Systematic Review and Meta-analysis of Tanshinone Capsule in the Treatment of Polycystic Ovary Syndrome
Bing WEI, Yi ZHANG, Ningning ZHAO, Shiying TANG
Institute of Traditional Chinese Medicine, Chengde Medical University/Key Laboratory of Traditional Chinese Medicine Research and Development of Hebei Province, Chengde Medical University, Chengde 067000, China
Abstract [Objectives] To study the efficacy and safety of tanshinone capsule in the treatment of polycystic ovary syndrome (PCOS). [Methods] A randomized controlled trial or semi-randomized controlled trial of tanshinone capsule in the treatment of polycystic ovary syndrome was included. Two researchers screened the retrieved literature, extracted the data, assessed the risk of bias of the included literature, and then carried out meta-analysis. [Results] A total of 5 articles were included, involving 350 patients. The results of meta-analysis showed that compared with the control group, tanshinone capsule could reduce total cholesterol[MD=-0.73, 95% CI (-0.86,-0.60), P<0.000 01], triglyceride[MD=-0.40, 95% CI (-0.57,-0.23), P<0.000 01], low density lipoprotein cholesterol[MD=-0.47, 95% CI (-0.73,-0.53), P<0.000 01], follicle stimulating hormone[MD=-0.63, 95% CI (-0.73,-0.53), P<0.000 01], increase high density lipoprotein cholesterol[MD=0.28, 95% CI (0.20, 0.35), P<0.000 01], the effective rate (P=0.06) and luteinizing hormone (P=0.07) in the treatment of polycystic ovary syndrome. [Conclusions] Tanshinone capsule could effectively improve the level of lipid metabolism and follicle stimulating hormone in patients with polycystic ovary syndrome.
Key words Polycystic ovary syndrome, Tanshinone capsule, Meta-analysis, Systematic review
1 Introduction
Polycystic ovary syndrome (PCOS) is a common disease caused by complex endocrine and metabolic abnormalities in women of childbearing age, and it is the most common endocrine disease in women. The incidence of it in women of childbearing age is 6%-25%[1]. The common complications are infertility, depression, diabetes, endometrial cancer and so on. Western medicine treatment is mainly lifestyle changes, drug treatment (ethinylestradiol cycloproesterone tablets, deoxypregnenylestradiol tablets, Diane-35,etc.), surgical treatment[2]. Tanshinone capsule is an effective ingredient extracted by modern technology ofSalviamiltiorrhiza, and the main active ingredient is cryptotanshinone. Modern studies have shown that cryptotanshinone can reduce androgen and regulate glucose and lipid metabolism[3]. There are some clinical studies on tanshinone capsule in the treatment of polycystic ovary syndrome, but there is no meta-analysis in this field. This study collected all relevant research literature at home and abroad, and comprehensively and systematically evaluated the therapeutic effect of tanshinone capsule in the treatment of polycystic ovary syndrome, in order to provide reliable evidence-based medicine for the clinical application of tanshinone capsule in the treatment of polycystic ovary syndrome.
2 Research methods
2.1 Inclusion criteria
2.1.1Research types. Randomized controlled trials or semi-randomized controlled trials. Chinese or English.
2.1.2Object of study. The patients diagnosed with polycystic ovary syndrome are not limited in age and course of disease.
2.1.3Intervention measures. The control group was treated with placebo, untreated or routine western medicine, and the treatment group was treated with tanshinone capsule or tanshinone capsule on the basis of the control group.
2.1.4Outcome index. Metabolic indices: TC, TG, HDL-C, LDL-C; sex hormones and related indices: FSH and LH; the occurrence of adverse reactions.
2.2 Exclusion criteriaRepeatedly published literature; reviews, animal experiments, individual cases; intervention measures mixed with other TCM techniques, including acupuncture, massage,etc.; literature in which outcome index cannot be obtained.
2.3 Retrieval strategySeven major databases were selected for literature retrieval: Pubmed, EMBASE,Cochrane Library, CNKI, Wanfang Database, CBMDisc and VIP Database. The retrieval time is from the establishment of the database to February 9, 2020.
Chinese key words: 多囊卵巢综合征, 丹参酮胶囊, 丹参酮.
English key words: tanshinone, danshentong, polycystic ovarian syndrome, POCOS.
2.4 Data collection and analysis
2.4.1Literature screening and data extraction. The literature was screened by two researchers. If the two people do not agree with each other, they may consult and, if necessary, a third party will make a decision. Data extracted include: year, author, number, intervention measures, outcome index, course of treatment.
2.4.2Risk of bias assessment. The risk of bias of the included literature was assessed according to the method recommended by the Cochrane manual. It includes the following seven items: the generation of random sequence, whether to conceal allocation, whether to implement blinding method (subjects and researchers), whether to implement blinding method (outcome surveyor), whether the result data is complete, whether it is selectively published, and whether there are other biases. Each item was evaluated with "high risk", "unknown risk" and "low risk", which was carried out by two researchers, respectively.
When there was a disagreement, it could be judged by a third party.
2.5 Statistical analysisIn this study, RevMan 5.3 software was used for meta-analysis. The relative risk degree (RR) or absolute risk degree (OR) was used for counting data. For the measurement data, the mean difference was used to calculate the effect size. All adopted 95% confidence interval. Heterogeneity test:P≥0.1 andI2≤50%, indicating that there was homogeneity among studies, then a fixed effect model was used. IfP>0.1 andI2≥50%, or ifP<0.1 andI2≤50%, it indicated that there was heterogeneity among studies, and random effect model could be used. When the included literature quantity was more than 9, the funnel plot could be drawn to judge whether there was bias.
3 Results
3.1 Basic characteristics of included literatureThrough the search of Chinese and English databases, a total of 105 articles were retrieved and imported into the software Noteexpress. There were 67 articles left after double check. After reading the title and abstract, 62 articles that did not meet the inclusion criteria or met the exclusion criteria were excluded, and finally there were 5 articles left[4-8], as shown in Fig.1. Among the included articles, 4 reported TC, TG, HDL-C and LDL-C[4,6-8], 2 reported FSH and LH[4,6], 2 reported effective rate[5,7]and 1 reported adverse reactions[8]. Details are shown in Table 1.
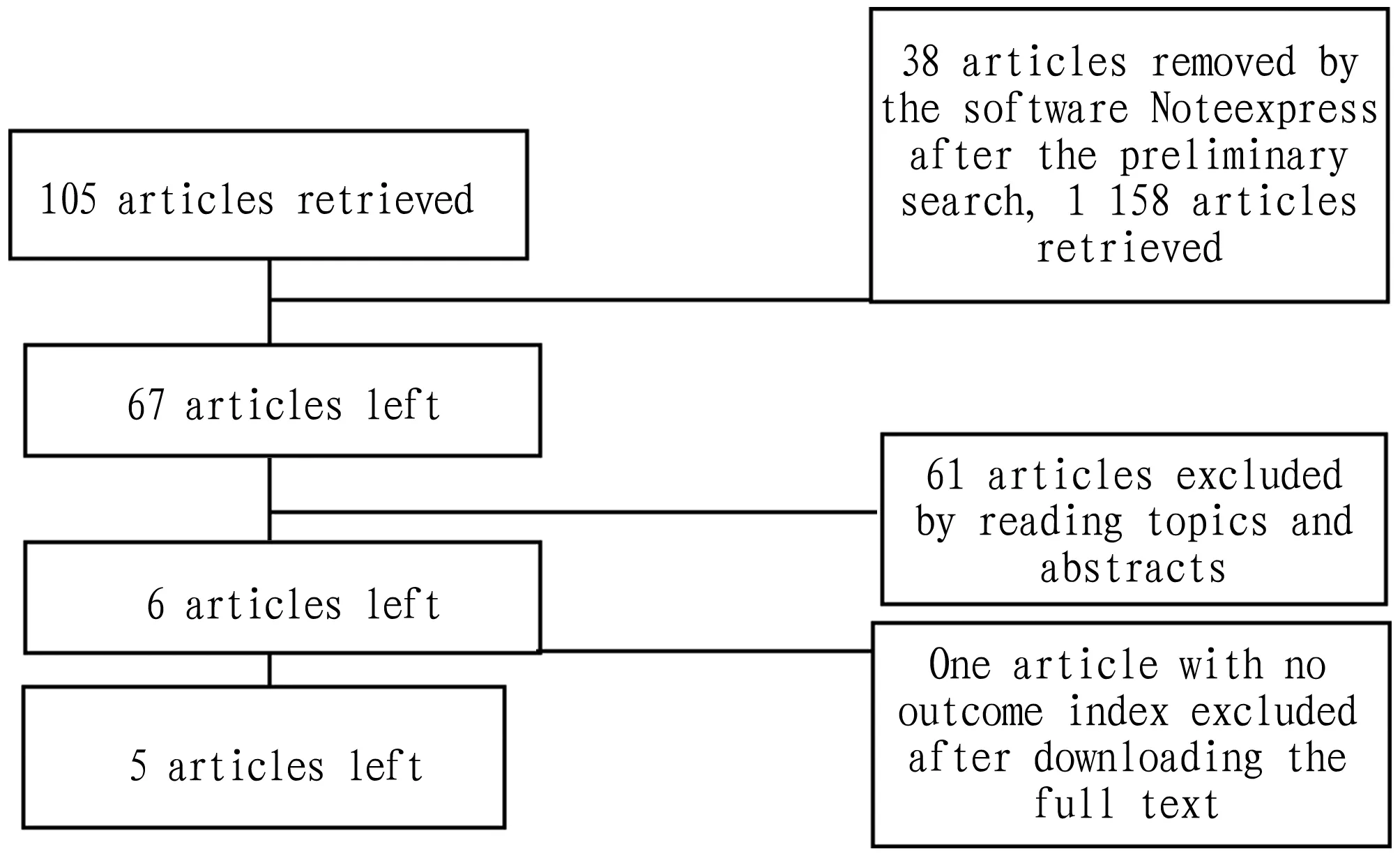
Fig.1 Literature screening process
Table 1 Basic characteristics of literature
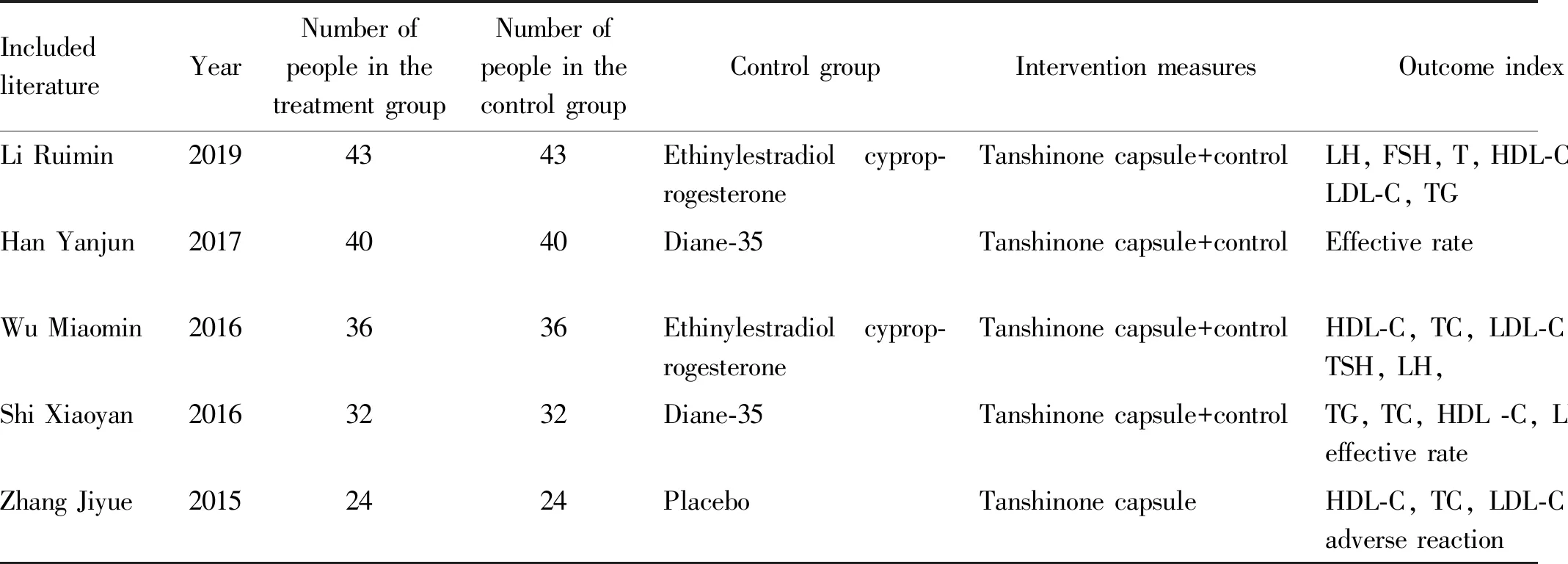
IncludedliteratureYearNumber ofpeople in thetreatment groupNumber ofpeople in thecontrol groupControl groupIntervention measuresOutcome indexCourse oftreatmentLi Ruimin20194343Ethinylestradiol cyprop-rogesteroneTanshinone capsule+controlLH, FSH, T, HDL-C, TC, LDL-C, TG3 monthsHan Yanjun20174040Diane-35Tanshinone capsule+controlEffective rate2 monthsWu Miaomin20163636Ethinylestradiol cyprop-rogesteroneTanshinone capsule+controlHDL-C, TC, LDL-C, TG, TSH, LH, 3 monthsShi Xiaoyan20163232Diane-35Tanshinone capsule+controlTG, TC, HDL -C, LDL-C, effective rate2 monthsZhang Jiyue20152424PlaceboTanshinone capsuleHDL-C, TC, LDL-C, TG, adverse reaction3 months
3.2 Literature quality evaluationAmong the 5 articles included, 3 used the random number table method, 2 only described the random method, and did not mention which random method; there was no mention of whether or not to conceal the allocation; one article described the use of placebo; there was no mention of whether the blinding method was used to measure the outcome; there was no mention of whether it was published selectively or not; all five articles described the comparability of the baseline between the treatment group and the control group (Fig.2).
4 Meta-analysis
4.1 Total cholesterolA total of 4 articles were included. Heterogeneity test:I2=62,P=0.05, which indicated that there was heterogeneity among the studies, so the random effect model was used. The combined statistics MD=-0.73,95% CI (-0.86,-0.60),P<0.000 01, and the difference was statistically significant, indicating that the total cholesterol of tanshinone capsule was lower than that of the control group, as shown in Fig.3.

Fig.2 Summary chart of Cochrane risk of bias in included literature
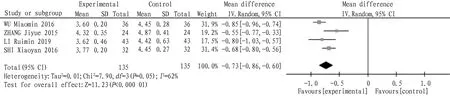
Fig.3 Comparison of total cholesterol between the experimental group and the control group
4.2 TriglyceridesA total of 4 articles were included, and the heterogeneity test:I2=94,P<0.000 01, which indicated that there was heterogeneity among the studies, so the random effect model was adopted. The combined statistics MD=-0.40,95% CI (-0.57, -0.23),P<0.000 01, indicating that the triglyceride of tanshinone capsule was lower than that of the control group, as shown in Fig.4.
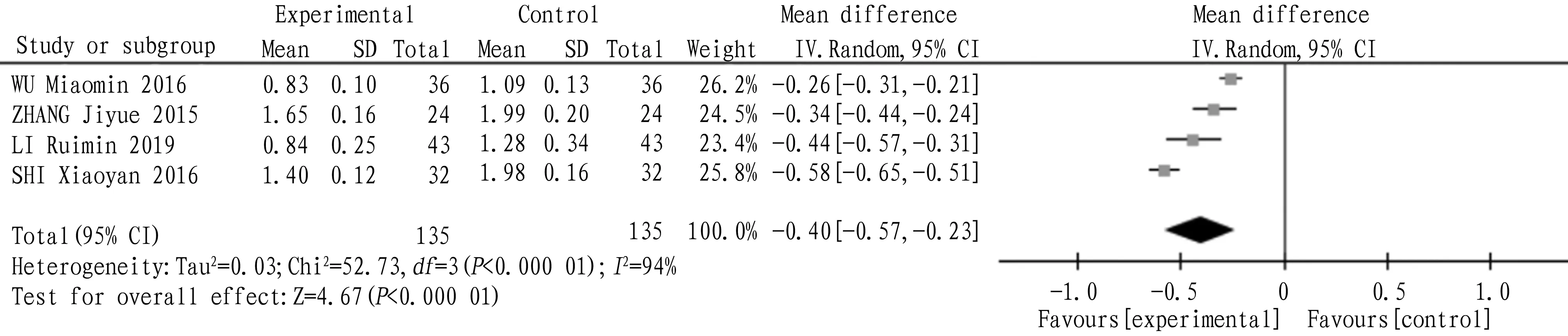
Fig.4 Comparison of triglycerides between experimental group and control group
4.3 High density lipoprotein cholesterolA total of 4 articles were included, and the heterogeneity test:I2=75,P=0.008, which showed that there was heterogeneity among the studies, so the random effect model was adopted. The combined statistics MD=0.28,95% CI (0.20,0.35),P<0.000 01, and the difference was statistically significant, indicating that the total cholesterol of tanshinone capsule was higher than that of the control group, as shown in Fig.5.

Fig.5 Comparison of high density lipoprotein cholesterol between the experimental group and the control group
4.4 Low density lipoprotein cholesterolA total of 4 articles were included, and the heterogeneity test:I2=90,P<0.000 01, which indicated that there was heterogeneity among the studies, so the random effect model was adopted. The combined statistics MD=-0.47,95% CI(-0.51,-0.43),P<0.000 01, and the difference was statistically significant, indicating that the low density lipoprotein cholesterol of tanshinone capsule was lower than that of the control group, as shown in Fig.6.
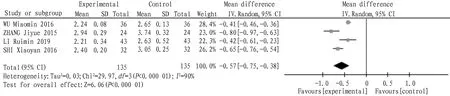
Fig.6 Comparison of low density lipoprotein cholesterol between the experimental group and the control group
4.5 Follicle stimulating hormoneA total of 2 articles were included, and the heterogeneity test:I2=16,P=0.27, which showed that there was homogeneity among the studies, so the fixed effect model was adopted. The combined statistics MD=-0.63,95% CI(-0.73,-0.53),P<0.000 01, and the difference was statistically significant, indicating that the follicle-stimulating hormone of tanshinone capsule was lower than that of the control group, as shown in Fig.7.
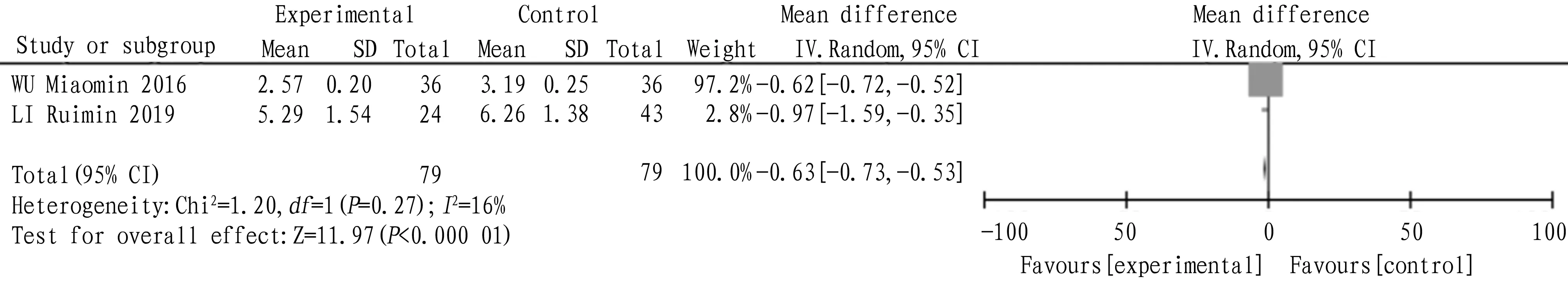
Fig.7 Comparison of follicle-stimulating hormone between the experimental group and the control group
4.6 Luteinizing hormoneA total of 2 articles were included, and the heterogeneity test:I2=98,P<0.000 01, which indicated that there was heterogeneity among the studies, so the random effect model was adopted. The combined statistics MD=-2.80,95% CI(-5.82,0.22),P=0.07, and the difference was not statistically significant, indicating that the luteinizing hormone of tanshinone capsule was similar to that of the control group, as shown in Fig.8.

Fig.8 Comparison of luteinizing hormone between the experimental group and the control group
4.7 Effective rateA total of 2 articles were included, and the heterogeneity test:I2=69,P=0.07, which indicated that there was heterogeneity among the studies, so the random effect model was adopted. The combined statistics MD=1.28,95% CI(0.99,1.65),P=0.06, and the difference was not statistically significant, indicating that the effective rate of tanshinone capsule was similar to that of the control group, as shown in Fig.9.
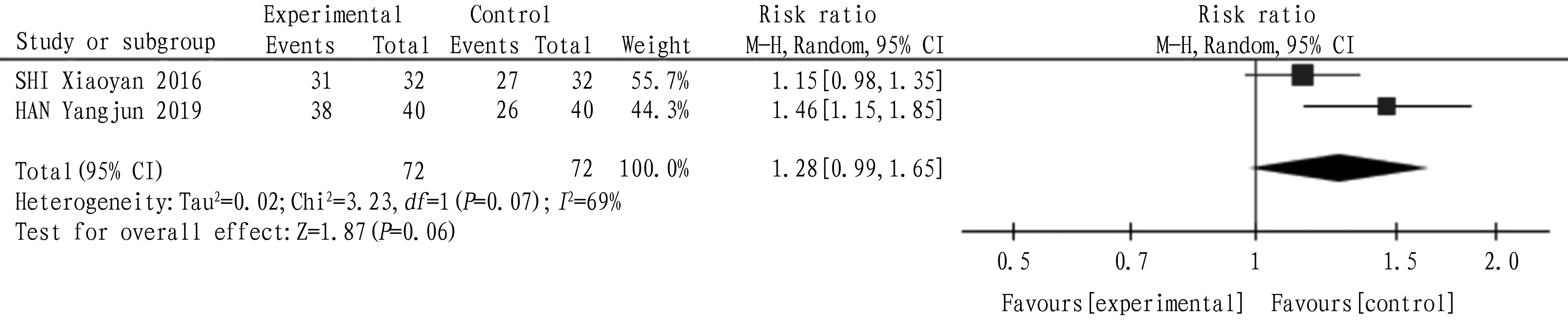
Fig.9 Comparison of effective rate between the experimental group and the control group
5 Descriptive analysis
Only one literature described the occurrence of adverse reactions. In the course of treatment, there were no adverse reactions such as gastrointestinal discomfort, rash, and dizziness.
6 Discussion
Polycystic ovary syndrome falls within the category of "late menstruation", "amenorrhea" and "infertility" in traditional Chinese medicine. Western medicine has some limitations in the treatment of polycystic ovary syndrome. With the development of traditional Chinese medicine, traditional Chinese medicine is becoming more and more popular. And tanshinone capsule, as a proprietary Chinese medicine, has a certain effect in the treatment of polycystic ovary syndrome.
A total of 5 articles were included in this study, and the results showed that tanshinone could reduce total cholesterol, triglyceride, low density lipoprotein cholesterol and follicle stimulating hormone. It was similar to that in the control group in terms of follicle stimulating hormone and effective rate. Limitations of the study: there were few articles included in this study, and the quality of the literature was generally not high; the heterogeneity of the outcome index was high, and the subgroup analysis was not carried out because of the small number of articles; since only one article mentioned adverse reactions, it was impossible to evaluate the safety of tanshinone capsules according to the current research.
- Medicinal Plant的其它文章
- Application of Chaihu plus Longgu Muli Decoction in Treatment of Physical and Mental Diseases
- Study on Pharmacological Effects of Calycosin in Astragali Radix
- Advances in Research on Treatment of Heart Failure with Yangxinshi Tablet
- Advances in Chemical Constituents and Pharmacological Activity of Pholidota spp.
- Treatment of Arthralgia Syndrome from Zang and Fu
- Effects of Zingber mioga Aqueous Extract on Hepatic Anti-alcoholism in Mice

