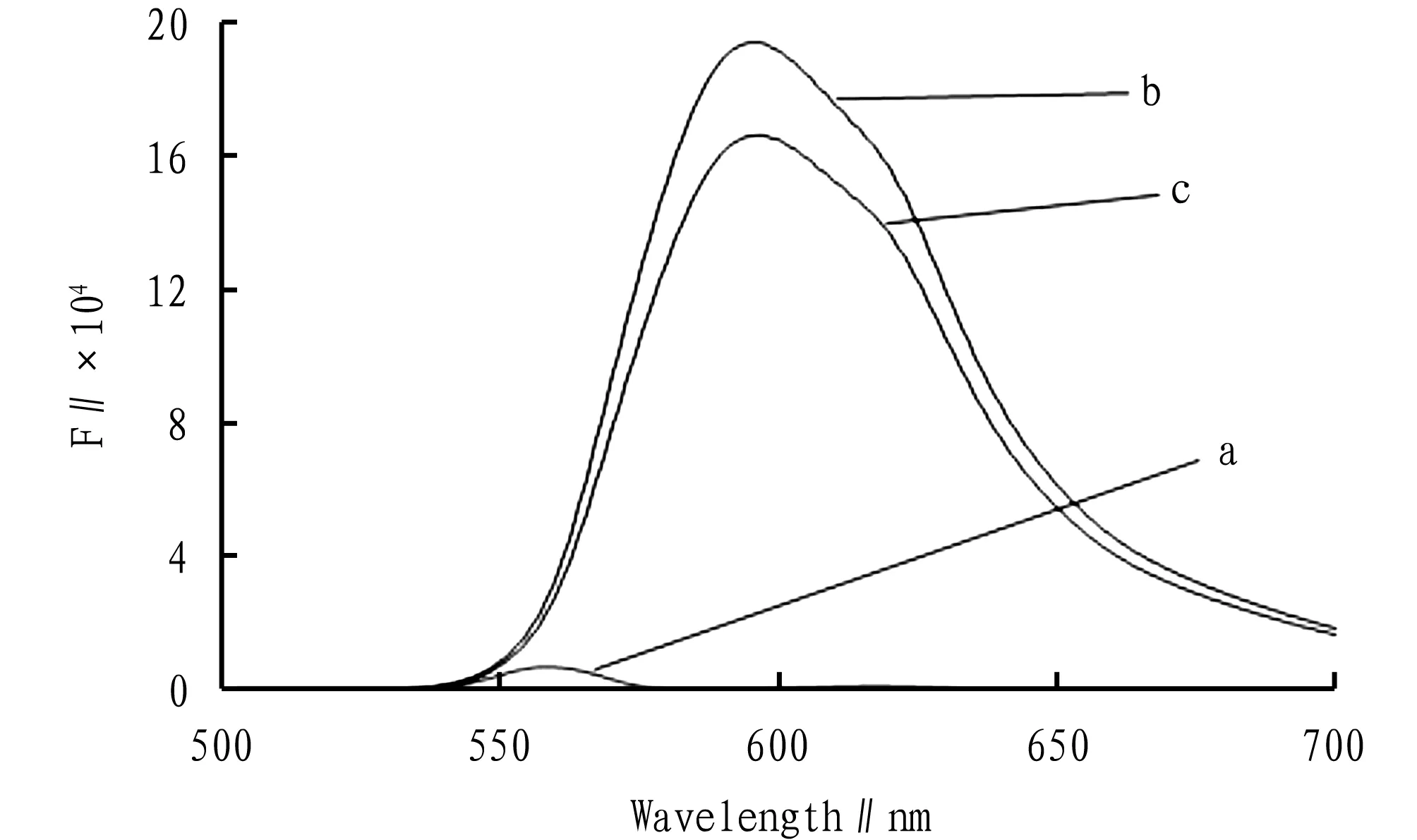
Note: a. pH=9.2 buffer+2.00×10-4mol/L of Cu2+; b. 6.00×10-5mol/L of bisoprolol fumarate solution+pH=9.2 buffer solution; c. 6.00×10-5mol/L of bisoprolol fumarate+pH=9.2 buffer+2.00×10-4mol/L of Cu2+.
Fig.1 Fluorescence spectra of different solutions
Fixed the added amount of bisoprolol fumarate and the buffer solution, add a certain amount of 1.00×10-3mol/L Cu2+solution in sequence, and measured the fluorescence intensity of the system according to the experimental method in Section3.2, as shown in Fig.2. With the increase of added Cu2+solution, the degree of fluorescence quenching of bisoprolol fumarate increased.
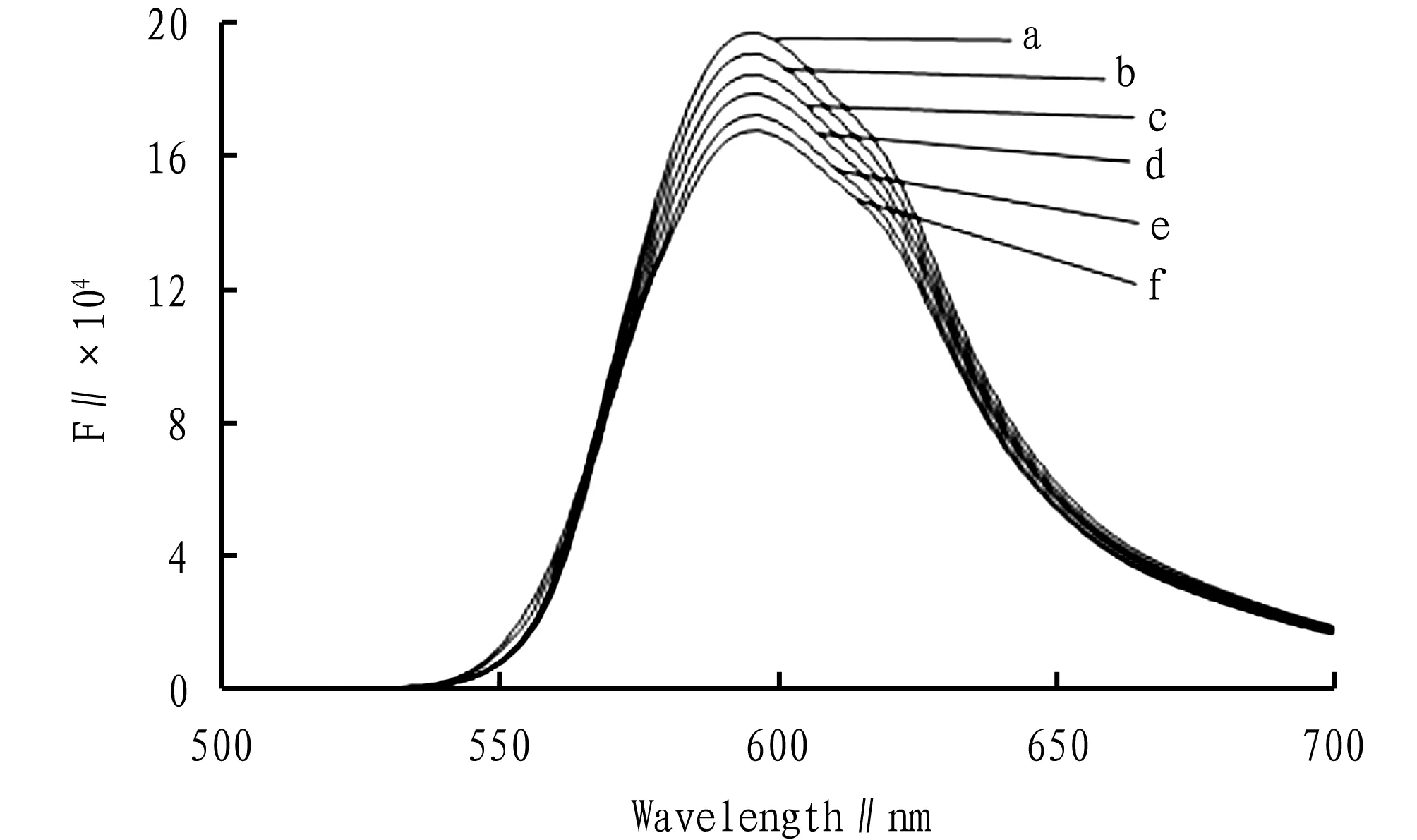
Note: a. 6.00×10-5mol/L of bisoprolol fumarate+0.50 mL of pH 9.2 buffer solution; b, c, d, e, f are added in sequence 5.00×10-5mol/L of Cu2+, 1.00×10-4mol/L of Cu2+, 1.50×10-4mol/L of Cu2+, 2.00×10-4mol/L of Cu2+, 2.50×10-4mol/L of Cu2+into a.
Fig.2 Fluorescence spectra of complexed Cu2+solutions with bisoprolol fumarate
3.4Methodologyexamination
3.4.1Determination of linear range and limit of detection. Separately and precisely pipetted different concentrations of bisoprolol fumarate standard solutions, and then added 0.50 mL of ammonia water and ammonium chloride buffer solution with pH=9.2 and 2.00 mL of 1.00×10-3mol/L of Cu2+solution in sequence, and measuredΔFusing the experimental method . In the range of 15.39-76.93 μg/mL, the linear equationΔF=146.7C+482.1,r=0.998 8, and the limit of detection was 0.139 1 μg/mL.
3.4.2Precision test. Precisely pipetted 3.00 mL 2.00×10-4mol/L of bisoprolol fumarate standard solution, and then added 0.50 mL of ammonia water and ammonium chloride buffer solution with pH=9.2 and 2.00 mL of 1.00×10-3mol/L of Cu2+solution to a 10 mL volumetric flask, and measured the 6 groups of solutions using the method in Section3.2, and obtainedRSD=1.4%.
3.4.3Stability test. Precisely pipetted 3.00 mL 2.00×10-4mol/L of bisoprolol fumarate test solution, 0.50 mL of ammonia water and ammonium chloride buffer solution with pH=9.2 and 2.00 mL of 1.00×10-3mol/L of Cu2+solution, to a 10 mL volumetric flask, and measured the fluorescence intensityFat different times using the method in Section3.2, and calculatedΔF. The results show that with the extension of time, the fluorescence emission signal of the system was basically stable, and theRSDwas 1.2%.
3.4.4Reproducibility test. Precisely pipetted 3.00 mL 2.00×10-4mol/L of bisoprolol fumarate test solution, 0.50 mL of ammonia water and ammonium chloride buffer solution with pH=9.2 and 2.00 mL of 1.00×10-3mol/L of Cu2+solution, to a 10 mL volumetric flask, measured the 6 groups of solutions in parallel using the method in Section3.2, and obtainedRSD=2.5%.
3.4.5Measurement of samples. Pipetted 2.00 mL of the bisoprolol fumarate test solution using the method in Section3.1and calculatedΔFusing the experimental method in Section3.2(Table 1).
Table 1 Measurement results of bisoprolol fumarate samples (n=6)
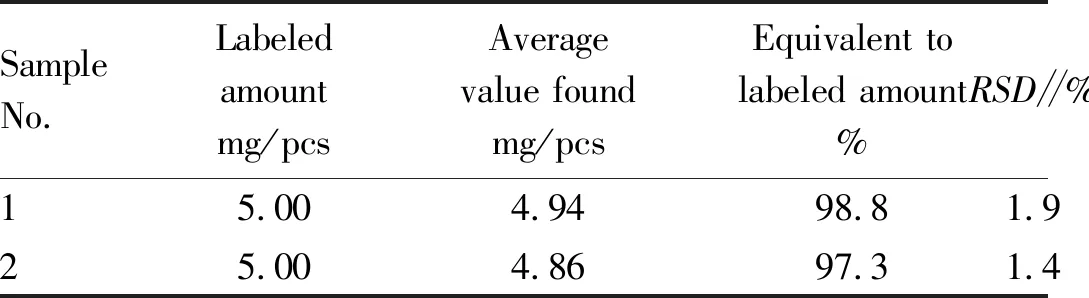
SampleNo. Labeledamountmg/pcsAveragevalue foundmg/pcsEquivalent tolabeled amount%RSD∥%15.004.9498.81.925.004.8697.31.4
Note: Sample 1: Beijing Wellso Parmaceutical Co., Ltd. (National Medicine Permit No. H10970082, specification: 5 mg/tablet); Sample 2: Chengdu Easton Biopharmaceuticals Co., Ltd. (National Medicine Permit No.H20083008, specification: 5 mg/tablet) .
3.4.6Sample recovery test. Pipetted 2.00 mL of the prepared bisoprolol fumarate test solution using the method in Section3.1and performed the sample recovery test using experimental method in Section3.2(Table 2.)
4 Discussions
4.1ScreeningofmetalionsFixed the amount of bisoprolol fumarate standard solution, added the same concentration of Cu2+, Mn2+, Al3+, Mg2+, Co2+, Ni2+, and measured the fluorescence intensityFof the test solution after the volume was fixed.
Table 2 Results of sample recovery test (n=9)
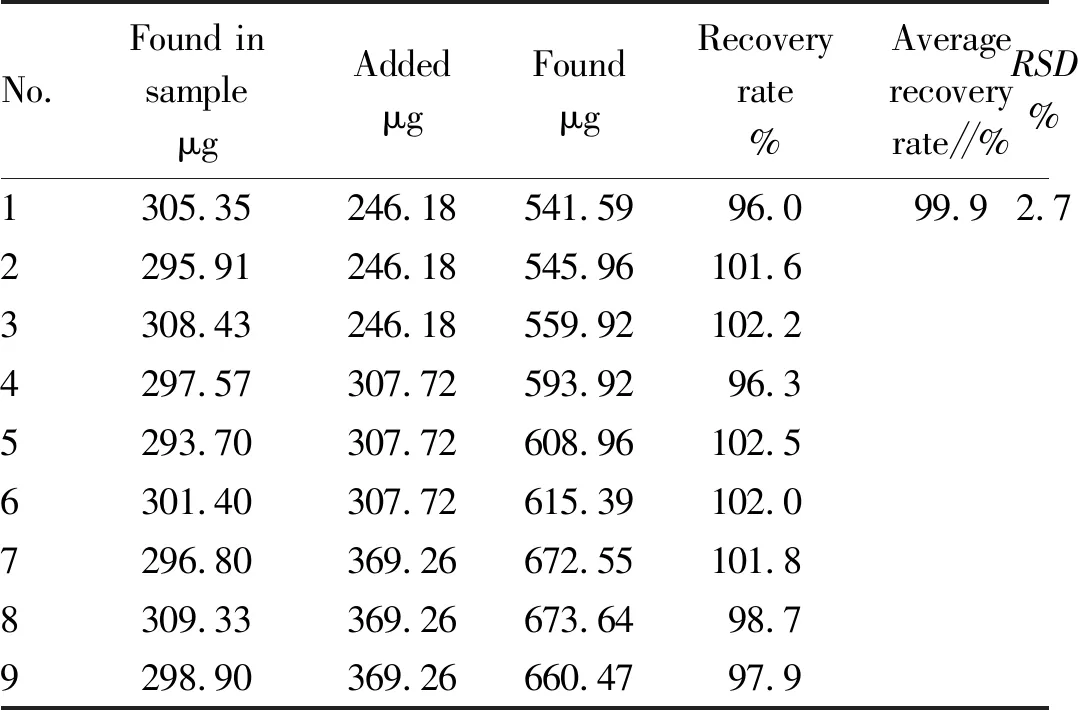
No.Found insampleμgAddedμgFoundμgRecoveryrate%Averagerecoveryrate∥%RSD%1305.35246.18541.5996.099.92.72295.91246.18545.96101.63308.43246.18559.92102.24297.57307.72593.9296.35293.70307.72608.96102.56301.40307.72615.39102.07296.80369.26672.55101.88309.33369.26673.6498.79298.90369.26660.4797.9
Added an equal amount of bisoprolol fumarate standard solution to a colorimetric tube as a reagent blank, measured the fluorescence intensityF1under the same conditions, and calculated △F=F1-F. The results show that Cu2+had the largest △Fand the highest sensitivity. Therefore, we selected Cu2+in this study.
4.2OptimizationofsolutionpHPrecisely pipetted 3.00 mL of 2.00×10-4mol/L of bisoprolol fumarate standard solution and an appropriate amount of 1.00×10-3mol/L of Cu2+solution into a 10-mL colorimetric tube, using 0.10 mol/L NaOH and 0.10 mol/L HCl to adjust the pH, and measured using the experimental method in Section3.2. The results show that when the pH was in the range from 3 to 7, theΔFvalue decreased with the increase of pH; when the pH ≥ 9, theΔFvalue tended to be stable. Therefore, we selected ammonia buffer solution with pH=9.2 to control the pH of the system in this study.
4.3OptimizationofbuffersolutionvolumeFixed the volume of bisoprolol fumarate and Cu2+solution, and added different volumes of buffer solution of ammonia water and ammonium chloride with pH=9.2, the experiment performed using the method in Section3.2showed that when Vbuffer<0.50 mL, theΔFincreased with the increase in the volume of buffer solution; when Vbuffer=0.50 mL, theΔFwas the largest; when Vbuffer>0.50 mL, theΔFdecreased with the increase of the volume of buffer solution. Therefore, we selected 0.50 mL of ammonia water and ammonium chloride buffer solution with pH=9.2 in this experiment to adjust the pH of the system (Fig.3).
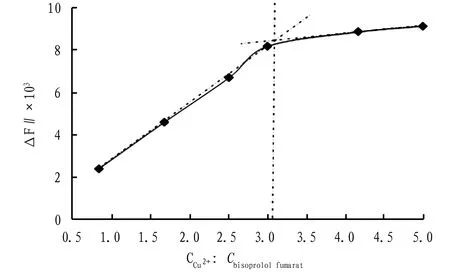
Fig.3 Curve for reaction ratio
4.4DiscussionofreactionratioFixed the volume of bisoprolol fumarate standard substance solution and ammonia buffer solution, changed the added volume of Cu2+, the experiment performed using the method in Section3.2showed that with the increase of CCu2+,ΔFgradually increased; when CCu2+:Cbisoprolol fumarate=3,ΔFwas the largest, indicating that the complexation effect of bisoprolol fumarate and Cu2+was the best at this time; when CCu2+:Cbisoprolol fumarate>3, with the increase of CCu2+, the increase ofΔFbecame gradually gentle, indicating that the added Cu became saturated.
4.5OptimizationofreagentadditionsequenceWhen the other conditions unchanged, changed the sequence of reagent addition, the sequence is as follows: (i) bisoprolol fumarate standard solution, Cu2+solution, ammonia water and ammonium chloride buffer solution; (ii) Cu2+solution, ammonia water and ammonium chloride buffer solution, bisoprol fumarate standard solution; (iii) bisoprolol fumarate standard solution, ammonia water and ammonium chloride buffer solution, Cu2+solution. According to the method in Section3.2, calculatedΔF, obtained (iii)>(ii)>(i). Therefore, the final reagent selection sequence is: bisoprolol fumarate standard solution, pH=9.2 ammonia water and ammonium chloride buffer solution, and Cu2+solution.
4.6EffectofreactiontemperatureWith other conditions unchanged, after changing the reaction temperature, determinedΔFaccording to the experimental method in Section3.2. WhenTwas between 30 ℃ and 40 ℃,ΔFwas the largest; whenT>40 ℃,ΔFdecreased with the increase of the reaction temperature, which may be due to the increase of the molecular movement speed, which increased the probability of molecular collision, and reduced the fluorescence intensity of the drug itself. Therefore, we adopted the room temperature to conduct the experiment.
4.7TestofreactiontimeAccording to the experimental method in Section3.2, with other conditions unchanged, we explored the effect of different reaction time on the complexation of bisoprolol fumarate and Cu2+. The results indicate that whenTreaction<30 min,ΔFincreased with the increase ofTreaction; and when ,Treaction= 30 min,ΔFwas the largest and stable. Therefore, we selected 30 min as the reaction time.
4.8InterferenceexperimentUnder the optimized experimental conditions, we investigated the effects of different excipients, including starch, talc, lactose, microcrystalline cellulose, Mg2+,etc. In the range of relative error ≤ ± 5%, the interference of talc, lactose and microcrystalline cellulose was small, while the interference of starch and Mg2+was large, but Mg2+generally existed in the form of magnesium stearate. Both starch and magnesium stearate were insoluble in water and can be removed by filtration without affecting the experiment.
5 Conclusions
In this experiment, we applied the fluorescence quenching method to indirectly determine the content of bisoprolol fumarate by measuring the difference in fluorescence intensity between bisoprolol fumarate and Cu2+complex. When using this method in determi-
nation of the content of bisoprolol fumarate sample preparation, we obtained satisfactory results.
- Medicinal Plant的其它文章
- Study on Chemical Constituents of Ethanol Extract from Yao Medicine Cissampelopsis spelaeicola
- Optimization of Extraction of Total Flavonoids from HERBA BLUMEAE RIPARIAE Using Response Surface Methodology
- Comparison of Polysaccharides Content among Common Fruits
- Systematic Review and Meta-analysis of Tanshinone Capsule in the Treatment of Polycystic Ovary Syndrome
- Extraction Process of Volatile Oil of Artemisiae Scopariae Herba
- Comparative Identification between Kadsura coccinea and Kadsura heteroclite

