Effects of CO2-water interaction with coal on mineral content and pore characteristics
Qiuho Du ,Xioli Liu ,*,Enzhi Wng ,Jinping Zuo ,Weimin Wng ,Yujie Zhu
a State Key Laboratory of Hydro-Science and Engineering,Tsinghua University,Beijing,100084,China
b State Key Laboratory of Coal Resources and Safe Mining,China University of Mining and Technology,Beijing,100083,China
Keywords:Carbon dioxide(CO2)sequestration Abandoned coalmines Mineral content Pore structure
A B S T R A C T There are a large number of abandoned coalmines in China,and most of them are located around major coal-fired power stations,which are the largest emission sources of carbon dioxide(CO2).Considering the injection of CO2 into abandoned coalmines,which are usually in the flooded condition,it is necessary to investigate the effect of CO2-water-coal interaction on minerals and pore structures at different pressures,temperatures and times.It reveals that the CO2-water-coal interaction can significantly improve the solubility of Ca,S,Mg,K,Si,Al,Fe and Na.By comparing the mineral content and pore structure before and after CO2-water-coal interaction,quartz and kaolinite were found to be the main secondary minerals,which increased in all samples.The structures of micropores and mesopores in the range of 1.5-8 nm were changed obviously.Specific surface areas and pore volumes first increased and then decreased with pressure and time,while both increased with temperature.By using the Frenkel-Halsey-Hill model,the fractal dimensions of all samples were analyzed based on Ds1 and Ds2,which reflected the complexities of the pore surface and pore volume,respectively.The results show that fractal dimensions had very weak positive correlations with the carbon content.Ds1 had a positive correlation with the quartz and kaolinite contents,while Ds2 had a negative correlation with the quartz and kaolinite contents.
1. Introduction
Carbon dioxide(CO2)capture and storage is considered as a potential and feasible method to mitigate climate change by reducing greenhouse gas concentrations in the atmosphere.Depleted oil and gas reservoirs,deep saline aquifers,deep unmined coal seams,other geological formations,or the ocean can provide huge storage spaces for geological storage of CO2(White et al.,2005).As an important part of the national power grid in China,coal-fired thermal power plants are the main source of CO2emissions,and there are a large number of closed/abandoned coalmines located around major power stations.It is estimated that by 2020,the number of closed/abandoned mines in China will reach 12,000,and by 2030,that number will reach 15,000.Due to technical and economic issues, currently abandoned mines in China have coal resources up to 42 billion tons.Unfortunately,the coal resources and underground spaces of these abandoned mines have not been effectively utilized,but they are the potential targets for CO2storage with depths over 800 m.Below the 800 m depth,the formation pressure and temperature would cause CO2to reach a supercritical state.
During the process of coal mining,the overlying strata will destress,which results in the collapse of immediate roof,followed by the subsidence of the basic roof.Goafs,collapsed areas,remaining shafts and major roadways have formed large spaces for CO2storage in abandoned coalmines (Li et al., 2018). Abandoned coalmines have also been used for storage of natural gas(Tauziede et al., 2001, 2002), and CO2sequestration in deep reservoirs(Piessens and Dusar,2004;Jalili et al.,2011;Ray and Dey,2018).After abandonment,since the pumping and drainage systems stop running,coalmines begin to accumulate water into the goafs,main shafts,and drifts from surrounding coal and rock strata.As such,it is possible for CO2to escape from channels formed by coal excavation around storage location(Li et al.,2014).Therefore,abandoned coalmines have complex stress, hydrodynamic, and chemical fields.The permeability of the coal pillar determines whether CO2will leak horizontally into the surrounding area.Abandoned coal resources and empty spaces could have naturally stored methane(CH4)(Karacan,2015),which can be displaced by CO2injection since the Lennard-Jones potential function of CO2is greater than that of CH4(Sun and Duan,2005).The possibility of using abandoned mine methane as an energy source may partially offset the CO2geological sequestration expenses.
Collapsed rock from the overlying strata and the remaining coal resources(such as pillars)coexist in the goaf.In general,the minerals in coal generally include quartz,clay minerals(e.g.kaolinite,illite and interstratified illite/smectite),feldspars,carbonates(e.g.siderite,calcite and dolomite),and sulfide minerals(e.g.pyrite)(Ward,2002).The pores and fractures are generally filled with minerals,which reduce the internal surface area of the coal by mineralizing pores and fractures(Flores,2014;Ramandi et al.,2016).CO2injected into an abandoned coalmine will dissolve in formation water,and carbonic acid will be formed if there is high partial pressure of CO2.This will cause CO2-water-coal(rock)interactions until the acid solution is neutralized.This may not only affect water quality and mineralogy,but also result in acid leaching of primary minerals and organically bound metal ions within the coal as well as the adjacent host rock.Numerical results showed that significant increases in porosity occurred in the acidified zones due to mineral dissolution(Li et al.,2011).In addition,it would change the pore shape and fracture apertures,which would also affect the permeability of coal subsequently(Beni et al.,2012;Farquhar et al.,2015;Luquot et al.,2016;Pearce et al.,2016;Saldaña et al.,2016).It has been accepted that the spaces of micropores,macropores as well as fractures could be increased after treatment of supercritical CO2(Gathitu et al.,2009;Zhang et al.,2016a;Meng and Qiu,2018).
The solubility of CO2in a fluid,as described by Henry’s law,typically decreases with temperature and ionic strength,but increases with pressure(Charlet et al.,2017).Some studies(e.g.Wang et al., 2016a, b) showed that CO2-brine-rock interaction can significantly mobilize major elements from the dissolution of carbonate and silicate minerals,distinctly changing the mineralogical compositions.Several elements,including Al,Ca,Fe and Mg,have been found to be more mobile during CO2-water-coal interaction experiments in comparison to that of water-only experiments from the same samples under 9.5 MPa CO2partial pressure and 40°C for 72 h(Dawson et al.,2015).Acid dissolution of reactive minerals such as calcite may improve the permeability and enhance the coal pore volume after CO2injection(Massarotto et al.,2010;Dawson et al.,2011).An increase in pore volume may increase the CO2sorption capacity of the coal,which potentially can increase coal swelling and decrease permeability(Mazumder and Wolf,2008),because volumetric swelling has both a linear and nonlinear relationship with sorption under all pressure ranges(Mukherjee and Misra,2018).In addition,the reaction of CO2-H2O with calcite causes considerable porosity changes through calcite dissolution,and the mobilization of kaolinite may block pore throats and decrease permeability(Xu and Pruess,2010;Beni et al.,2012;Pearce et al., 2016). The supercritical CO2-H2O-coal reaction mainly led to increased micropores and either damaged or created ink-bottle-shaped pores and affected interactions within the pores(Liu et al.,2010;Sun et al.,2016).Interactions within pores in a CO2-H2O-coal system have indicated desorption of water molecules from the surfaces of coal micropores and mesopores.Some studies of the effect of water-CO2treatment or gas sorption revealed that sorption/desorption-induced swelling/shrinkage dramatically alters the porosity, excess adsorption, and microstructure of the coal,which distinctly changes the gas-or water-relative permeability(Massarotto et al.,2010;Jing et al.,2016;Zhang et al.,2016b,c;Ibrahim and Nasr-El-Din,2017;Ni et al.,2017).In other words,previous studies have mainly focused on element migration in CO2-water-coal interaction and CO2-coal interaction-induced microstructure change.However,little attention has been paid to the influence of the CO2-water-coal interaction on mineral inorganic composition and microscopic pore properties.In addition,the CO2excess sorption of wet coal changes from 8 MPa to 10 MPa of CO2partial pressure(Weniger et al.,2012;Mukherjee and Misra,2018).The amount of adsorption increases and the expansion does not change over 8-10 MPa of CO2pressure.It is necessary to study the effects of CO2-water-coal interaction on pore properties and minerals when the CO2partial pressure is less than or equal to 10 MPa.
Due to CO2sorption, diffusion and dissipation, the partial pressure of CO2is not constant after injection,and the ambient temperature of the injection zone may also change.However,it is not clear how the CO2-water-coal interaction changes the micropore and mineral content under different temperature and pressure conditions.Therefore,it is meaningful to measure the mineral content and pore structure under different experimental conditions of the CO2-water-coal system.In this paper,the effects of the CO2-water interaction with coal on its pore structure changes and mineral content are studied with respect to the partial pressure of CO2,temperature and time.These results may provide reference information for CO2sequestration in abandoned coalmines.
2. Materials and methods
2.1. Materials and apparatus
Coal samples were collected from the#2 coal seam in the Xiaojihan coalmine in Yulin,Shaanxi Province,China.The#2 coal seam is located in the Ordos Basin and is of low-rank bituminous,containing gangue minerals.The coal was packed in bags and sealed immediately to prevent contamination and oxidation.The petrographic parameters of the coal samples are shown in Table 1,in which wt% and% represent the mass fraction and volume fraction,respectively.
Fig.1 schematically shows the experimental apparatus.The reaction kettle was built to operate at temperatures ranging from 0°C to 300°C.The maximum pressure of the reaction kettle is 16 MPa,and the volume is 500 mL.The carbon dioxide employed had a purity of 99.995% .An air compressor and a booster pump were linked with a carbon dioxide cylinder and batch reactor so that the pressure of the CO2could be increased to a preset value.
2.2. Experimental scheme
In order to compare the effects of pressure,temperature and time on the CO2-water-coal interaction,the experimental scheme is shown in Table 2,where sample N0 represents the original coal sample,and DH2O represents the deionized water.Samples N0-N12 are with the same quality treated at different conditions of pressure,temperature and time.
2.3. Experimental procedure
The experimental procedure is described as follows:

Table 1 Petrographic parameters of the coal samples.

Fig.1.Schematic diagram of the experiment system.
(1)To measure the pore structure of the coal samples,powdered samples with a microparticle size of 75-80 μm(180-200 mesh)were prepared for testing by using nitrogen adsorption isotherms at 77.35 K(-195.8°C).In order to prevent high temperature coking from affecting the test results,the degassing temperature of nitrogen adsorption was 80°C.
(2)X-ray diffraction(XRD)was used to obtain the material components of the coal samples before and after the reaction.As these samples were derived from the same coal block and were then ground and mixed completely,no repeated experiments were needed.The XRD was used on powdered samples over a measuring angle(2θ)of 3°-70°at routine scan steps.Coal powder samples were dried at 80°C for 48 h in an oven before testing.The carbon content of samples was detected with an X-ray fluorescence(XRF)spectrometer.
(3)A mixture of coal powder and deionized water with a coal/water ratio of 1:20 was poured into the reactor(Wang et al.,2016b).Deionized water was employed to simplify the experiments,and the reactor was washed with deionized water three times after each reaction.The CO2gas was injected first at a pressure of 1 MPa to evacuate the chamber.After each experiment was completed,the reactor was opened after the reaction kettle naturally cooled down to the ambient temperature.Then,25 mL of reaction solution(10.5% nitric acid)was poured into a reagent bottle to form an acidizing environment and prevent chemical precipitation.Finally,the fluid chemistry was analyzed using an inductively coupled plasma optical emission spectrometer(ICP-OES).
3. Results and discussion
3.1. Changes in ion concentration of the reaction solution
Since the initial reaction solution used in this experiment was deionized water,the concentration of the conventional metal ions was regarded as zero.After the CO2-water-coal interaction was completed,the reaction solution was placed in an acidic environment and immediately sealed for inspection.The ion concentrations of Ca2+,Fe2+,S2-,Si4+,Mg2+,Al3+,K+and Na+were then measured.The results are shown in Table 3.
Comparing the conditions of samples N1-N5,it is clear that samples N1-N3 are in a subcritical state and samples N4-N5 are in a supercritical state.As shown in Fig.2,with rising CO2pressure,the ion concentrations of Ca2+,S2-,Mg2+,K+and Si4+gradually increase.The ion concentrations of Ca2+and S2-change most obviously, and acid treatment preferentially removes Ca2+(Ozdemir,2016).In addition,the ion concentrations of Al3+,Fe2+and Na+also increase at different pressures,but the changes are irregular.Comparing the ion concentrations of the detected elements,it was found that the ion concentration after supercritical CO2-water-coal interaction was higher than that after subcritical CO2-water-coal interaction.This is mainly because the solubility of CO2in water increases with pressure,resulting in a lower pH value and a more severe chemical reaction.
Fig.3 shows that the ion concentration increases as the temperature rises,indicating that chemical reactions were more severe at high temperatures under supercritical CO2-water-coal interaction.Temperature is an important factor affecting chemical reactions.With increasing temperature,the dissolved amount of CO2decreases,which causes the pH value to gradually increase.However,chemical reaction rates increase with elevated temperature,leading to a rapid dissolution of minerals.
Fig.4 shows the relationship between the ion concentration and reaction time.As the reaction time increases,ion concentrations of the detection elements nearly rise,except for Fe2+and Na+in N11 and N12.As the reaction time increases,the concentration of HCO-3begins to decline,the pH value begins to rise,and the change in the acidic environment results in a decrease in the Fe2+concentration(Wang et al.,2016b).This may also result in a decrease in Na+concentration.Changes in theion concentrationwith time illustratethat the mineral chemical reaction is gradually enhanced over time.
3.2. Changes in mineral content
Fig.5 compares the XRD diffraction patterns for the unreacted and reacted coal powder in different test conditions.It can be seen that quartz(SiO2),calcite(CaCO3),kaolinite(Al2Si2O5(OH)4),albite(NaAlSi3O8)and potassium feldspar(KAlSi3O8)are the main minerals in the raw coal sample.After the CO2-water-coal interaction,the mineral content of the coal displayed a significant change.The peak intensity of calcite changes obviously,while that of quartz remains unchanged,which is mainly dependent on its strong chemical stability.Besides,albite and potassium feldspar have small changes in peak intensity.The dissolution of calcite,potassium feldspar and albite can be described by the following chemical reactions:
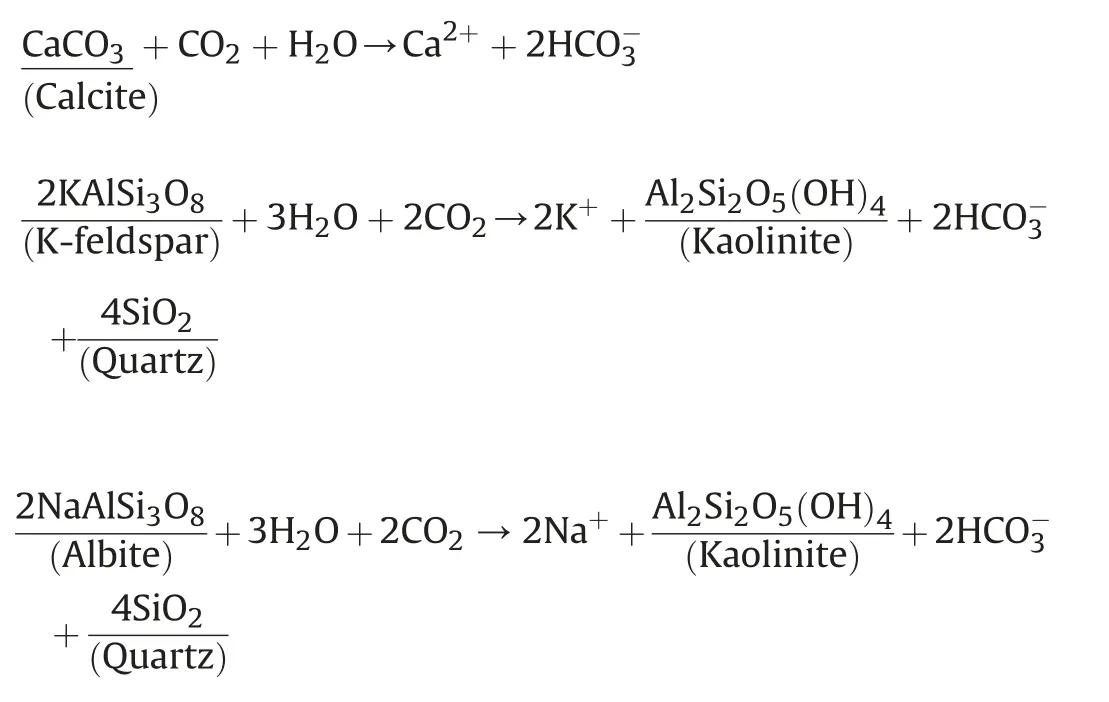
Table 4 shows that the quartz and kaolinite contents increase significantly due to the geochemical reaction of K-feldspar and albite.In the subcritical CO2-water-coal interaction,the content of quartz increases with pressure.Compared with the lower partial pressure of CO2,the relative content of calcite at 10 MPa of CO2partial pressure is increased,due to the fact that the precipitation of calcite is related to the partial pressure of CO2(Przybylinski,1987).The increase in partial pressure is not conducive to precipitation of calcite,and the pressure decreases the precipitation rate of calcite.Ca2+carbonation efficiency is directly affected by the CO2pressure,and it is found that Ca2+carbonation efficiency is in direct relationship with CO2pressure(Azdarpour et al.,2015).Besides,Table 3 shows that the Fe2+concentration of sample N5 is lower than that of N4,while S2-concentration of N5 is higher than that of N4.This means that Fe2+reacted in the reactor.When the Fe2+concentration is much lower than the Ca2+concentration,Fe2+could easily react with CO2to form precipitation(Azdarpour et al.,2015).After the test was stopped and depressurized,it showed that pressure reduction accelerated precipitation of calcite.This may be caused by higher calcite content of N5 than that of N4 at supercritical CO2-water interaction.
The increase in the content of calcite results in a decrease in the incremental contents of quartz and kaolinite.For calcite dissolved layer-by-layer,the next layer would have begun to dissolve after the complete dissolution of the top layer(Na et al.,2015).With rising temperature,the calcite content decreases,and the quartz and kaolinite contents increase.As the reaction time increases,the calcite,K-feldspar and albite contents decrease gradually,and the quartz and kaolinite contents increase gradually.
The secondary minerals formed by the supercritical CO2-water-coal interaction were not determined.For the primary minerals,the quartz content increased,and the kaolinite,carbonate minerals and pyrite decreased(Jiang and Yu,2019).Combined with the chemical reaction of the CO2-water-minerals,the quartz and kaolinite contents should increase. However, the structure of kaolinite may be destroyed by fluids and cannot be detected.Balucan et al.(2016)considered whether the newly formed fluoridated precipitates have sufficient capacity to protect the newly generated seepage pathways,because the neofluoride minerals have a larger size and higher mechanical strength than the primary minerals do.In this experiment,quartz and kaolinite are mainly the secondary minerals formed after the CO2-water-coal interaction.Quartz can be used as the high-strength mineral to support newly opened cracks.The formation of the secondary kaolinite,however,results in neither greater resistance to stress compaction nor better maintenance of permeability channels,because clay minerals have weaker mechanical strength than quartz and calcite do.Theoretical analysis can predict formation of carbonate.In addition,previous studies(e.g.Gysi and Stefánsson,2008;Dawson et al.,2011;Wang et al.,2016b)numerically and experimentally reported that carbonate minerals could be generated.By comparing the test conditions,it is revealed that minerals mainly undergo dissolution in a relatively short reaction time period,and the water/coal ratio used in this experiment is relatively larger to speed up the reaction rate in the experiment.This adversely affects the formation of carbonate secondary minerals owing to the Ca2+,Mg2+and Fe2+ion concentrations becoming relatively smaller.
3.3. Changes in pore structure and surface area
3.3.1. Adsorption isotherms and surface area
The low-temperature liquid nitrogen adsorption and desorption isotherms are shown in Fig.6.The variation trend of the adsorption curves is very similar at low relative pressure(P/P0<0.1)and middle pressure(P/P0=0.1-0.9),where P and P0are the equilibrium pressure and saturated vapor pressure, respectively. By contrast,the adsorption curves clearly change at high pressure(P/P0=0.9-1).According to the six types of physical adsorptionisotherms proposed by International Union of Pure and Applied Chemistry(IUPAC)(Polanyi,1963),by combining the experimental adsorption-desorption isotherm,the adsorption isotherm is close to the horizontal axis at low relative pressure.The weak adsorption force between N2and coal results in lower adsorption capacity at low pressures.In contrast,the isotherm dramatically increases at high relative pressure.It can therefore be conclusive that the adsorption isotherm belongs to type-III isotherm,multi-molecular layer adsorption occurs,and the pore types are of micropore(<2 nm)and mesopore(2-50 nm).The adsorption-desorption isotherm rises slowly during the middle pressure stage.The hysteresis loop reflects the fact that the isotherm is an H4type,according to the classification criteria of IUPAC.The CO2-water-coal interaction does not noticeably change the coal isotherm type.Fig.6 shows the relationship between the incremental surface area and pore diameter. The specific surface area increment ds is calculated by Eq.(1).The curves indicate that the CO2-water treatment in different conditions mainly changes the structure of the micropores and mesopores,especially for pores in the range of 1.5-8 nm.
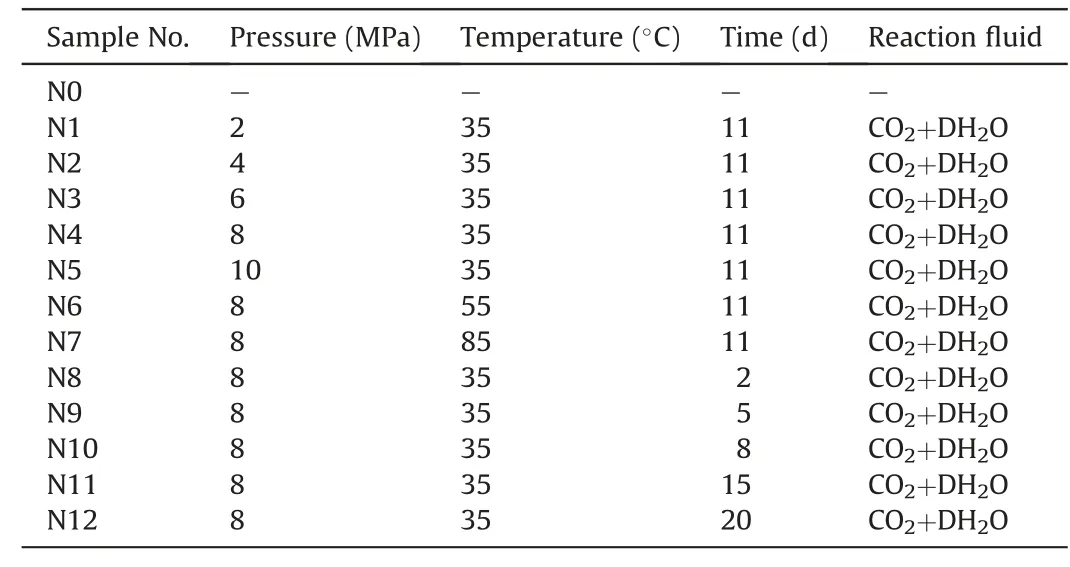
Table 2 Experimental scheme.

Table 3 Ion concentration of main elements in the reaction solution.

Fig.2.Variation of ion concentration with pressure at 35°C.As the reaction pressure increases,the ion concentration generally appears to increase gradually.

where Siand Si+1are the cumulative surface areas at the pressure point(Pi)and a higher pressure point(Pi+1),respectively;and diand di+1denote the pore diameters where the cumulative surface areas are Siand Si+1,respectively.
3.3.2. Pore characteristics of a coal sample
The specific surface and pore size analysis is conducted using the equipment Autosorb SI,Quantachrome.The Barrett-Joiner-Halenda(BJH)model is the widely used method for pore size distribution(Wang et al.,2015).According to the BJH model and de Boer thickness equation,the specific surface area,pore volume and pore diameter of all samples calculated from the adsorption isotherm are tabulated in Table 5.
Fig.7 shows that during a subcritical CO2-water-coal interaction,the specific surface area and pore volume gradually increase with elevated pressure; however, when transitioning from subcritical to supercritical state,the specific surface area and pore volume decrease as the pressure increases.According to the ion concentrations of Ca2+,S2-and Fe2+in solution,the chemical reaction was violent during the supercritical CO2-water-coal interaction,but the specific surface area and pore volume decreased at a supercritical state.The specific surface area and pore volume are quadratic functions of pressure.
It is assumed that the coal sample has remarkable adsorption swelling during chemical reaction in a supercritical CO2-watercoal interaction. The experimental study confirmed that the wettability of the mineral surface altered towards less water-wet conditions from subcritical to supercritical CO2conditions(Espinoza and Santamarina,2010;Saraji et al.,2012).The contact angles also decreased with increasing pressure,indicating that the coal became more CO2-wet,and the relationship between the contact angle and pressure was approximately linear(Sakurovs and Lavrencic,2011).As the contact angle decreased,CO2was better able to penetrate into the coal quickly.Changes in wettability may result in coal matrix swelling,because the CO2-water-coal interaction at higher pressures can release more pore volume due to mineral dissolution,and the released pore space can be occupied by CO2adsorption.The change also has a relationship with the coal rank,mineral type and mineral content,pore size,water content,temperature and pressure.
As shown in Fig.8,it is assumed that the N0 data were measured at a room temperature of 25°C.It was found that the temperature had a significantly positive effect on the specific surface area and pore volume of the coal sample,which increased exponentially with increasing temperature.

Fig.3.Variation of ion concentration with temperature.For samples N4,N6 and N7,their reaction pressure is 8 MPa,and temperatures are 35 °C,55 °C and 85 °C,respectively.

Fig.4.Variation of ion concentration with reaction time.For samples N4,and N8-N12,their reaction pressure and temperature are 8 MPa and 35°C,respectively.As the reaction time increases,the ion concentration gradually increases.The changes of Ca2+and S2-are more obvious.
Gas adsorption is an exothermic process,thus the increased temperature opposes the coal adsorption to CO2,reducing the effective exchange between adsorbed water and CO2(Sun et al.,2016). Additionally, swelling was slightly lower at higher temperatures than that at lower temperatures(Kim et al.,2011),but increased temperatures can accelerate molecular motion and chemical reaction rates.The increased temperature will also cause thermal expansion and temperature stress in minerals(organic and inorganic)due to different thermal expansion coefficients to open pore channels.In addition,it can be seen from the change in mineral contents that the proportion of quartz increases with temperature,and quartz has a positive effect on pore volume,while clay has a negative effect on pore volume(Liu et al.,2019).These may be the reasons accounting for the increases in pore volume and specific surface area at high temperatures.

Fig.5.XRD patterns of all samples.

Table 4 Relative content percentage of minerals.
As shown in Fig.9,the specific surface area and pore volume gradually increase with the reaction time before 11 d,and then begin to decrease.They decrease at 2 d reaction time period due to adsorption swelling at the initial injection.The specific surface area and pore volume have a quadratic function relationship with the reaction time.
The average pore diameters of all coal samples increase after CO2-water-coal interaction. This is mainly due to the filling minerals in pores being dissolved after the CO2-water-coal interaction,resulting in opened pores that were originally closed or semi-closed.The average pore size reduced only at 20 d reaction time period.This may be due to the secondary minerals(e.g. kaolinite/quartz) precipitating into refilled pores. CO2-brine-rock interaction experiment found that precipitation of kaolinite,quartz as well as new mineral phases consisted of C,O,Na,Cl,Al and Si.The pore throats were accumulated by clay particles(Yu et al.,2012).
3.4. Fractal behavior from N2 adsorption

Fig.6.Adsorption isotherm and specific surface area increment of all shale samples at(a)different pressures of 2 MPa,4 MPa,6 MPa,8 MPa and 10 MPa;(b)different temperatures of 35°C,55°C and 85°C;and(c)different reaction times of 2 d,5 d,8 d,11 d,15 d and 20 d.The specific surface area increment is sensitive to the pore diameter in the range of 1.5-8 nm.After CO2-water-coal interaction,the specific surface area increment increases with corresponding pore except for N5(10 MPa)and N8(2 d).
N2adsorption can be measured in pore sizes less than 200 nm by using the Frenkel-Halsey-Hill(FHH)model,which is the most effective method for analyzing the fractal characteristics of porous media(Peng et al.,2017;Lu et al.,2018).The pore fractal dimension has been determined by the N2adsorption method with shale and coal (Yao et al., 2008; Liu et al., 2019), and the fractal dimension of the pore after coal reacts with CO2-water can be obtained.The formula for calculating the fractal dimension is written as follows:

where V is the total adsorption volume,C is a constant,and D is the fractal dimension.

Table 5 Specific surface area(Sa),pore volume(Va)and average pore volume(PV)of all coal samples.

Fig.7.Correlations between specific surface area(SSA),pore volume and pore diameter with pressure.

Fig. 8.Correlations between specific surface area (SSA), pore volume and pore diameter with temperature.
The fractal characteristic analysis of sample N4 is shown in Fig.10.Taking ln{ln[1/(P/P0)]}=0 as the partition point,the data can be divided into two fitted linear regions,that is,two fractal dimension values of Ds1and Ds2.The reason for this may be that P/P0= 0.5 is believed to be the demarcation point of capillary condensation(Yao et al.,2008).The isotherm behavior is dominated by the behavior of van der Waals forces at lower pressure and capillary condensation at higher pressure(Yao et al.,2008).Ds1can represent the complexity of the pore surface,while Ds2can reflect the complexity of the pore volume(Yao et al.,2008;Liu et al.,2019).The fractal dimension of all samples is summarized in Table 6.Comparing the fractal dimensions of all samples,Ds2is larger than Ds1,which reveals that the pore structure is more complicated than the pore surface.
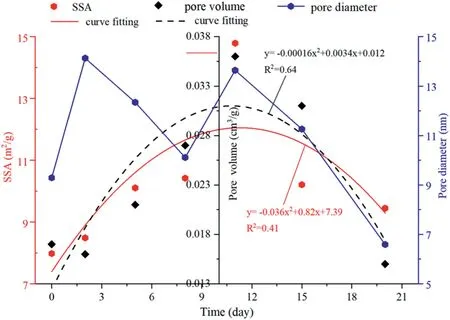
Fig. 9.Correlations between specific surface area (SSA), pore volume and pore diameter with reaction time.

Fig.10.Plots of lnV vs.ln{ln[1/(P/P0)]}and fractal dimension from the N2 adsorption isotherms of sample N4.

Table 6 Fractal dimension of the samples from N2 adsorption.
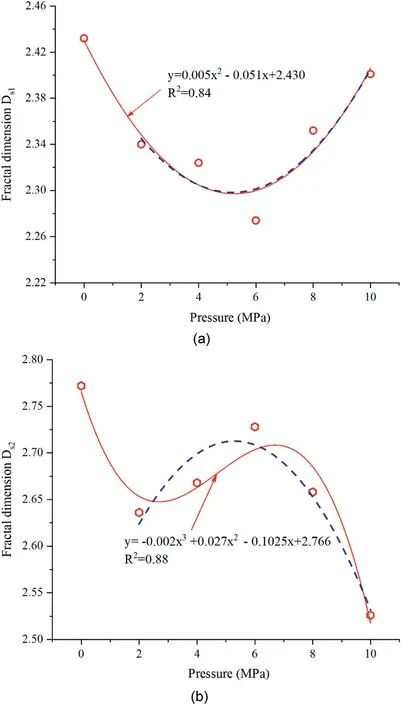
Fig.11.Relationships between fractal dimension and reaction pressure.
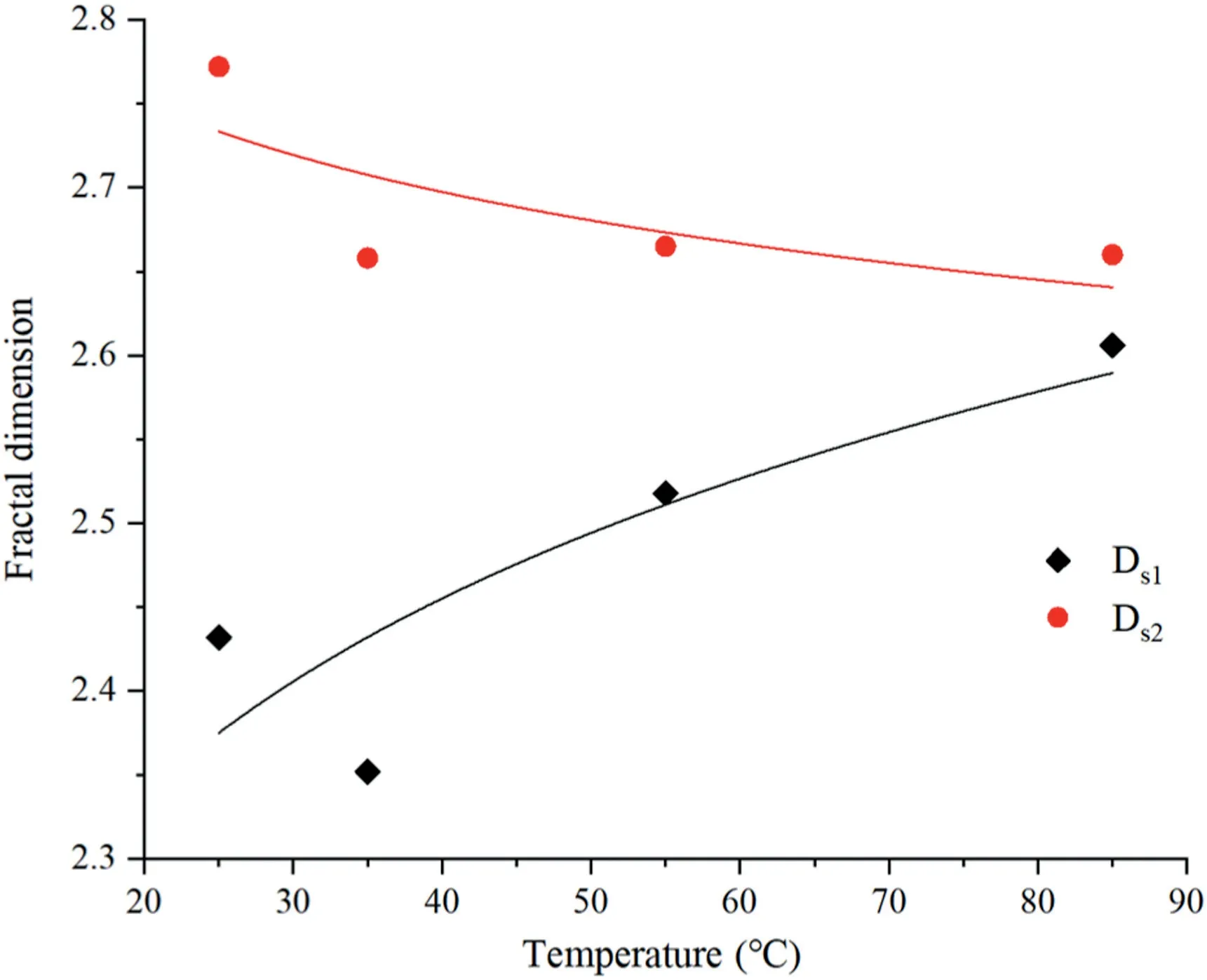
Fig.12.Relationships between fractal dimension and reaction temperature.

Fig.13.Relationships between fractal dimension and reaction time.
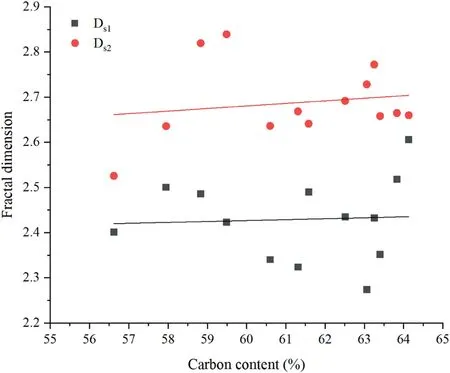
Fig.14.Correlations between fractal dimension and carbon content.
The relationships between the fractal dimension and CO2partial pressure,temperature,and time are shown in Figs.11-13.It can be seen that Ds1and Ds2are lower than the original values of sample N0 after the CO2-water interaction reacting with coal under different pressures.The mineral particles usually adhere to the surface of pores,increasing the irregularities of pores.As the minerals gradually dissolve,the surface and shape of the pores begin to become smooth and regular,resulting in decreases in DS1and DS2.Ds1decreases first and then increases,and the change trend in the subcritical and supercritical CO2-water-coal interaction is different. When sample N0 is not considered, Ds2changes in a manner exactly opposite to that of Ds1,as shown by the blue dotted line in Fig.11.The supercritical CO2is a nonpolar solvent for the supercritical extraction process (Zhang et al.,2013);therefore,organic matter in the coal is extracted to form new micro-cavities,resulting in an increase in Ds1.As the temperature increases,Ds1increases,but Ds2decreases.As the reaction time increases, Ds1gradually decreases and reaches a minimum at 11 d, and then it begins to increase gradually;however,Ds2first increases and then reduces to a stable level.The results indicate that the CO2-water-coal interaction can change the microscopic structure of the pores.
According to fractal theory,the smaller the fractal dimension,the more regular the pore shape and the smoother the pore surface.Carbon content,coal rank,coal composition and pore diameter are the main factors determining the surface fractal dimensions.The fractal dimension D decreases with an increase in carbon and hydrogen contents(Zhang et al.,2014);other scholars,however,suggest that a high carbon content corresponds to a higher D(e.g.Sun et al.,2015).In this experiment,the fractal dimension has a very weak positive correlation with carbon content(see Fig.14).Furthermore,Ds1has a positive correlation with the quartz and kaolinite contents,while Ds2has a negative correlation with the quartz and kaolinite contents(Fig.15).This means the formation of kaolinite and quartz reduces the complexity of the pore volume and increases the irregular,convoluted surfaces of the pore.
4. Conclusions
In this paper,changes in mineral contents and pore structures of coal samples owing to the CO2-water-coal interaction were observed and analyzed.A series of experiments,including ICP-OES,XRD analysis,and low-temperature N2adsorption,were conducted to investigate the properties of coal samples before and after CO2-water treatment. According to the experimental results, the following conclusions were obtained:
(1)Quartz,calcite,kaolinite,potassium feldspar and albite were the main reactive minerals during the CO2-water-coal interaction.The test results showed that pressure,temperature and time can significantly affect the dissolution and formation of minerals in coal.After the CO2-water-coal interaction,the mineral contents of the coal displayed a significant change,and quartz and kaolinite were the main secondary minerals in the reaction.
(2)The CO2-water-coal interaction mainly modified the microporous and mesoporous structures of the coal samples,especially for pores in the range of 1.5-8 nm.During the subcritical CO2-water-coal interaction, the specific surface area and pore volume increased with pressure.After a supercritical CO2-water-coal interaction, the specific surface area and pore volume decreased with pressure.The specific surface area and pore volume had quadratic function relationships with the pressure and reaction time,while they had exponential function relationships with temperature.
(3)By using the FHH model,the fractal dimensions of all samples from N2adsorption were analyzed based on Ds1and Ds2.It is found that Ds1and Ds2have a very weak positive correlation with the carbon content.For the formation of minerals during the CO2-water-coal interaction, Ds1had a positive correlation with the quartz and kaolinite contents,while Ds2had a negative correlation with the quartz and kaolinite contents.Due to the limited pore size range of the nitrogen adsorption test (1.4-200 nm), this paper only analyzed the pore characteristics of micropores to mesopores.The fractal characteristics of the pore structure in the full pore size range need further studies.
Declaration of Competing Interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
The National Key Research and Development Plan(Grant No.2016YFC0501104)and the National Natural Science Foundation of China (Grant Nos. 51522903 and 51479094) are gratefully acknowledged.
 Journal of Rock Mechanics and Geotechnical Engineering2020年2期
Journal of Rock Mechanics and Geotechnical Engineering2020年2期
- Journal of Rock Mechanics and Geotechnical Engineering的其它文章
- Sugarcane press mud modification of expansive soil stabilized at optimum lime content:Strength,mineralogy and microstructural investigation
- Dynamic compression characteristics of layered rock mass of significant strength changes in adjacent layers
- Reliability analysis of earth dams using direct coupling
- Centrifuge model test and numerical interpretation of seismic responses of a partially submerged deposit slope
- Anisotropic surface roughness and shear behaviors of rough-walled plaster joints under constant normal load and constant normal stiffness conditions
- Determination of full-scale pore size distribution of Gaomiaozi bentonite and its permeability prediction
