Comparison of Polysaccharides Content among Common Fruits
Jun WANG, Feng MIAO, Xiurong YANG
1. College of Life Science, Cangzhou Normal University, Cangzhou 061001, China; 2. Cangzhou Academy of Agriculture and Forestry Sciences, Cangzhou 061001, China
Abstract [Objectives] Polysaccharides in common fruits were determined and compared. [Methods] Polysaccharides in fresh bananas, apples, grapes and strawberries purchased from local markets were extracted using water extraction-alcohol precipitation method and determined using 3,5-dinitrosalicylic acid (DNS) method. [Results] The content of polysaccharides in banana was relatively high. The optimal conditions for DNS colorimetry were as follows: measurement wavelength of 510 nm, color developer of 1.0 mL, color development temperature of 90 ℃, color development time of 5min and stabilization time within 30 min. [Conclusions] The reproducibility and recovery rate of this method are ideal. It shows that the method is highly precise and is helpful for the research of fruit polysaccharides. This study provides a certain reference value for the development and application research of fruit polysaccharide resources in China.
Key words Fruit, Polysaccharide, Application
1 Introduction
An important component in fruits is polysaccharides, which have a variety of functions such as immune regulation and anti-oxidation[1]. Therefore, fruit polysaccharides have extremely high application and promotion value. The in-depth research on fruit polysaccharides is helpful to the development and utilization of fruit resources[2]. Predecessors have studied about fruit polysaccharides, but focusing on improving and maintaining the sensory quality of fruits and their processed products. With the continuous advancement and expansion of research content and scope, people are more and more inclined to research on the extraction and application of fruit polysaccharides[3]. A large amount of fruit polysaccharides have been discovered and extracted for use. One after another, polysaccharides are extracted from apples (Malusdomestica)[4], grapes (Vitisvinifera)[5], bananas (Musanana)[6]and strawberries (Fragariaananassa)[7]. There are many ways to extract polysaccharides from fruits[8]. The most traditional method is hot water extraction. It is easy to operate and the disadvantage is that it takes time[9]. Fruit polysaccharides have a variety of biological activities and have been used in a variety of food and drug development[10]. In the industrial production process of polysaccharides, it is necessary to determine the content of polysaccharides[11]. There are also various determination methods[12]. Currently, the commonly used method is 3,5-dinitrosalicylic acid method. This method is accurate, reliable and widely used[13]. Different fruits have different polysaccharide contents[14-16]. Chen Guanglinetal.[17]investigated the total sugar content of 52 kinds of fruits, such as pineapple, banana and watermelon, using high-performance liquid chromatography. The results show that the total sugar contents of banana, litchi, and pineapple were high, and the total sugar contents of watermelon, kumquat and other fruits were low[17]. So far, there are few studies on fruit polysaccharide content. Therefore, the study of polysaccharide content in different fruits is beneficial to the development and utilization of polysaccharides in different fruits.
In this study, fresh bananas, apples, strawberries and grapes were used as raw materials.
Because the technology of extracting polysaccharides by traditional hot water extraction method in China is quite mature and the hot water extraction method is easy to operate, the water extraction and alcohol precipitation method was used for extraction. The content of the extracted polysaccharides was determined by 3,5-dinitrosalicylic acid method. The contents of polysaccharides in various fruits were compared, in order to provide a certain reference value for the development and application research of fruit polysaccharides in China.
2 Materials and methods
2.1 Fruits and reagentsApples, bananas, grapes, strawberries, anhydrous glucose, 95% ethanol, anhydrous ethanol, acetone, distilled water, DNS reagent, sodium hydroxide, potassium sodium tartrate, crystalline phenol, sodium sulfite and diluted hydrochloric acid.
2.2 Apparatus and instrumentsElectric constant temperature water bath (HH-4), visible spectrophotometer (723N), vacuum drying box (DZ-1BC type), mortar, desktop low-speed centrifuge (TD4), glass rod, volumetric flask (10, 50, 100, 1 000 mL), beaker, pH test paper, electronic balance, pipette and dropper.
2.3 Methods
2.3.1Effect of soaking time and temperature on extraction rate of crude polysaccharides. After washing the fruits with clear water, 5 portions of each of the fruits, 5 g for each portion, were weighed. Each portion of the fruits was placed in a beaker, and added with 100 mL of distilled water, respectively. Each kind of the fruits was soaked for 20, 30, 40, 50 and 60 min, respectively, and then subjected to polysaccharides extraction.
After washing the fruits with clear water, 5 portions of each of the fruits, 5 g for each portion, were weighed. Each portion of the fruits was placed in a beaker, and added with 100 mL of distilled water, respectively. Each kind of the fruits was soaked for 30 min at 20, 30, 40, 50 and 60 ℃, respectively for extraction of polysaccharides.
2.3.2Extraction of crude polysaccharides. After soaked in 50 mL of water, each kind of the fruits was ground to homogenate and placed in a constant-temperature water bath (apple: 95 ℃, 4 h, one time; banana: 90 ℃, 3 h, 2 times; grape: 90 ℃, 2 h, 3 times; strawberry: 90 ℃, 1 h, 2 times) to obtain an extract.
The extract was centrifuged at 4 000 r/min for 10 min, and filtered directly with gauze. Then, the filtrate was concentrated under reduced pressure to a quarter of the original volume, the pH of the liquid was adjusted with acid, and 4 times the amount of 95% ethanol was slowly added to the liquid while stirring for about 0.5 h. The liquid was let stand at room temperature overnight and centrifuged at 4 000 r/min for 10 min to obtain the crude polysaccharide precipitation of the four fruits. The precipitation was washed with 95% ethanol, acetone and absolute ethanol in success according to the following procedure (Fig.1), which was repeated 2-3 times. The final precipitate obtained was the crude polysaccharides.
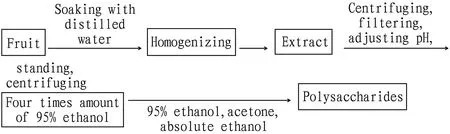
Fig.1 Extraction process of polysaccharides from fruits
2.3.3Solution preparation. (i) Reducing sugar solution. A certain amount (50 mg) of the crude polysaccharides of each fruit was dissolved in appropriate volume of distilled water and then diluted to 25 mL. (ii) Total sugar solution. A certain amount (50 mg) of the crude polysaccharides of each fruit was dissolved in a certain volume of distilled water in a beaker, added with 10 mL of hydrochloric acid (6 mol/L), placed in boiling water for 10 min, cooled, added with 10% NaOH solution to adjust the pH to neutral, and finally diluted to 100 mL with water for use.
2.3.4Determination of test conditions for DNS colorimetry. (i) Optimal colorimetric wavelength. In a 10-mL volumetric flask, 1 mL of DNS reagent solution and 1 mL of glucose standard solution were added. The volumetric flask was placed in boiling water for 5 min. After taking out from boiling water, the volumetric flask was cooled to room temperature with tap water, and then the liquid in the flask was diluted to 10 mL with water. The diluent was scanned with a visible spectrophotometer in the range of 300-1 000 nm. Taking the DNS reagent as a control, the absorption curves of glucose+DNS and DNS+blank were obtained to determine the optimal colorimetric wavelength.(ii) Dosage of color developer. Six parts of glucose standard solution, 1 mL for each part were transferred to six 10-mL volumetric flasks, which were then added with 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4 mL of DNS reagent, respectively. The flasks were placed in boiling water for 5 min and cooled, and the solutions in the volumetric flasks were diluted to 10 mL, respectively. The absorbances of the diluents were measured. (iii) Color development time. Certain volumes of glucose standard solution (1 mL) and DNS reagent (1 mL) were added to each of the five 10-mL volumetric flasks, which were placed in boiling water for 1, 3, 5, 7 and 9 min, respectively. After 30 min of cooling, the solutions were diluted to 10 mL, respectively, and the absorbances of the diluents were measured. (iv) Color development temperature. Certain volumes of glucose standard solution (1 mL) and DNS reagent (1 mL) were added to each of the five 10-mL volumetric flasks, which were placed in water bath at 60, 70, 80, 90 and 100 ℃ for 5 min, respectively. After 30 min of cooling, the solutions were diluted to 10 mL, respectively, and the absorbances of the diluents were measured. (v) Drawing of standard curve. Certain volumes (0, 1, 2, 4, 6, 8 and 10 mL) of glucose standard solution were diluted to 25 mL, respectively. A certain volume (1 mL) of each of the diluent was added with 1 mL of DNS reagent, placed in boiling water bath for 5 min, cooled for 30 min, and diluted to 10 mL, respectively. The absorbances of the new diluents were measured, and the standard curve was drawn.
2.3.6Sample detection. Certain volumes of reducing sugar solution (1 mL) and total sugar solution (1 mL) were transferred into a 10-mL volumetric flask, added with 1 mL of DNS reagent, heated in 90 ℃ water bath for 5 min, cooled for 30 min, and diluted to 10 mL in success. The absorbance of the diluent was measured, and the content of polysaccharides was calculated.
2.3.7Methodological investigation. (i) Stability test. Certain volumes of glucose standard solution (1 mL) and DNS reagent (1 mL) were transferred to each of five 10-mL volumetric flasks, heated in boiling water for 5 min, cooled for 5, 10, 20, 30 and 40 min, and diluted to 10 mL, respectively. The absorbances of the diluents were measured. (ii) Recovery test. Certain volumes of reducing sugar solution (1 mL) and total sugar solution (1 mL) were mixed, and four replicates were arranged. The mixtures were added with 1 mL of glucose standard solution (1 mg/mL) and 1 mL of DNS reagent, respectively, and then, the absorbances of the new solutions were measured.
3 Results and analysis
3.1 Working drawing of standard curveAs shown in Fig.2, the regression equation of the glucose standard curve for this experiment was:
y=0.004 7x+0.089 6,R2=0.997 4.
3.2 Determination of the optimal colorimetric wavelengthThe measurement results are shown in Fig.3. The two had the highest absorbance at 470 nm. However, the absorbance value of DNS+blank at this time was also relatively large, which had a certain interference with the measured value, so the optimal colorimetric wavelength was set to another absorption peak of DNS+glucose at 510 nm.
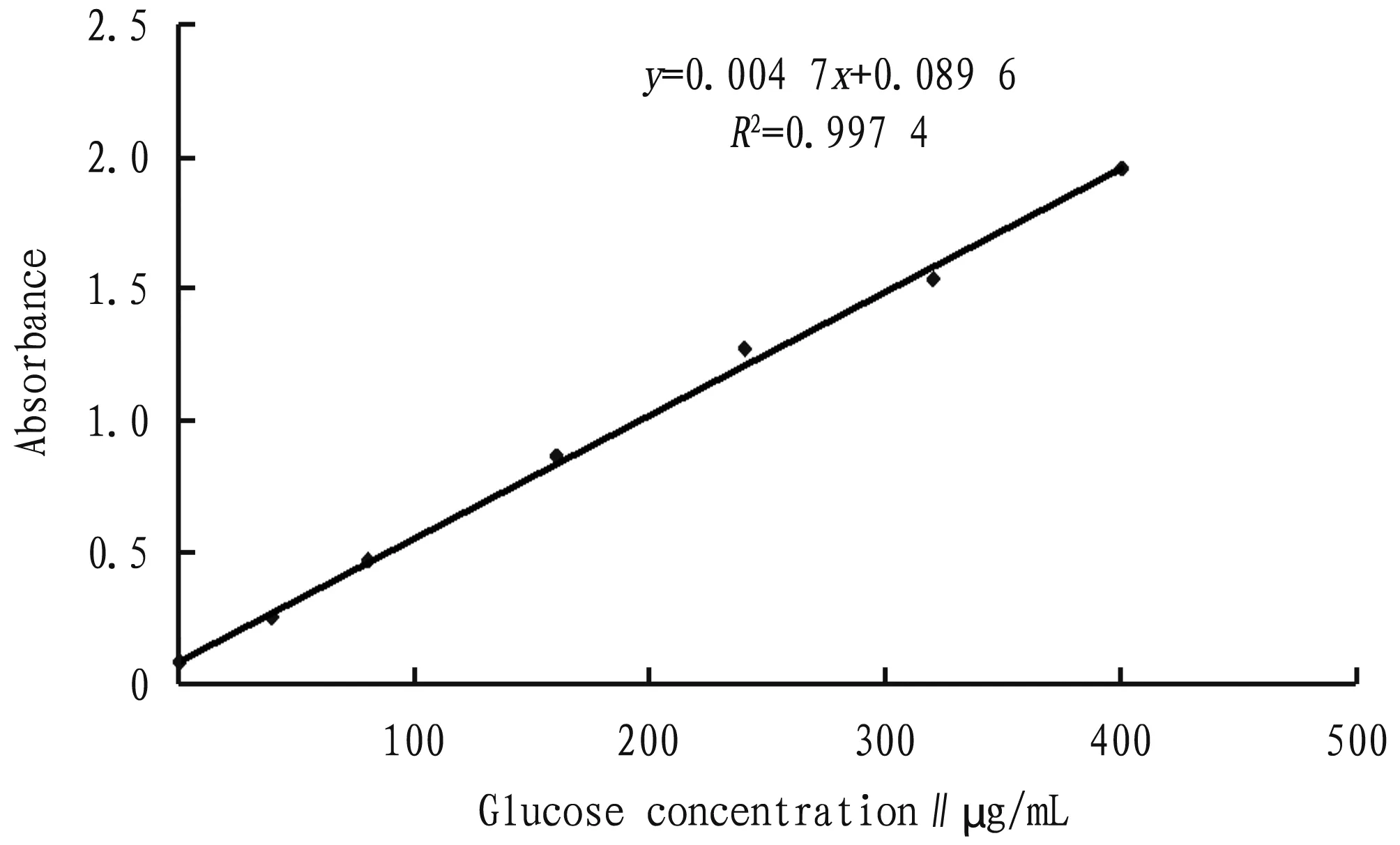
Fig.2 Glucose standard curve
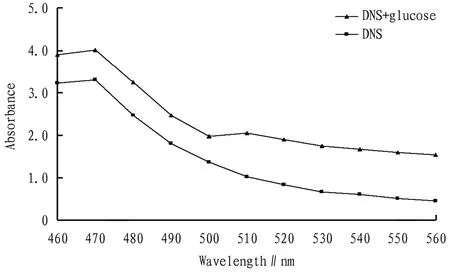
Fig.3 Absorption curve of DNS and DNS+glucose standard solutions
3.3 Effects of soaking time and temperature on extraction rate of crude polysaccharides
3.3.1Soaking time. As shown in Table 1, with the increase of the soaking time, `the extraction rate of crude polysaccharides showed a downward trend; and after the soaking time exceeded 30 min, the extraction rate of crude polysaccharides decreased sharply.
Table 1 Effect of soaking time on extraction rate of polysaccharides from four kinds of fruits
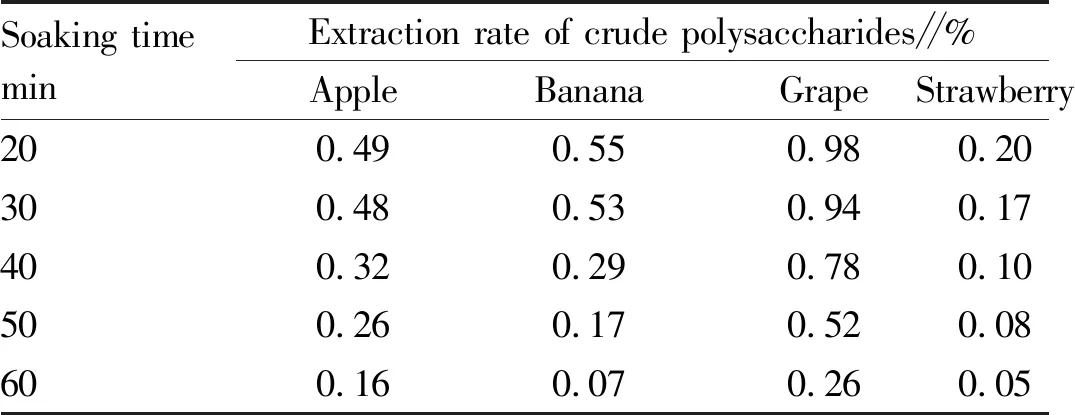
Soaking timeminExtraction rate of crude polysaccharides∥%AppleBananaGrapeStrawberry200.490.550.980.20300.480.530.940.17400.320.290.780.10500.260.170.520.08600.160.070.260.05
3.3.2Soaking temperature. As shown in Table 2, as the soaking temperature increased, the extraction rate of crude polysaccharides continued to increase; and after the temperature exceeded 40 ℃, the extraction rate of crude polysaccharides decreased gradually.
3.4 Dosage of color developerAs shown in Fig.4, when the addition amount of DNS reagent was in the range of 0.4-1.0 mL, the absorbance increased with the increase in the addition amount of DNS reagent; and when it exceeded 1 mL, the absorbance decreased, so the addition amount of DNS reagent was determined as 1.0 mL.
Table 2 Effect of soaking temperature on extraction rate of polysaccharides from four kinds of fruits
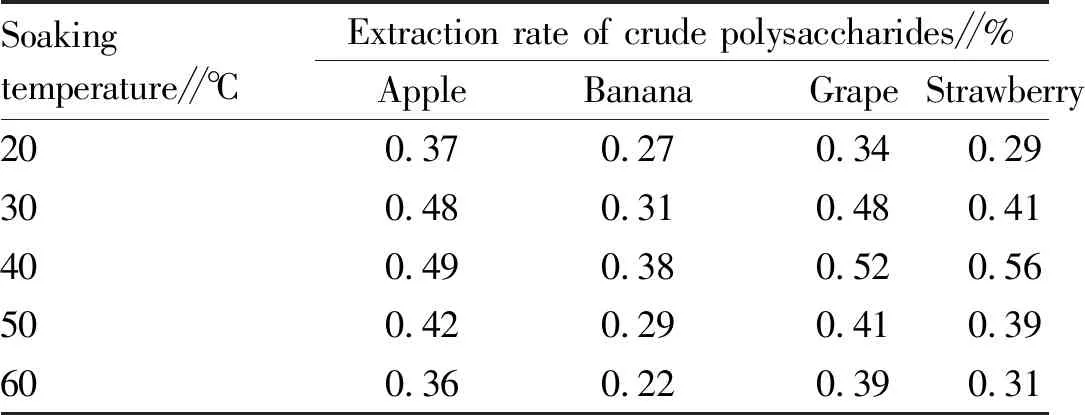
Soakingtemperature∥℃Extraction rate of crude polysaccharides∥%AppleBananaGrapeStrawberry200.370.270.340.29300.480.310.480.41400.490.380.520.56500.420.290.410.39600.360.220.390.31
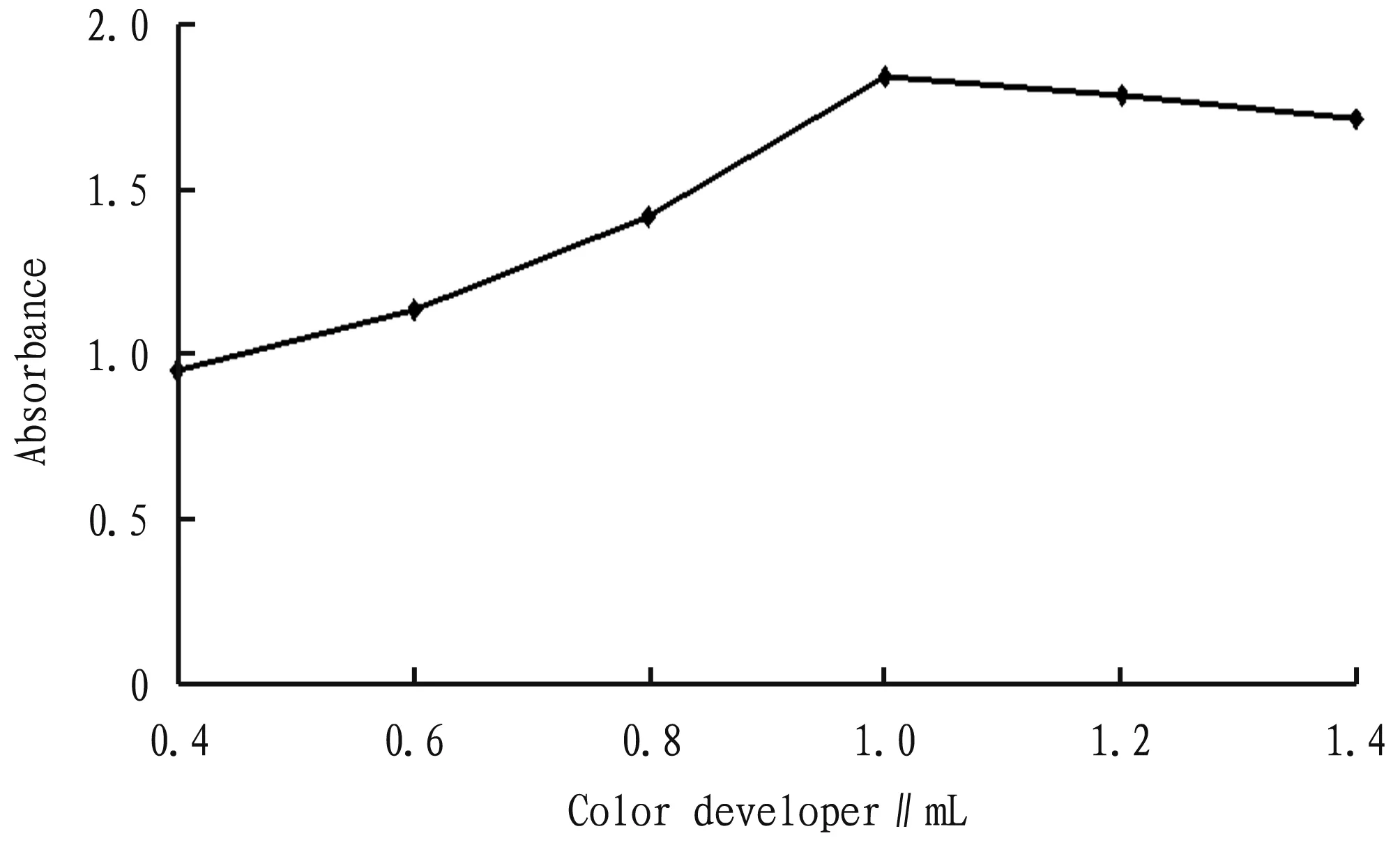
Fig.4 Relationship between dosage of color developer and absorbance
3.5 Color development timeAs shown in Fig.5, within 3 min, the absorbance increased slowly; in the range of 3-5 min, the absorbance increased significantly with time; and after 5 min, the absorbance remained in a stable state. Therefore, the color development time was preferably 5 min.
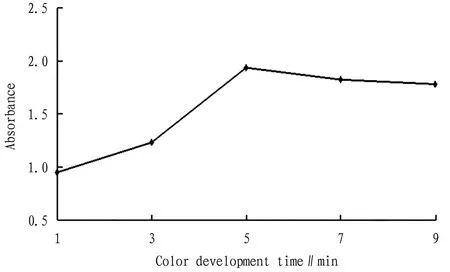
Fig.5 Relationship between color development time and absorbance
3.6 Determination of color development temperatureAs shown in Fig.6, when the temperature was lower than 90 ℃, the absorbance increased with increasing temperature; and after the temperature exceeded 90 ℃, the absorbance showed a downward trend. Therefore, the color development temperature was selected to be 90 ℃.
3.7 Results of stability testAs shown in Fig.7, within a range of 5-30 min, the absorbance gradually increased with time; and after 30 min, the absorbance decreased with time. Therefore, the standing time was determined to be 30 min.
3.8 Detection results of samples
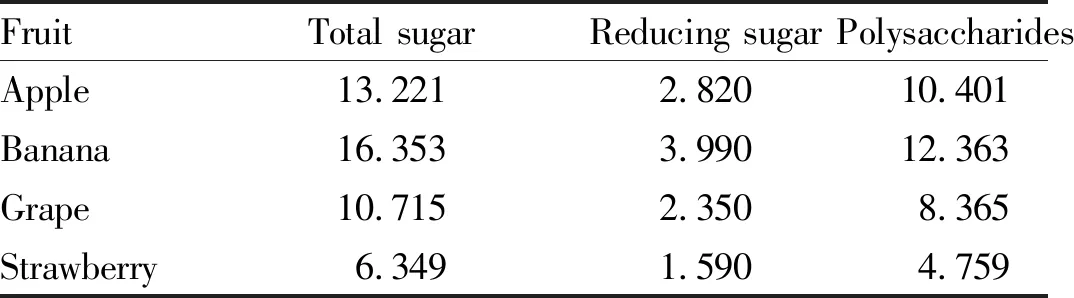
3.8.1Reproducibility test. According to the water extraction and alcohol precipitation method, the absorbances of the total sugar solution and reducing sugar solution of each fruit were measured separately and repeated 4 times to verify the reproducibility of the test method. The test results are shown in Tables 3 and Table 4. Among total sugar solutions, the total sugar solution of banana showed the best reproducibility, while those of other fruits showed lower reproducibility. Although theRSDvalues were larger, they were all less than 5% and were all in the valid range. In term of reducing sugar solution, grape showed better reproducibility. Although theRSDvalues of the four fruits were generally large, they were also within the valid range. Table 5 shows that the total polysaccharides content of the four fruits is different. Among them, the polysaccharides content of banana fruit was the highest among the four fruits, reaching 12%, followed by that of apple fruit (around 10%) and grape fruit (8%), and the polysaccharides content of strawberry fruit was the lowest, below 5%.
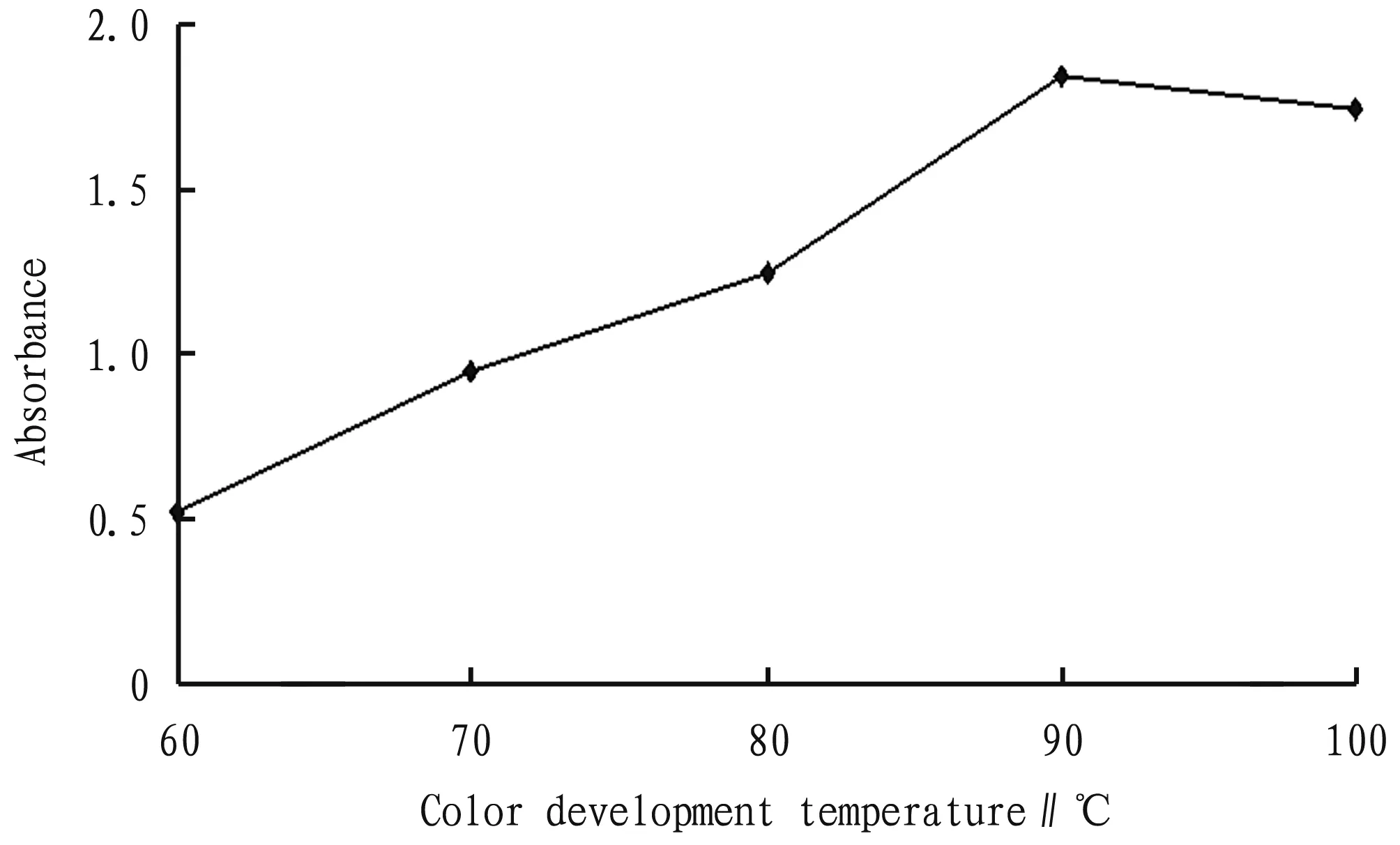
Fig.6 Relationship between color development temperature and absorbance
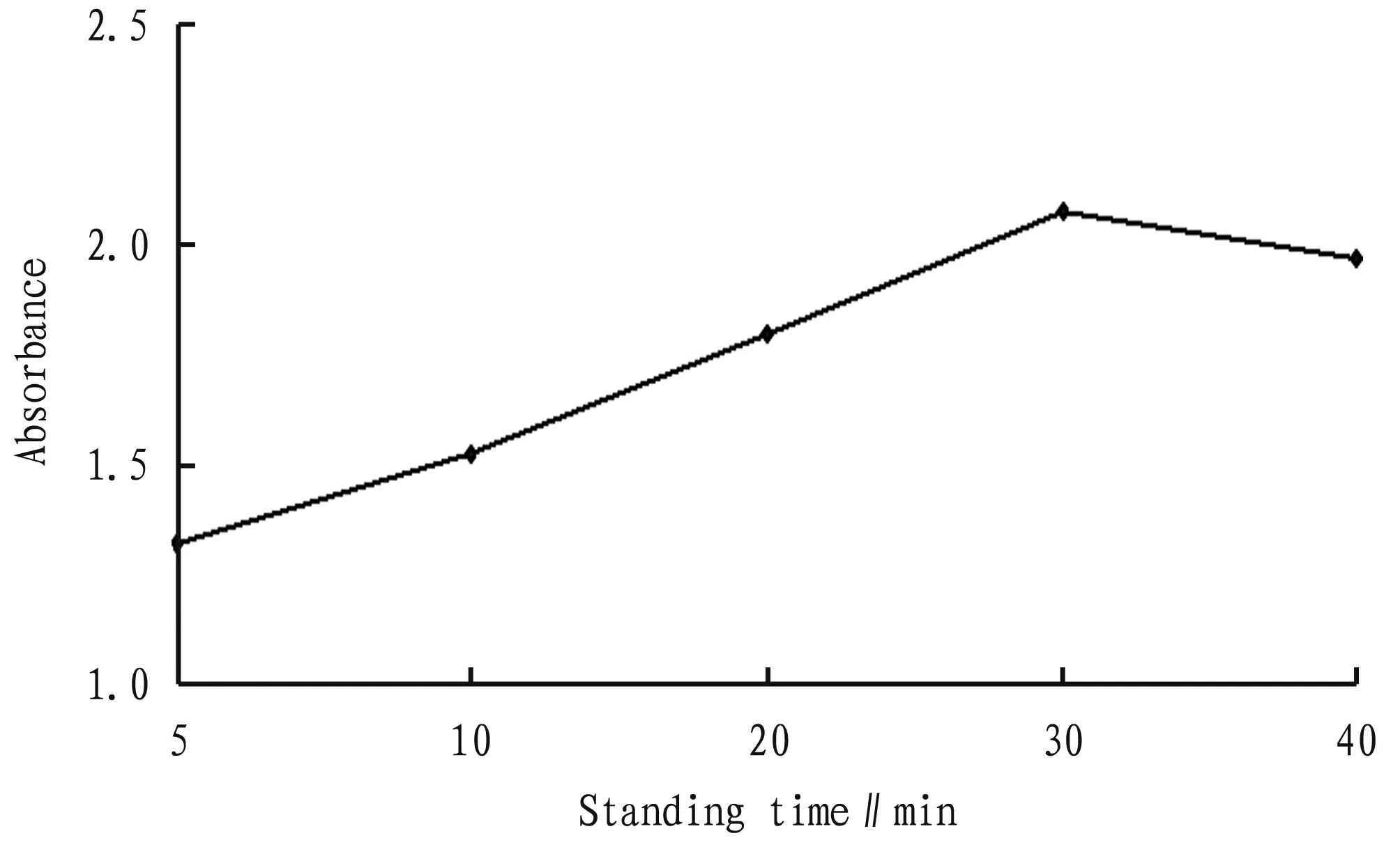
Fig.7 Relationship between standing time and absorbance
Table 3 Reproducibility results of determination of total sugar in four kinds of fruits
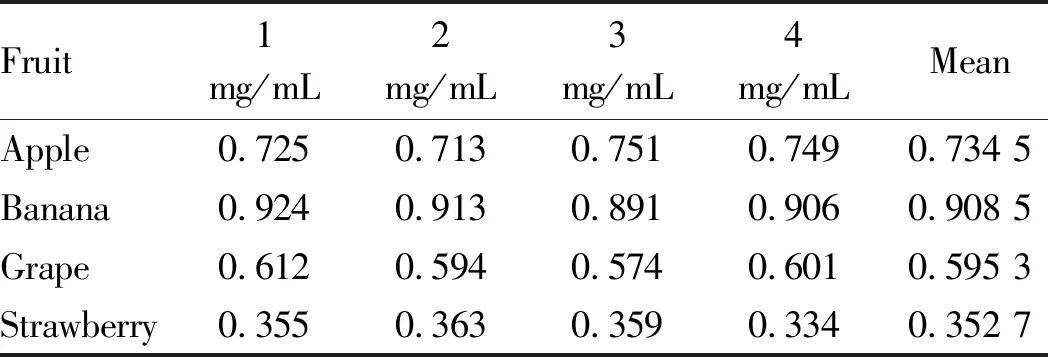
Fruit1mg/mL2mg/mL3mg/mL4mg/mLMeanRSD%Apple0.7250.7130.7510.7490.734 52.53Banana0.9240.9130.8910.9060.908 51.52Grape0.6120.5940.5740.6010.595 32.69Strawberry0.3550.3630.3590.3340.352 73.67
Table 4 Reproducibility results of determination of reducing sugar in four kinds of fruits
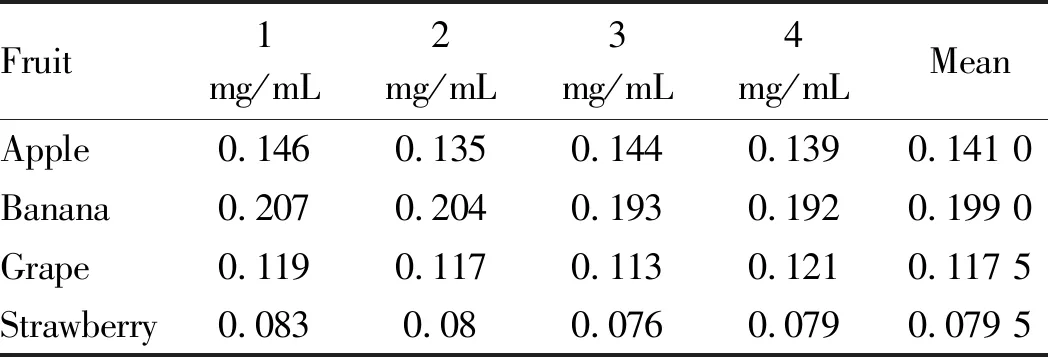
Fruit 1mg/mL2mg/mL3mg/mL4mg/mLMeanRSD%Apple0.1460.1350.1440.1390.141 03.52Banana0.2070.2040.1930.1920.199 03.83Grape0.1190.1170.1130.1210.117 52.91Strawberry0.0830.080.0760.0790.079 53.63
Table 5 Determination results of total polysaccharides from four kinds of fruits
%
3.8.2Results of recovery test (taking apple fruit as an example). According to Table 6, the recovery rate of the total sugar solution was in the range of 101.2%-103.2%, with an average value of 102.12%. The recovery rate of the reducing sugar solution was in the range of 96.9%-100.3%, with an average value of 98.6%. TheRSDvalues of total sugar and reducing sugar were 0.83% and 1.41%, respectively, indicating that the test results were ideal.
Table 6 Test results of sample recovery rate
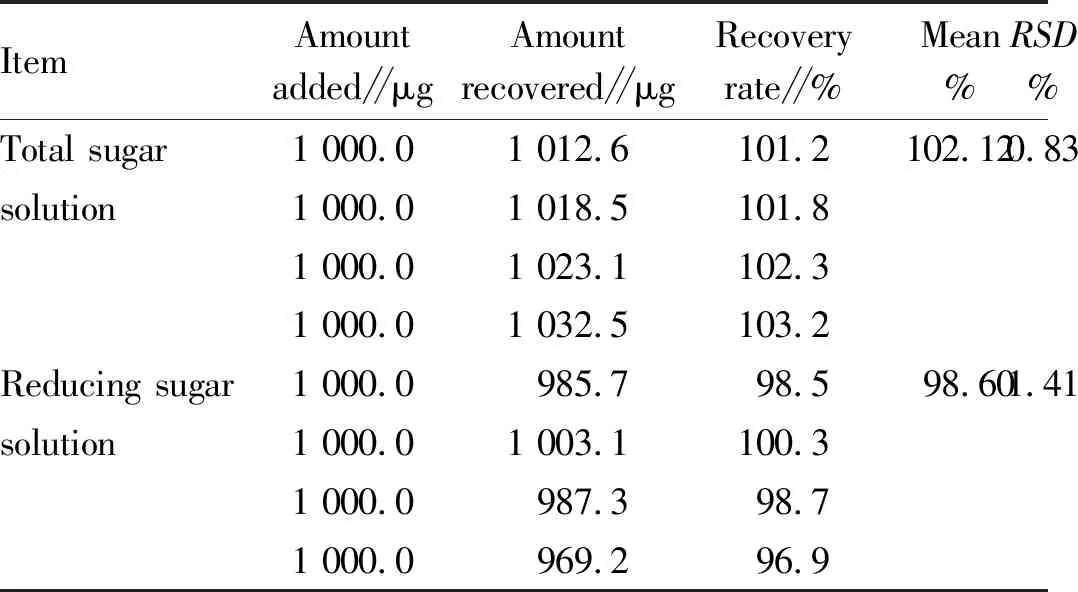
ItemAmountadded∥μgAmountrecovered∥μgRecoveryrate∥%Mean%RSD%Total sugar 1 000.01 012.6101.2102.120.83solution1 000.01 018.5101.81 000.01 023.1102.31 000.01 032.5103.2Reducing sugar 1 000.0985.798.598.601.41solution1 000.01 003.1100.31 000.0987.398.71 000.0969.296.9
4 Discussion and conclusions
According to the test results, soaking time and soaking temperature had a certain effect on the extraction rate of crude polysaccharides from fruits. The extraction rate of crude polysaccharides did not decrease within 30 min; and when the soaking temperature exceeded 40 ℃, the yield of crude polysaccharides decreased significantly. Therefore, the fruit soaking time should not exceed 30 min, and the soaking temperature should not exceed 40 ℃, because the harmful substances such as pesticides remaining on the fruits have been basically removed within 30 min of soaking time.
In the investigation of DNS test conditions, the determination conditions of polysaccharides content in fruits were determined: color developer of 1.0 mL, test temperature of 90 ℃, water bath time of 5 min and stabilization time of 30 min. The optimal detection wavelength for this test was 510 nm, different from the 540 nm used in the general method, which might be caused by different sensitivity and performance of instruments. In addition, the results of the reproducibility and recovery tests showed that the reproducibility and recovery of this method are ideal. Therefore, it can be said that the determination of polysaccharides by the 3,5-dinitrosalicylic acid method is feasible.
In this test, the total sugar content and reducing sugar content were measured. The difference between the two was regarded as polysaccharides content. This method has certain operability and safety. The results showed that under the optimal detection conditions, the polysaccharides content of the four fruits was different. The bananas had the highest polysaccharides content among the four fruits, followed by apples, grapes and strawberries. To this end, the polysaccharides in bananas and apples can be further studied to give full play to their biological activity, thereby improving the quality of life.
In recent years, scientists have found that fruit polysaccharides are of great significance for maintaining people’s health. Scientists from various countries have begun to research and develop new types of green health products using polysaccharides extracted from some fruits. In fact, the application of polysaccharides can be seen everywhere in our daily lives, and they have been applied to various foods for humans, such as juice drinks, oral liquids and capsules. In addition, polysaccharides can also be used as various injections to treat diseases such as cancer and diabetes. The application prospect of fruit polysaccharides is very broad.
- Medicinal Plant的其它文章
- Application of Chaihu plus Longgu Muli Decoction in Treatment of Physical and Mental Diseases
- Study on Pharmacological Effects of Calycosin in Astragali Radix
- Advances in Research on Treatment of Heart Failure with Yangxinshi Tablet
- Advances in Chemical Constituents and Pharmacological Activity of Pholidota spp.
- Treatment of Arthralgia Syndrome from Zang and Fu
- Effects of Zingber mioga Aqueous Extract on Hepatic Anti-alcoholism in Mice

