Regulatory effects of moxibustion on ubiquitin and NLRP3 proteins in colon of ulcerative colitis rats
Li Xi-ying (李茜莹), Yang Guang (杨光), Wu Li-jie (吴丽洁), Hong Jue (洪珏), Zhao Yue (赵越), Liu Jie (刘婕),Kong Xie-he (孔谐和), Dong Xiao-qing (董小庆), Zhi Fang-yuan (智方圆), Ma Xiao-peng (马晓芃),, Yang Ling (杨玲),Zhang Dan (张丹)
1 Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
2 Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China
Abstract Objective: To observe the effects of moxibustion on colonic inflammation, and the expressions of ubiquitin and nucleotide-binding oligomerization domain (Nod)-like receptor protein 3 (NLRP3) proteins in rats with ulcerative colitis(UC), and to explore the anti-inflammatory mechanism of moxibustion in the UC treatment.Methods: Clean grade male Sprague-Dawley (SD) rats were randomly divided into a normal group (NG), a model group(MG), a moxa-stick moxibustion group (MSMG) and a Western medicine group (WMG). UC model was prepared by freely drinking 35 g/L dextran sulfate sodium (DSS) solution. Bilateral Tianshu (ST 25) were selected for mild moxibustion treatment in the MSMG; mesalazine solution was intragastrically administrated in the WMG. Rats in the NG and MG were only grasped and fixed as in the MSMG without any treatment. After treatment, hematoxylin-eosin(HE) staining was performed to observe and score the colonic pathological damage under light microscope;immunofluorescence method was used to determine the expression of colonic ubiquitin protein; immunohistochemical method was used to detect the expressions of colonic interleukin (IL)-1β and NLRP3 proteins.Results: The colon tissue was severely injured, and the pathological score was significantly increased in the MG than in the NG (P<0.01), and the protein expressions of ubiquitin, NLRP3 and IL-1β in the colon were significantly increased (all P<0.01). Compared with the MG, the colonic damage was repaired, the inflammation and pathological scores were reduced, and the ubiquitin, NLRP3 and IL-1β protein expressions were decreased in the MSMG and WMG (all P<0.01).Correlation analysis revealed that the ubiquitin protein expression was correlated with the colonic pathological score and the NLRP3 protein expression (r=0.677, P<0.01; r=0.536, P<0.05).Conclusion: Moxibustion can down-regulate the protein expressions of ubiquitin, NLRP3 and IL-1β in the colon of UC rats, which may be one of the mechanisms to promote the repair of colonic inflammatory lesions and exert anti-inflammatory effects.
Keywords: Moxibustion Therapy; Moxa Stick Moxibustion; Point, Tianshu (ST 25); Colitis, Ulcerative; Ubiquitin; NLRP3 Protein; Interleukin-1beta; Rats
Ulcerative colitis (UC) and Crohn's disease (CD) are two common types of inflammatory bowel diseases(IBD), with chronic colonic nonspecific inflammation as the basic pathological damage. UC is a common gastrointestinal disease, mainly manifested by abdominal pain, diarrhea and bloody stools. The lesions mainly affect the colonic mucosa and submucosa. The condition of this disease is complex, recurrent, and refractory. Some patients may present with extra-intestinal manifestations such as enteropathic arthritis, skin and eye lesions, and primary sclerosing cholangitis. The risk of colorectal cancer in UC patients is significantly increased[1], which seriously affects their quality of life and prognosis[2]. Therefore, it is of great clinical significance to prevent and cure UC early,promote clinical remission, and delay disease progression. Although the pathogenesis of UC remains unclear, colonic inflammation is the generally considered as the underlying pathogenesis. The secretion of multiple pro-inflammatory factors and epigenetic interactions including genetic and environmental factors may be involved in the formation of chronic intestinal inflammation[3-4]. As one of the important epigenetic modifications, ubiquitination plays an important role in the occurrence and development of IBD[3,5-8]. Studies have shown that the abnormal activation of nucleotide-binding oligomerization domain(Nod)-like receptor protein 3 (NLRP3) inflammasome inducing the release of downstream inflammatory factor interleukin (IL)-1β is one of the important mechanisms of colon barrier defense and inflammatory damage; and regulating the activity of NLRP3 inflammasome helps to suppress the inflammatory response and promote the immune homeostasis of the colon mucosa[9-10]. Ubiquitination modification has been found as an effective way of regulating NLRP3 protein.Studies have shown that promoting damaged mitochondrial ubiquitination degradation can inhibit NLRP3 protein activation or directly enhance the ubiquitination degradation of NLRP3 protein, or influence the NF-κB inhibitory protein (IκB)ubiquitination in NF-κB signaling pathway and indirectly regulate the transcription of the NLRP3 protein, thereby affecting the release of downstream inflammatory factors such as IL-1β and IL-18 to regulate the inflammatory response[11-13]. Results described above suggest that regulating NLRP3 protein ubiquitination may be an important mechanism to control the occurrence and development of colonic inflammatory response. Moxibustion can significantly improve the clinical symptoms, promote colonic damage repair and relieve the inflammation in UC[14-15]. Whether moxibustion exerts anti-inflammatory effects by affecting NLRP3 protein activity via regulating ubiquitination modification is worth further study.Therefore, this study intended to observe moxibustioninduced regulation of NLRP3 and ubiquitin proteins in the colon of UC model rats, and to explore the immune mechanism of its anti-inflammatory effect in the treatment of UC.
1 Materials and Methods
1.1 Experimental animals
Forty-three clean grade male Sprague-Dawley (SD)rats with body weight of (150±10) g were provided by Shanghai Slark Experimental Animal Co., Ltd. [SCXK(Shanghai) 2012-0002] and bred at the Laboratory Animal Center of Shanghai University of Traditional Chinese Medicine [constant temperature: (22±2) ℃,constant humidity: 64%, 12 h day and night alternation]with ventilation of 8-12 times/h. The rats had free access to food and water, and were adaptively fed for 3 d. The experiment started when the rats appeared healthy in terms of diet, activity, posture and back hair.All experimental operations strictly referred to the
Guiding Opinions on the Treatment of Experimental Animals issued by the Ministry of Science and Technology of the People's Republic of China. This experiment had been reviewed by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine (No. PZSHUTCM18111612).
1.2 Main reagents and instruments
1.2.1 Main reagents
Dextran sulfate sodium (DSS) (MDL number:MFCD00081551, MP Biomedicals, UK);paraformaldehyde (product number: 80096618,Sinopharm Chemical Reagent Co., Ltd., China);pentobarbital sodium (CAS number: 57-33-0, Sigma,USA); refined moxa stick (model: HY-JJAT-02-02,Nanyang Hanyi Moxa Factory, China); mesalazine enteric-coated tablets (China Food and Drug Administration approval number: H19980148,Sunflower Pharmaceutical Group Jiamusi Luling Pharmaceutical Co., Ltd., China); anti-NLRP3 rabbit polyclonal antibody (product number: ab214185),anti-IL-1β rabbit polyclonal antibody (product number:ab205924), anti-ubiquitin mouse polyclonal antibody(product number: ab205924) and Alexa Fluor 647 goat anti-mouse IgG H&L (product number: ab150115),(Abcam, UK); Dako REAL EnVision rabbit and mouse universal secondary antibody detection kit (product number: K5007, Dako, Denmark); hematoxylin-eosin(HE) staining kit (product number: D006-1-3, Nanjing Jiancheng Biological Co., Ltd., China); 4', 6-diamidino-2-phenylindole (DAPI) (product number: C1005,Biyuntian Biotechnology Co., Ltd., China).
1.2.2 Main instruments
ASP300S tissue dehydrator, EG1160 tissue embedder,RM2235 paraffin slicer and HI1210 baking sheet machine (Leica Company, Germany); BX53 optical microscope and image analysis system (Olympus Company, Japan); PL203 electronic balance (METTLER TOLEDO Company, Switzerland).
1.3 Model preparation
Eleven of the 43 SD rats were randomly selected as the normal group (NG), and the remaining 32 rats freely drank35g/LDSSsolution (molecularweight: 36000-50000Da) for7 dto establish the UC model[16].One rat from the NG and 2 rats from the MG were randomly selected after the model was established, and the pathological changes of the colon tissues by HE staining were observed under microscope to identify whether the model was successful. After successful modeling,the rats were further randomly divided into a model group (MG), a moxa-stick moxibustion group (MSMG)and a Western medicine group (WMG), with 10 rats in each group.
1.4 Treatment by groups
moxibustiontreatmentintheMSMG[17].The moxa sticks (4 mm in diameter and 12 cm in length) especially for animal experiments were ignited and suspended 2-3 cm above the acupoints for 10 min each time, once a day for 8 consecutive days.
BilateralTianshu(ST25) wereselected formild
Mesalazine solution was intragastrically administrated in the WMG. The daily dosage was based on the ratio of adult human (70 kg body weight) to rat(200 g body weight) 1:0.018[18], once a day for 8 consecutive days.
Rats in the NG and MG did not undergo any treatment, but were only grasped and fixed same as in the MSMG.
1.5 Sample collection and processing
During the treatment, 1 rat in the MSMG died and severe colon injury was found by dissection. After the treatment, rats in each group were fasted while having free access to water for 24 h. Rats were anesthetized by intraperitoneal injection of 1% sodium pentobarbital at 30 mg/(kg·bw). A piece of colon tissue of 5-8 cm was separated upward from the anus, and rinsed in cold saline at 4 ℃. One third of the colon tissue was collected and fixed in 4% paraformaldehyde solution for 24 h.
1.6 Observation items and methods
1.6.1 Colon histopathology observation
With HE staining, the morphological changes of colon tissues in all groups were observed under light microscope. Conventional dewaxing and hydration: the tissue slices were dewaxed in xylene Ⅰ and Ⅱ, and incubated in 100%, 90%, 80% and 70% gradient ethanol for hydration in order; stained with hematoxylin,washed with running water, and differentiated by 1%hydrochloric acid ethanol for 3 s, and then washed with running water; stained with eosin, then incubated in gradient ethanol 70%, 80%, 90% and 100%, and then in xylene Ⅰ and Ⅱ for transparency. Finally, the slides were examined and scored under microscope after being sealed with neutral gum[19].
1.6.2 Detection of colonic protein expressions of NLRP3 and IL-1β
Immunohistochemical method (Envision) was used to detect the protein expressions of NLRP3 and IL-1β as follows: paraffin sections of rat colon tissues were dewaxed, hydrated, antigen repaired, and blocked;primary antibodies (NLRP3 1:300; IL-1β 1:50) were added dropwise and incubated overnight at 4 ℃ in refrigerator; rinsed with phosphate buffer saline (PBS)and dropwise added secondary antibody (1:200) and incubated at room temperature for 1 h; stained the nuclei with hematoxylin for 1 min, differentiated,dehydrated, cleared and mounted. Cells with tan granules in the cytoplasm were positive ones. Five fields of view were randomly selected from each slice to be photographed at a fixed light intensity, and analyzed with Image-Plus Pro 6.0 software to calculate the integral optical density (IOD) of the positive target of each photo, the average value was used as the IOD value of the slice.
1.6.3 Detection of colonic ubiquitin protein expression
The ubiquitin protein expression was detected by immunofluorescence method as follows: paraffin sections of rat colon tissues were dewaxed, hydrated,antigen repaired, and blocked. Primary antibody(ubiquitin 1:500) was added dropwise and incubated overnight at 4 ℃ in refrigerator; the next day, the sections were placed in a 37 ℃ constant temperature oven for 30 min and rinsed with PBS. The following steps were performed in order in dark: added secondary antibody (1:200) and incubated at 37 ℃ for 45 min; counter-stained the nuclei with DAPI staining solution for 10 min; rinsed with PBS; covered. The excitation light of the corresponding wavelength for excitation was selected at a fixed field of view, then photographed and merged. The results showed a blue DAPI-stained nuclei and red ubiquitin protein expression. Five fields of view were randomly selected from each slice. Semi-quantitative analysis was performed by Image J software to calculate the IOD of the positive target and the average value was calculated.
1.7 Statistical methods
All experimental data were analyzed using SPSS version 25.0 statistical software. First, the normal distribution test was performed on the measurement data, and the measurement data for normal distribution were presented as mean ± standard deviation (x ±s);the measurement data for skewed distribution were presented as median (interquartile range). Second,one-way ANOVA was used for the comparison between groups, and least significant difference (LSD) was used for the pairwise comparison among the homogeneity and normal distribution data. Games-Howell test was used for data with heterogeneity of variance.Non-parametric test was used for skewed distribution data. Pearson test was used for correlation analysis. The size of test was α=0.05. The difference was statistically significant with P<0.05.
2 Results
2.1 Histopathological observation of colon
NG: intact colonic mucosal epithelium and regularly arranged glands without obvious congestion and inflammatory cell infiltration. MG: severe necrosis of the colonic mucosa and submucosa, as well as discontinuous epithelial distribution with a large number of inflammatory cell infiltration, hyperemia and some lost glands. MSMG: intact colonic mucosal epithelium, obvious hyperplasia and regularly arranged glands, as well as few hyperemia and infiltration of inflammatory cells in the mucosa and submucosa.WMG: basically complete colonic mucosal epithelium with inflammatory cells infiltrating the mucosa and submucosa (Figure 1).
Compared with the NG, the histopathological score of colon in the MG was significantly increased (P<0.01).Compared with the MG, the histopathological scores of colon in the MSMG and the WMG were reduced (both P<0.01). Compared with the WMG, the histopathological score of colon in the MSMG was significantly reduced (P<0.01), (Figure 2).

Figure 1. HE staining of rat colon tissue in each group (×200)
2.2 Expression of IL-1β protein in colon tissues
The IL-1β protein was mainly expressed in the cytoplasm, and the positive cells were mostly intestinal mucosal epithelial cells, submucosal and lamina propria lymphocytes (round, smaller size, high ratio of nuclear and plasma), monocytes/macrophages (ellipse or irregular, huge size, relatively low ratio of nuclear and plasma), (Figure 3).
Compared with the NG, the expression of IL-1β protein in the rat colon of the MG was significantly increased (P<0.01). Compared with the MG, the expressions of IL-1β protein in rat colons of the MSMG and WMG were significantly reduced (both P<0.01).Compared with the MSMG, there was no significant difference in the IL-1β protein expression of the rat colon in the WMG (P>0.05), (Figure 4).

Figure 3. Immunohistochemical staining of IL-1β protein of rat colon tissue in each group (×200)
2.3 Expression of NLRP3 protein in rat colon tissues in each group
NLRP3 protein was mainly expressed in the cytoplasm, and the positive cells were mostly intestinal mucosal epithelial cells, submucosal and lamina propria lymphocytes, and monocytes/macrophages (Figure 5).
Compared with the NG, the expression of NLRP3 protein in the rat colon of the MG was increased, and the difference was significant (P<0.01). Compared with the MG, the expressions of NLRP3 protein in rat colons of the MSMG and WMG were significantly decreased(both P<0.01). Compared with the MSMG, the expression of NLRP3 protein in rat colon of the WMG was not significant (P>0.05), (Figure 6).

Figure 2. Comparison of pathological score of rat colon tissues among groups
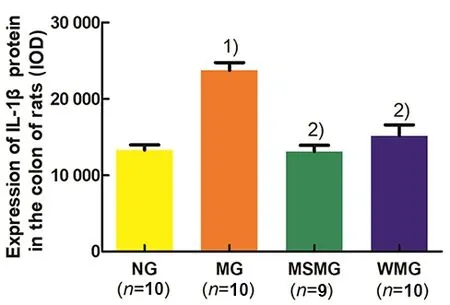
Figure 4. Comparison of the IL-1β protein expression in rat colon tissues among groups

Figure 5. Immunohistochemical staining of NLRP3 protein in the rat colon of each group (×200)
2.4 Ubiquitin protein expression in rat colon of each group
The ubiquitin protein expression was mainly concentrated in the intestinal lamina propria, and weakly positive in the mucosal epithelium (Figure 7).
Compared with the NG, the ubiquitin protein expression in the rat colon tissue of the MG was significantly up-regulated (P<0.01). Compared with the MG, the expressions of ubiquitin protein in rat colon tissues of the MSMG and WMG were significantly down-regulated (both P<0.01). Compared with the MSMG, the expression of ubiquitin protein in the rat colon of the WMG was not significantly different(P>0.05), (Figure 8).
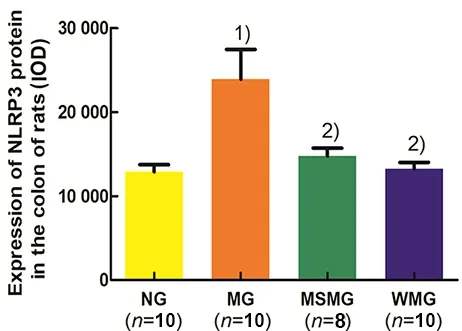
Figure 6. Comparison of the NLRP3 protein expression in rat colon among groups
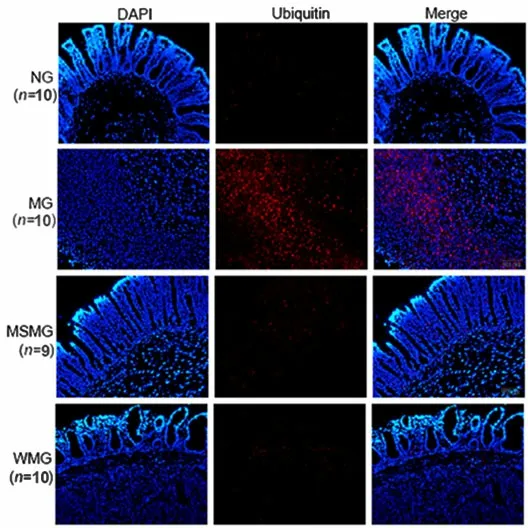
Figure 7. Immunofluorescence staining of ubiquitin protein in rat colon tissue of each group (×200)

Figure 8. Comparison of the ubiquitin protein expression in rat colon among groups
2.5 Correlation analysis between the ubiquitin protein expression with the pathological score and the NLRP3 protein expression in colon
In general, the relevance between variables was determined by the following value ranges: correlation coefficient r≥0.8, but <1.0 was highly correlated; r≥0.6,but <0.8 was strongly correlated; r≥0.4, but <0.6 was moderately correlated; r≥0.2, but <0.4 was weakly correlated; r<0.2 was very weakly correlated or uncorrelated[20]. Correlation analysis in this study showed that the colonic pathological score of rats was strongly and positively correlated with the ubiquitin protein expression (r=0.677, P<0.01); the expressions of colonic ubiquitin protein and NLRP3 protein were moderately and positively correlated (r=0.536, P<0.05),(Table 1).
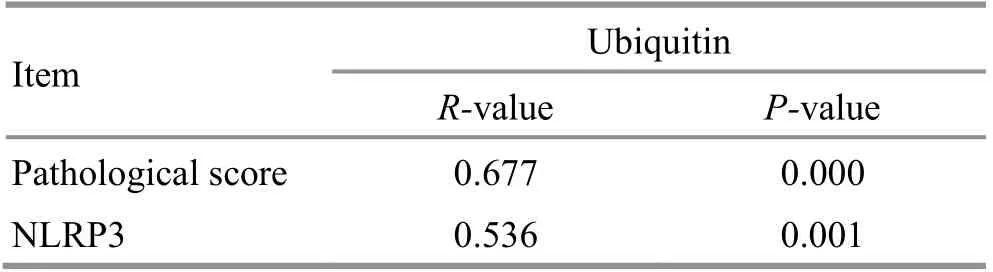
Table 1. Correlation analysis of the colonic ubiquitin protein expression with the colonic pathological score and the NLRP3 protein expression
3 Discussion
Ulcers and inflammation are the basic lesions of UC.Anti-inflammation and promoting mucosal healing are the fundamental principles in clinical treatment of UC[21]. In recent years, studies have confirmed the clinical efficacy of moxibustion for mild-to-moderate UC[14-15], which is worth to pay attention and recommend for clinical application. However, the mechanism in moxibustion treatment of UC is not so clear, which limits its application and promotion.
NLRP3 protein belongs to the Nod-like receptors(NLRs) family, which is assembled to form a largemolecule multi-protein complex with an apoptosisassociated speck-like protein (ASC, harboring a CARD in the adaptor molecule) and pro-Caspase-1. It is called the NLRP3 inflammasome and can be activated by a variety of pathogens or damage-related molecular patterns, thereby regulating the function of innate immune system[22]. NLRP3 inflammasome plays an important role in identifying intestinal commensal bacteria, maintaining a stable intestinal environment,and regulating intestinal inflammatory responses.Studies have shown that two SNPs, rs10754558 and rs10925019, on the NLRP3 gene in the Chinese Han population were found to be associated with the pathogenesis of UC, suggesting that the NLRP3 gene may play an important role in UC susceptibility in China[23]. In recent years, many literatures have reported that the abnormal activation of NLRP3 inflammasome is closely related to the pathogenesis of UC[24]. The NLRP3 inflammasome plays a major inflammatory role in the occurrence of colitis. Various pathogenic factors act on the intestinal mucosa of UC patients to cause abnormal activation of NLRP3 inflammasome and promote the expressions of inflammatory factors such as IL-1β, IL-18, thus to cause the local intestinal mucosal cell damage; inhibition of the NLRP3 protein activity helps alleviate the inflammatory response of the colon[25-27]. Our current study showed that compared with the MG, the NLRP3 and IL-1β expressions of rat colon in the MSMG were significantly reduced, which was consistent with the colon injury and pathological scores, suggesting that moxibustion at Tianshu (ST 25) may reduce the IL-1β protein expression and the inflammatory response by down-regulating NLRP3 protein activation in the colon of UC rats, thus to promote the repair of intestinal tissues.
Ubiquitination modification is one of the posttranscriptional modifications in proteins. It regulates many signal-mediated inflammatory responses and is closely related to inflammatory diseases such as IBD[9-10]. Ubiquitination modification is the process of ubiquitin proteins covalently bound to the target proteins under the catalysis of a series of enzymes, with the main function of degradation and clearance[28-30].Ubiquitin protein is an important signal molecule of the ubiquitination modification pathway indicating the degradation of target protein[31]. Therefore, the ubiquitin protein expression is closely related to ubiquitination modification and target protein expression. Studies have shown that compared with the NG, ubiquitin was significantly increased in the colon tissue of rats with colitis induced by trinitrobenzenesulfonic acid (2,4,6-trinitrobenzenesulfonic acid solution, TNBS), and associated with inflammation[32].This study found that the ubiquitin protein expression in colon tissues of UC rats induced by DSS was also significantly up-regulated, and the ubiquitin protein expression was strongly correlated with the colonic pathological injury score. Moxibustion treatment significantly repaired the colon injury, reduced the inflammation response and the abnormally increased colonic protein expressions of ubiquitin and NLRP3 with moderate correlation. It is suggested that ubiquitination modification may be involved in moxibustion-regulated NLRP3 protein expression in UC colon, thus to promote colonic inflammatory injury repair by down-regulating the expression of colonic NLRP3 to reduce IL-1β protein.The results provided some scientific basis for unveiling the mechanism of moxibustion treatment of UC.Nonetheless, how to exert the anti-inflammatory effect by regulating the NLRP3 protein expression through ubiquitination modification needs further study.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by Natural Science Foundation of Shanghai ( 上海市自然科学基金项目, No.19ZR1451600); the Budgeted Scientific Research Project of Shanghai University of Traditional Chinese Medicine(上海中医药大学预算内项目, No. 18LK050); National Natural Science Foundation of China (国家自然科学基金项目, No. 81674073, No. 81202754); Excellent Academic Leader Training Program Project of Shanghai Health and Family Planning System (上海市卫生计生系统优秀学科带头人培养计划项目, No. 2017BR047).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria.
Received: 30 July 2019/Accepted: 2 September 2019
 Journal of Acupuncture and Tuina Science2020年2期
Journal of Acupuncture and Tuina Science2020年2期
- Journal of Acupuncture and Tuina Science的其它文章
- Effects of electroacupuncture on the behaviors and expressions of hippocampal neurotransmitters and Bax/Bcl-2 proteins in rat models of anxiety disorder
- Effect of An-pressing manipulation on post-stroke muscle spasticity in rats and its mechanism study
- Clinical study on intradermal needle therapy in treating urinary retention after cervical cancer surgery
- Clinical observation of deep electroacupuncture at Baliao points for female stress urinary incontinence
- Clinical observation on prevention of chemotherapy infection in gastric cancer by moxa-stick moxibustion plus rhG-CSF and its effect on immune function
- Clinical observation of acupuncture plus repetitive transcranial magnetic stimulation in the treatment of post-stroke insomnia
