Preparation of reduced sensitivity co-crystals of cyclic nitramines using spray flash evaporation
Mrinl Ghosh ,A.K.Sikder *,Shibl Bnerjee ,M.B.Tlwr ,N.Sikder
a High Energy Materials Research Laboratory,Pune 411 021,India
b Defence Institute of Advanced Technology,Pune 411 025,India
Keywords:Cyclic nitramine Solubility Spray evaporation Co-crystal Reduced sensitivity
ABSTRACT The present day weapon technology demands novel energetic materials that exhibit simultaneous high explosive yield and reduced sensitivity.This article demonstrates application of spray evaporation to prepare reduced sensitive co-crystals of high performance nitramine explosives like HMX and CL-20 with a relatively less insensitive explosive 1,1-diamino-2,2-dinitroethylene or FOX-7.Stronger intermolecular hydrogen bonding in FOX-7 is responsible for limited solubility in most of organic solvents.Large solubility differences of FOX-7 with HMX and CL-20 restricts it's co-crystallization through classical methods that yields thermodynamically favorable product.Spray flash evaporation,a kinetic crystallization method,has been therefore adopted and could successfully produce CL-20/FOX-7(2:1)and HMX/FOX-7(4:1)co-crystals.The fine powdered materials obtained were characterized by SEM,powder XRD,Raman spectroscopy,DSC-TGA etc.Multipoint Raman spectra showed consistent occurrence of spectral features indicating stoichiometric co-existence of ingredients in the crystal lattices.DSC analysis showed absence of all thermally assisted solidsolid phase transformation in the co-crystals as they were observed in pristine materials.The thermal stability calculated in terms of activation barrier for decomposition,revealed the CL-20/FOX-7 co-crystal to be intermediately stable on comparison to their constituents while,the HMX/FOX-7 co-crystal is more stable.Compared to pure HMX and CL-20,both the co-crystals have shown higher insensitivity to impact force,suggesting them to be suitable for future generation insensitive munitions.©2020 China Ordnance Society.Production and hosting by Elsevier B.V.on behalf of KeAi Communications Co.This is an open access article under the CCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
The most pronounced nitramine class of explosives such as hexahydro-1,3,5-trinitro-s-triazinane (RDX),tetrahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine(HMX)and 2,4,6,8,10.16-hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.0[3,11].0[5,9]]dodecane(CL-20)are characterized by velocity of detonation(VoD)well above 8.5 km/s.How ever,suffer from inherent limitations of their high sensitivity to mechanical stimuli.Explosive power and safety,often contradicting each other,are the two most concerned parameters in the field of high energy materials.The safest energetic materials(EMs) are least powerful such as trinitrotoluene (TNT),dinitrotoluene(DNT),trinitrotriaminobenzene(TATB)etc.,have VoD only near to 7.0 km/s[1].This has been a most apprehending circumstance w hile developing new energetic materials[2,3].Thus,there exists a grow ing demand for high performance and less sensitive(HPLS)energetic materials.As a matter of fact,only three energetic materials namely, nitrotriazolone (NTO), dinitrodiaminopyrazinoxide (LLM-105)and dinitrodiaminoethane(FOX-7)fall into this category with VoD above 8.5 km/s and impact insensitivity(h50%)more than 50 cm.Two pronounced approaches being adopted to reduce sensitivity of energetic materials are based on manipulating chemical stability[4-16](inclusion of-NH2,-OH,-COOH,conjugation,aromatic functionality etc.),and particle characteristics[17-19](reduced crystalline defects,polymorphism,spherocity,etc.).For existing energetic materials,how ever,chemical manipulation is limited and thus physical manipulation becomes the only option.A lot of efforts are being made to desensitize new age materials to improve their safety parameters however augmentation acquired in this direction is still inadequate.In recent days,co-crystallization has been widely accepted for solid modification in pharmaceuticals[20-22].The same concept is being experimented to manipulate hazard characteristics energetic materials.Co-crystals are built up owing to intermolecular nonbonding interactions such as hydrogen bonds,ionic bonds,π-π stacking and van der Waals forces.Presence of polar functional groups like-NO2,-NH2and a variety of acidic protons in nitramine explosive molecules greatly assist to form co-crystal.Progressively,new energetic co-crystals being produced opened up a potential route to high performance safer explosives and thus relaxing the contradiction[23-30].How ever,only a few of them such as CL-20/TNT(1:1);CL-20/HMX(2:1)and CL-20/BTF(1:1)have appreciable density and calculated Vo D above 8.5 km/s.Hence,further experiment to realize more powerful and better insensitive co-crystals of less sensitive EMs like FOX-7,TATBetc.,has become area of current interest.Among these,FOX-7,have most attractive performance indexes with density of 1.885 g/cm3,impact insensitivity upto h50%=126-159 cm,friction insensitivity>350 N and VoD of 9.1 km/s[31-34].Under this study,attempts were thus made to prepare co-crystals of CL-20 and HMX with FOX-7 as co-crystal former.Previously,Zhang et al.[29]proposed much attractive figures for densities of CL-20/FOX-7 and HMX/FOX-7 to be 1.993 g/cm3and 1.895 g/cm3,respectively.In a theoretical study published by Gao et al.[35]proposed high forming probability of 1:1 cocrystal of CL-20/FOX-7 system with a derived density of 2.001 g/cm3.As experimental studies are essential to practically realize such potential co-crystals of FOX-7,hardly any work is published in the recent literature.Using triamminotrinitrobenzene(TATB)as cocrystal former,a similar molecule with equivalent amino&nitro functionality like FOX-7,a 3:1 CL-20/TATB co-crystal is only reported till date[36].How ever,due to poor energetic performance of TATB,the overall activity of the co-crystal is not appreciable even though higher insensitivity could be expected.A previous study also reported HMX/TATBco-crystal with 8:1 stoichiometry[37]but,was later proved to be a physical mixture rather than co-crystal by morphological analysis[38].Attempt was thus made to prepare cocrystals of CL-20 and HMX with FOX-7 to impart a balance between insensitivity and performance.
Initial attempts were made to obtain desired co-crystals through classical methods as followed in preparation of most pharmaceutical co-crystals.A brief account of screening of method is described in experimental section.All these thermodynamically favoured methods were unsuccessful to yield expected co-crystals.Hence,kinetic crystallization method,spray flash evaporation(SFE)was attempted to enable forced co-crystallization.Fine crystalline powders of desired co-crystals could be obtained successfully with this method.The powdered materials thus obtained were well characterized using powder X-ray diffraction,Raman spectrometry and SEM for chemical composition and particle morphology.The insensitivity parameters pertaining to thermal initiation and initiation by mechanical stimuli measured as,improved activation energy of decomposition and minimum height of impact were obtained using differential scanning calorimetry(DSC)and BAM Impact tester.
2.Experimental
2.1.Materials
The energetic materials used for this study were obtained from various sources.ε-CL-20,HMX and FOX-7 were obtained from indigenous sources.All these materials were repeatedly recrystallized prior to their use to obtain chemical purity above 98.5%.All other chemical used were obtained from Thermo Fisher.UHP grade Nitrogen was used as pressurized gas for spray.
2.2.Screening of co-crystallization method
Screening out a suitable co-crystallization methodology has been the hard-hitting task under this study.Most effectively used techniques in different arena include solvent evaporation,grinding[39-46],slurry crystallization[47],melt extrusion[48],sonocrystallization[49,50]etc.Most of the energetic co-crystals realized so far have been prepared through solvent mediated process.High temperature melt extrusion how ever,not recommended for crystallization of thermally labile hazardous explosives.Wet grinding[51,52],a proven and safe method for preparation of fine energetic particulates was also expected to be useful.Thus,safer and thermodynamically favoured methods like solvent evaporation(fast and slow),w et grinding,ultrasound assisted cooling[53],vapour diffusion[54]etc.,have been attempted to co-crystallize FOX-7 with CL-20 and HMX.All these experiments were carried out using variety of solvents such as acetone,methanol,ethyl acetate,ethyl methyl ketone etc.Saturated solutions of explosive mixtures at room temperature were employed in each method.Thus,solvent volume was varied in all cases.However,each method yielded particulate mixtures rather than co-crystals as revealed by micro Raman analysis(Table 1).
Relative solubility of the constituents in the solvent system plays major role during co-crystallization.FOX-7 exhibits strong intra and intermolecular hydrogen bonding with average distance of 2.31Å[55]resulting in poor solubility in even polar solvents while,nitramines are appreciably soluble in the same medium.The homo intermolecular interaction in FOX-7 competed over to that in FOX-7/solvent and FOX-7/nitramine interactions resulting in separate homo molecular nucleation rather than forming hetero molecular clusters.In slow and fast evaporation,separated crystals were observed almost simultaneously.During vapour diffusion and ultra sound assisted cooling,in the first set of experiments(Table 1),FOX-7 partially crystallized out but CL-20 remained in solution. How ever, almost simultaneous but partial recrystallization of ingredients was observed for HMX/FOX-7 pair.These observations led to exclusion of thermodynamic methods for co-crystallization.
Rapid expansion of supercritical solutions(RESS)was developed as an alternate method to prepare nano-explosives[56].This specific technique how ever,offer constrains in the form of poor solubility of explosives in supercritical CO2.Spray flash evaporation(SFE)or commonly know n as spray drying has been successfully implicated to generate pharmaceutical nano materials[57-59]and also nano energetic materials[60-62].Feasibility of this methodology has been demonstrated vide continuous preparation of nanoco-crystals of 1:1 CL-20/TNT and 2:1 CL-20/HMX[63,64].In recent study by Yang et al.[65],fine co-crystals of 1:1 BTF/TNT has been realized through spray drying.The study suggested spray drying as a fast,simple and continuous methodology for preparation and scale-up of energetic co-crystal.SFE facilitates extraordinarily fast crystallization compared to classical techniques through rapid solvent evaporation,which favours enhanced interaction between chemical entities at molecular level to produce crystalline hybrids.Hence,this method is expected to provide an opportunity to cocrystallize solutes with in-concomitant solubility.How ever,in flash evaporation,sometimes depending on the nature of intermolecular interactions between the chemical pairs,different crystalline composites like separated particulate mix and core-shells and semi crystalline composites may also form.
Owing to advantages of rapid kinetic crystallization,SFE was attempted in this case and successfully yielded the desired cocrystals.The detailed experimental procedure is described in the section 2.3.
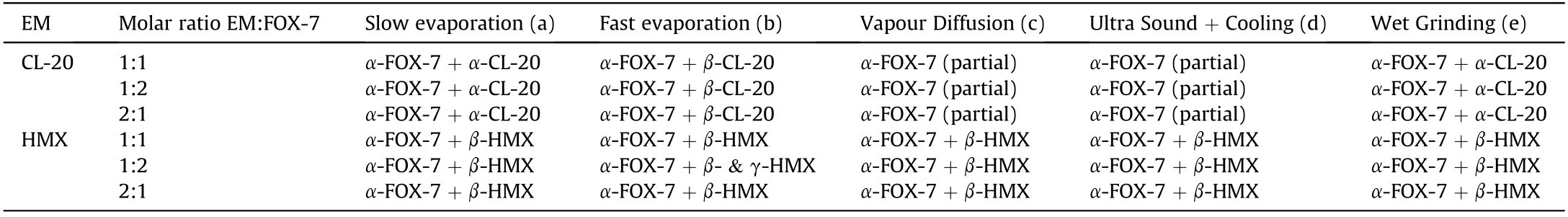
Table 1 The observations from the classical methods of crystallization.
2.3.Co-crystal preparation by SFE method
The sub-micron size co-crystals were prepared by means of SFE that allow s a continuous preparative process for spherical submicron particulates of sensitive energetic materials within a single processing step.The basis of this process is to sudden drop in spray liquid pressure in a preheated large chamber.Solvents with boiling point below 60°C were used to suit the process.The substances under study were taken as solution in acetone in stoichiometric ratio.Unlike the commercial equipments,in the present set up,neither a heated nozzle nor did any pre heated drying gas was used.Instead,the chamber is externally heated to a temperature of 70°C.This makes the air inside the chamber hotter and partially creates a low pressure zone.The solution was then nebulized inside the chamber using an UHP nitrogen overpressure of 75 kg/cm2through a single fluid hollow cone nozzle of 0.3 mm diameter.Under a state of sudden liquid pressure drop in a heated atmosphere,the thermodynamic equilibrium is displaced,making the liquid droplets unstable.To regain stability,the excess thermal energy is converted into latent heat,enabling ultra fast evaporation of the solvent.Ultra fast removal of solvent forces the solute molecules to arrange themselves into most favorable lattice positions to built a crystalline material.Such a crystal would thus contain stoichiometric ratio of both the solutes within same lattice,thus forming a co-crystal.A schematic representation of the method is shown in Fig.1.Only those free flowing particles settled at the bottom of drying chamber were collected for characterization.
Experiments were carried out in varied molar stoichiometry such as 6:1,4:1,2:1,1:1,1:2,1:4 and 1:6.The product from each experiment for both the explosive combinations i.e.CL-20/FOX-7 and HMX/FOX-7 were characterized by micro Raman spectrometer.Particle to particle probing helped to identify residual uncocrystallized materials.Multi point mapping has been extremely helpful to establish molar stoichiometry of complete cocrystallization.In this study,complete co-crystallization was obtained at 2:1 for CL-20/FOX-7 and 4:1 for HMX/FOX-7 combinations.Other stoichiometric combinations yielded mixture of cocrystal and pure crystals of the component that is in excess.Since,this study was aimed only to establish feasibility of spray technique to prepare co-crystals of non-concomitantly soluble energetic materials,quantitative calculation of yield has not been derived.
2.4.Co-crystal characterization
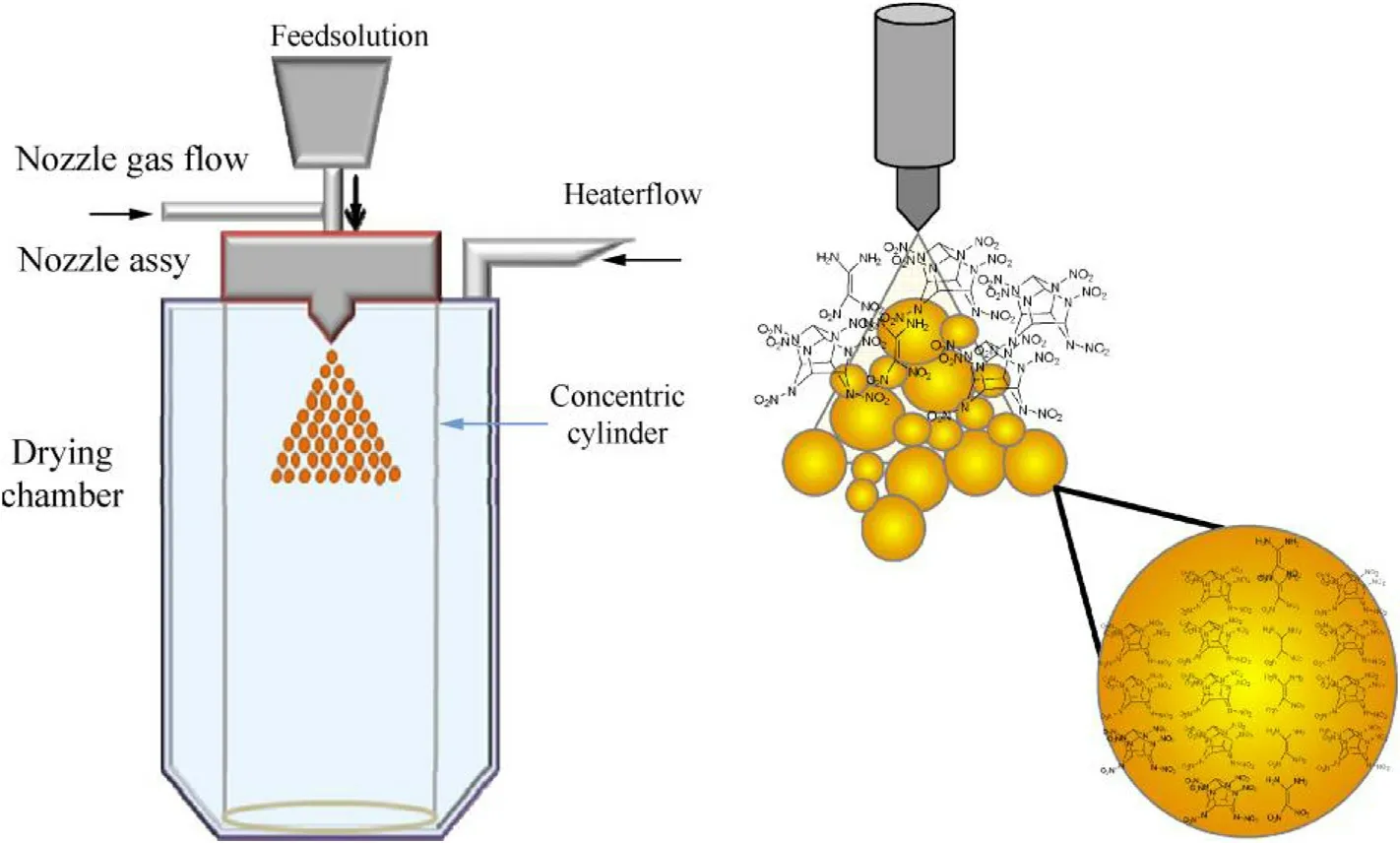
Fig.1.Experimental laboratory set-up and process scheme of Spray Flash Evaporation.
Particle morphology were recorded using Quanta 200,FEI Netherlands make ESEM.All the imaging was performed under low vacuum and beam accelerating potential of 12.5-15 k V.Raman spectra were collected by using a‘in Via Reflex’dispersive micro Raman spectrometer from Renishaw,UK with 514 nm Argon ion laser.The spectrometer is attached with a‘Leica’DM 250 research grade optical microscope with motorized stage having low est translational step size of 1μm.Spectra were collected in the Raman shift region of 100-3500 cm-1at 1 cm-1spectral resolution under 10%of laser pow er input to prevent sample damage and minimize thermal noise.FTIR spectrums were collected using a Nicolet 5700 FTIR spectrometer in the spectral range 400-4000 cm-1at 1 cm-1spectral resolution and 32 scans per spectrum.In order to avoid grinding of the co-crystal particles,which could alter the lattice properties,FTIR spectrums were collected in ATR mode using a single bounce Diamond ATR accessory.Powder X-ray diffraction data of pure and co-crystal samples were collected on Bruker D8 Advance diffractometer with nickel filtered Cu Kα radiation(λ=1.5406Å),run at 40 k V voltage and 40 n A current.Data were collected over diffraction angle range of 2θ=10°-70°at a scan rate of 0.1°/min.Thermal analysis were performed in a DSC 7,differential scanning calorimeter from Perkin Elmer,USA.Sample sizes were restricted below 0.5 mg for decomposition studies and>2 mg for phase transformation.Samples were taken in hermetically sealed Aluminum pans and were subjected to thermal profiling.A heating rate of 10°C/min was employed for solid-solid phase transition studies,while decomposition profiles were obtained at different heating rates viz.5°C/min,10°C/min,15°C/min and 20°C/min so as to derive kinetic parameters.
2.5.Sensitivity analysis
Insensitivity to impact force was measured by Bruceton Staircase method using a fall hammer weighing 2 kg.About 20 mg of the pure ingredients and their co-crystals were taken in stainless steel anvils and subjected to impact of the falling hammer from a certain height.Every impact test was noted for probable exposition.A positive result was marked to have occurred if there evidenced a loud explosion,ignition(flash and/or smoke),crackling,sparking and even brow n spots with burning smell from sample.In the event of no reaction,the test was repeated with a fresh sample from height 5 cm greater than previous and if a reaction occurred,fresh samples were tested at successively lower height.By using an up and down method and analyzing the data statistically using Bruceton staircase method,a height for 50%ignition probability designated as‘h50%’was determined.Ahigher value of h50%signifies better insensitivity.The experimental value of‘h50%’is obtained in‘cm’.How ever,for universal acceptability,the insensitivity to impact is expressed in Joules and is calculated as
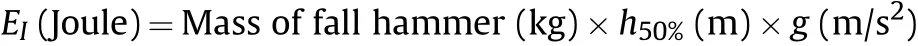
In the present measurement,fall hammer weight is 2 kg and value of g=9.8 m/s2.
3.Results and discussion
To demonstrate feasibility of to generate insensitive co-crystals of CL-20 and HMX with co-crystal former that differ largely in solubility,CL-20/FOX-7 and HMX/FOX-7 pairs were chosen.Selection of FOX-7 was made due to its energetic performance and insensitivity.FOX-7 with density 1.88 g/cm3and VoD 8870 m/sec[66]exhibited performance close to RDX,while,being significantly insensitive.Density has almost a linear co-relation to Vo D and to achieve VoD above 8500 m/sec,a density value of>1.78 g/cm3is expected for any energetic material[30].A co-crystal usually possesses density mutually compromised by the pure components but,is not always true[24,67].In cases,where significantly low dense co-crystal former is used,e.g.TNT in CL-20/TNT 1:1 co-crystal,both density and performance has been limited by the co-crystal former[68].FOX-7 as a co-crystal former thus would limit the extent of compromise in performance and impart superior insensitivity.
The proposed co-crystals were characterized by SEM for morphology,Raman&FTIR for conformational analysis and DSC for thermal stability&solid-solid phase transitions.It is well conceived that,spectroscopic and thermal analysis may not authenticate cocrystals formation as these analyses do not explain the lattice structure how ever,powder X-ray diffraction can support to some extent.Thus,it was attempted to carry out single-crystal X-ray diffraction(SC-XRD)analysis of both the proposed co-crystals.It was how ever,not a successful attempt owing very poor or no diffraction intensity from the tiny particulates.Further,smaller size and agglomeration characteristics of the particulates posed significant challenge to mount a single particulate and their stability under prolonged X-ray exposure.SC-XRD measurements is know n to provide explicit evidence towards formation of a co-crystal,how ever,this technique may not be suitable for all co-crystals prepared through different techniques.In absence of SC-XRD analysis,it would be difficult for accurate characterization of the proposed co-crystals.Therefore,extreme care has been taken to differentiate the proposed co-crystals from their pure constituents while analyzing by the conventional tools.
3.1.Solubility analysis
Solubility difference of CL-20 and HMX with FOX-7 restricted our initial attempt to co-crystallize by solvent evaporation method.All the three ingredients had their maximum solubility in acetone with values 75 g/100 ml,1.85 g/100 ml and 0.247 g/100 ml,respectively.Both,slow and rapid evaporation were futile to produce cocrystals,rather,components crystallized out separately.This can be understood by considering two main factors-(i)solubility difference and(ii)strong intermolecular hydrogen bonding in FOX-7.The measured solubility ratios in acetone at 298 K for CL-20/FOX-7 and HMX/FOX-7 pairs are 300:1 and 8:1,respectively.Such large solubility differences pushes FOX-7 to attain super saturation preferentially and hence crystallized out priory.The crystal structure of FOX-7,has been reported to exhibit wave shaped lattice layers[69]with intra-layer hydrogen bonding and inter-layer weaker van der Waals interactions.The inter-layer distance(~3Å)is shorter than observed in graphite(~3.5Å)and TATB(~6Å).Apart from that,two intra molecular hydrogen bonds exist within the molecule measuring bond distance 1.77Å.Such strong intermolecular forces in solid state,essentially lowers solubility of FOX-7 in moderately polar solvents like acetone.The same reason also explains extremely insensitive nature of FOX-7 towards mechanical stimuli.
3.2.Morphology and surface analysis
SEM micrographs of spray dried materials,of CL-20/FOX-7(Figs.2(a)-(c))and HMX/FOX-7 (Figs.2(d)-(e))co-crystals,respectively,show ed spherical particulates with smooth surface texture.However,very few particulates show ed non geometric morphology other than the majority of spherical particles.This is understood as possible amorphous content.The mean particle size measured for CL-20/FOX-7(2:1)and HMX/FOX-7(4:1)co-crystal systems are Xmean=3.3 and 4.6μm,respectively,with narrow size distribution.The SEM images were analyzed using available image analysis software“Nmage J1.52a”developed by National Institute of Health,USA.Being a two dimensional image processing tool,it could not generate spherocity,rather obtained the values for circularity and roundness,which are equivalent parameters to spherocity.The results are summarized in Table 2.
The results of image analysis suggest the CL-20/FOX-7 to be more spherical in shape than the HMX/FOX-7 particulates.However,the material collected from the wall of drying chamber show ed agglomeration with non-spherical particles as presented in Fig.2(g).This may be due to early particle adhesion facilitated by incomplete solvent evaporation before striking with the inner wall of drying chamber.This would thus be very important point to note for usefulness of this technique to generate spherical particles for improved the solid loading of composites drastically.
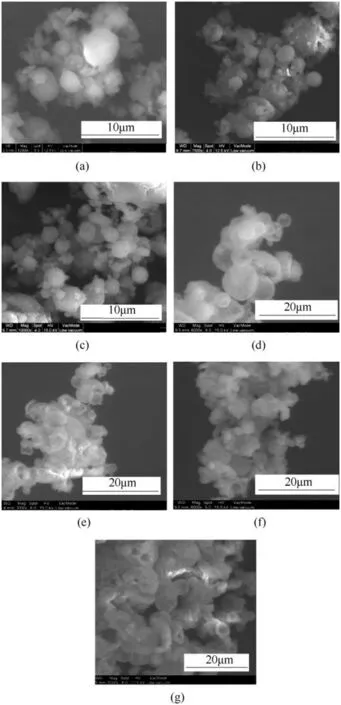
Fig.2.SEM micrographs of(a)-(c)CL-20/FOX-7;(d)-(f)HMX/FOX-7 co-crystals collected from collection chamber and(g)HMX/FOX-7 particulates collected form drying chamber wall.
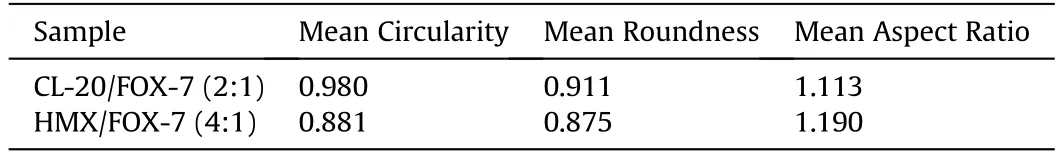
Table 2 Summary of results from image analysis.
In absence of SC-XRD analysis,careful analysis of crystal morphology by microscopic techniques may provide evidence of changes in unit cell geometries.For instance,by comparing the reported morphology of HMX/TATB co-crystal[37]and HMX morphology predicted using BFDH methodology,Wiscon et al.,could unambiguously prove the former to be re-crystallized HMX particles rather than co-crystals.How ever,the particles of proposed co-crystals generated under this study show much varied morphology than their pristine constituents.Fig.3 shows a schematic representation of morphological changes that occur during co-crystallization process.
ε-CL-20 is obtained as bi-pyramidal crystals while,FOX-7 crystallizes as prismatic or cuboid shaped particles and often show stacking of multiple plates.How ever,the particulates obtained through spray method are mostly spherical in nature.Similarly,the particulates obtained from HMX/FOX-7 system are spherical in shape,largely varied form shape of pure HMX and FOX-7.This indeed serves as useful evidence towards lattice alteration and thus formation of co-crystals.
3.3.Raman spectral analysis
The Raman spectra of the energetic/energetic co-crystals are given in Figs.4(a)-(b).FOX-7 is identified by a strong-ONO bending vibration band at 856 cm-1,two moderately strong bands at 1205 and 1351 cm-1for C-N and-ONO symmetric stretching,and NH stretching vibrations at 3332&3425 cm-1.The spectra of co-crystal show a band near 281 cm-1,a feature observed in α and β phases of CL-20,differentiates the conformation from pristine ε phase.A careful look into spectral pattern suggests the CL-20 conformation in co-crystal to be close to β phase.The-ONO bending vibrations in β-CL-20 and β-HMX are noted at 832&833 cm-1,respectively,while,the O-N-O symmetric stretching were marked near 1324 and 1309 cm-1.In both CL-20 and HMX,multiple bands originating from C-H bending vibrations,which are absent in case of FOX-7,are observed nearly at 1250 cm-1.Another set of distinguishing vibrational band,the N-N stretching vibrations in CL-20 and HMX appear in the region of 950 cm-1.Other distinguishing features in CL-20/FOX-7 co-crystal are C-H bending vibrations near 1270 cm-1,a single strong band at 859 cm-1showing a slight shift,a doublet at 318 cm-1,ring deformation band at 281 cm-1,C-H stretching at 3044 cm-1and N-H stretching at 3332 &3425 cm-1.All these,Raman peaks leads to probable conclusion of co-crystal formation with CL-20 being in β-phase conformation.As the SFE set up had not been isolated from atmosphere,presence of atmospheric moisture is unavoidable.Being metastable,an isolated particulate of β-CL-20 is highly susceptible for conversion to α phase.Retention of the conformation during the process and later is indicative of sufficient stabilization of the phase induced by intermolecular interactions with FOX-7 molecules in the co-crystal lattice.Similarly,the HMX/FOX-7 co-crystal can be identified by distinguished features like,a broad band at 1348 cm-1originating from O-N-O symmetric stretching with a blue shift compared to FOX-7 and HMX,a very sharp and strongest band at 866 cm-1,a doublet at 464&490 cm-1,singlet at 631 cm-1etc.Moreover,appearance of C-H stretching vibrations at 2977&3063 cm-1and N-H stretching at 3337&3431 cm-1,with slight blue shift are indicative of co-crystal formation.
In order to investigate homogeneity and therefore identifying isolated non-cocrystals,Raman mapping of both the proposed cocrystals were performed.An area of 60μm×60μm over the sample surface was mapped at 5μm step size thus generating 169 spectrums in totality.Otherwise,it can be understood that,Raman spectra were collected from 169 different locations confined within a 60μm×60μm area.The mappings were carried out using a 50×(NA 0.95)microscope eyepiece and 514 nm Ar ion laser source with approximate beam diameter of 330 nm at focal point,much low er than the observed particle size.This ensured collection of Raman spectrum even from smallest particles present in the sample.Collections were enabled with‘auto focus track’to optimize the working distance matching to focal distance before every collection.The Raman maps of sample surface thus generated are presented in Fig.5.
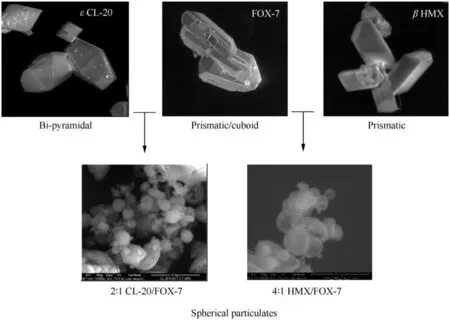
Fig.3.Morphological changes observed during co-crystallization of proposed co-crystals.
Color gradient from red to dark red represents low to high peak intensity of the vibrational band under consideration and a white spot would represent no scattered intensity.In case of CL-20/FOX-7 2:1 co-crystal,the Raman mapping(Fig.5(a))was obtained for the peak at 281 cm-1,ring deformation of β-CL-20 conformer.The color gradient shows change in intensity of the band at different location on the mapped surface.This may be due to random orientation of the particles and uneven surface as weak Raman scattered lights are prone to be absorbed by surrounding particles as well.Overall,all the positions on mapped surface show ed presence of vibrational band at 281 cm-1,signifying homogeneous presence of co-crystal particles.A corresponding map(Fig.5(b))for HMX/FOX-7 cocrystal was obtained for the vibrational band at 631 cm-1.This also showed similar kind of color gradient and thus overall homogeneity of co-crystal particles.These observations strongly corroborate to the condition of co-crystal formation.How ever,the results could not produce any clear indication of isolated particles.
3.4.Powder XRD analysis
To con f i rm the formation of co-crystals,powder X-ray diffraction pattern in the range of 2θ=10°-50°was considered for all the co-crystals and the pristine components.In each case,a comparative analysis is presented in Figs.6(a)-(b).The XRD pattern of FOX-7 show very few diffraction intensities with distinguishing features at 2θ=14.9°,20.0°,26.8°,27.8°,31.0°etc.with the band at 2θ=27.8°be the most intense.While,β-CL-20 is characterized by numbers of diffraction peaks with most of them are of low intensity.The most prominent peaks are at 2θ=13.7°(most intense),18.0°,19.0°,24.1°,28.3°,30.3°etc.In contrast,the co-crystal shows XRD pattern with bands at 2θ=13.6°,15.2°,18.1°,20.2°,24.0°,28.2°,30.2°,32.6°and 36.6°etc.The diffraction band at 28.2°and 30.2°is comparatively broadened,something not very similar to pristine materials.Interestingly,the diffraction pattern of co-crystal does not show bands matching directly to that pristine FOX-7.How ever,a closure look reveals a few bands with significant broadening such as at 2θ=15.2°,20.2°and 30.2°nearly matches to FOX-7.This is indicative of inclusion of FOX-7 in co-crystal lattice rather than formation of any amorphous powder.The XRD profile of pristine β-HMX shows prominent features at 2θ=15.0°,16.0°,18.7°,20.9°,23.4°,27.7°,30.1°,32.3°,37.5°41.7°and 42.7°with band at 2θ=32.3°to be the most intense.Whereas,the HMX/FOX-7 co-crystal exhibits new distinguishing features at 2θ=14.6°,16.9°,20.4°,22.9°,24.5°,26.0°,27.9°,29.5°,31.8°,35.0°,37.0°etc.Moreover,the entire pattern also shows specific alterations.In case of FOX-7 and β-HMX,most intense bands noted at 2θ=27.8°and 32.3°respectively,whereas,in the co-crystal,three almost equally intense peaks are observed at 2θ=20.4°,27.9°,31.8°.The peaks at 20.4°,22.9°and 29.5°are some new features observed in the cocrystal.FOX-7,however,show a medium intense doublet in this region.Moreover,the FWHM of all the features in the co-crystal are comparatively wider than the diffraction bands of pristine components.
Although,there may be several other factors that may influence peak broadening,formation of co-crystals with nano crystallite sizes cannot be ruled out.It is therefore likely that,the observed cocrystals particles may be of poly crystalline nature.Also,wide background is evident in the diffraction pattern of both the cocrystals,especially in case of HMX/FOX-7 co-crystal.It is therefore possible that,both the co-crystals may contain certain amount of amorphous content.The SEM images also show presence of particles with non-geometric shape,likely to be the amorphous phases.How ever,a quantitative analysis of amorphous content is beyond the scope of present study.
3.5.FTIR spectroscopic analysis
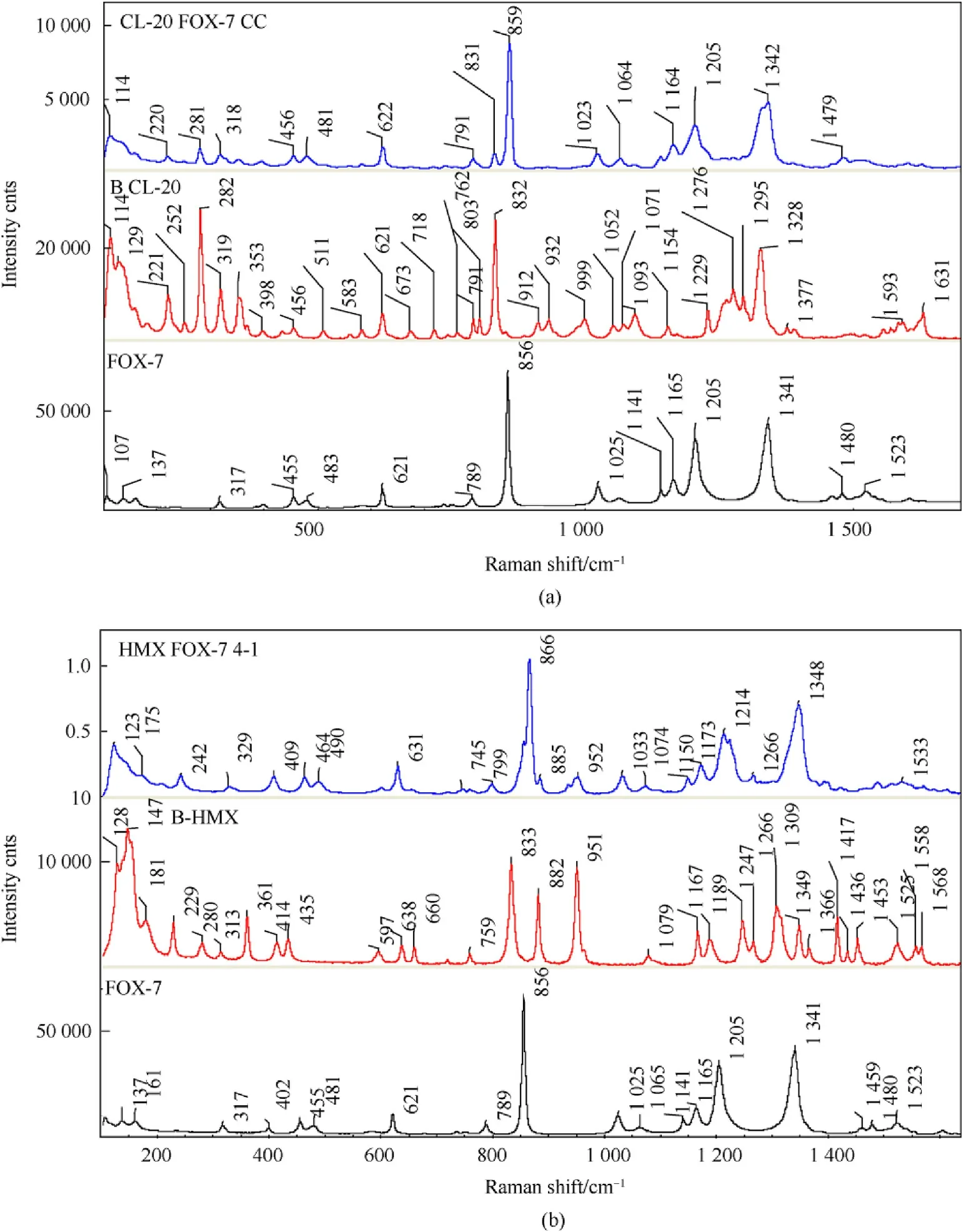
Fig.4.Comparative Raman spectra of CL-20/FOX-7 co-crystal(a),and HMX/FOX-7 co-crystal(b)in the range 100-1600 cm-1.
As the information obtained from Raman spectrometry and powder X-ray diffraction analysis of HMX/FOX-7 co-crystal were not sufficient to establish the claim,FTIR spectral analysis were carried out in support.FTIR spectra of the pow der material obtained from spray drying of 4:1 stoichiometric mixture of HMX/FOX-7,as presented in Fig.7,show some features distinguishable from the pristine materials.The new material essentially show no hydrate peak in the region of 3500 cm-1and above which nullify any possibility of formation separate HMX phases.
Furthermore,the new material contains vibrational bands at positions similar to that of β-HMX.The N-H stretching vibrations of FOX-7 at 3409 cm-1was observed to split into two distinguishable peaks at 3423 cm-1&3403 cm-1in the new material.Also,the strong N-H bending vibration of FOX-7 at 1623 cm-1has been manifested as two well separated peaks in the new material located at 1609 cm-1and 1637 cm-1.This indicates occurrence of two types of N-H stretching vibrations supposed to be originated from differently oriented intermolecular forces in the co-crystal.In a possible co-crystal lattice,the-NH hydrogen atoms of FOX-7 are believed to experience two types of intermolecular hydrogen bonds.One set of hydrogen bonds probably involved N-H&N-O of FOX-7 molecules(intermolecular)and other set involved N-H of FOX-7&N-O of neighboring HMX molecules in the same crystal lattice.The proposed intermolecular interactions are presentation in Scheme 1.
Similarly,the C-H stretching vibration of HMX at 3031 cm-1split into two new peaks at 3036&3027 cm-1.A minor broadening of peak at 2985 cm-1(C-H stretching,HMX)was also noted in the new material.Further,noticeable changes are observed in the regions of 1400-1500 cm-1,a multiplet structure at 1241 cm-1,two weaker but new peaks at 1110&1169 cm-1,broadening of the peak at 1022 cm-1,a strong and completely new vibrational peak at 908 cm-1and splitting of vibrational peaks in the region of 700-770 cm-1.All these distinguishable features supports to the possibility of formation of a co-crystal lattice rather than an amorphous physical mixture or a composite.
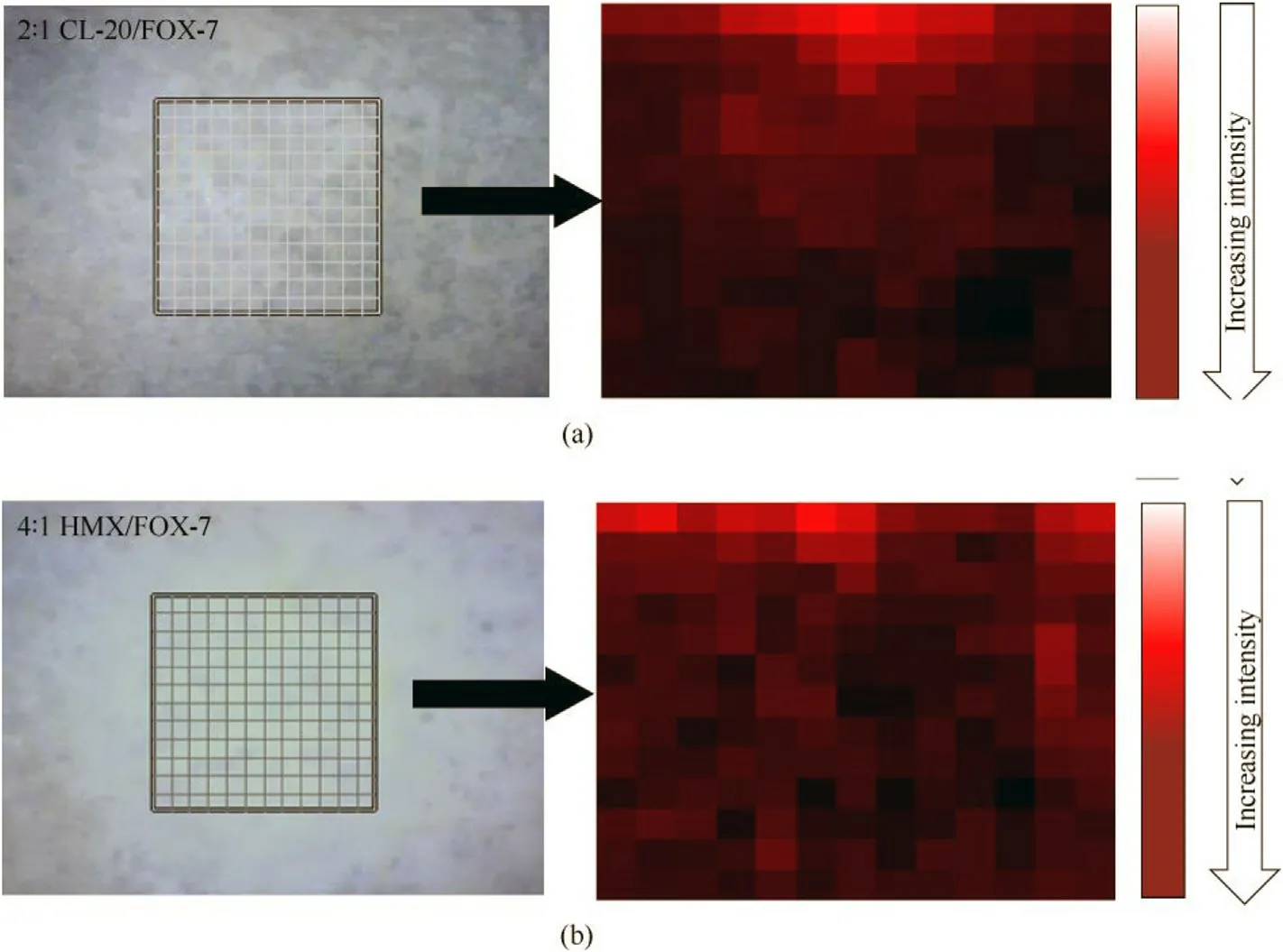
Fig.5.The Raman maps of sample surface.
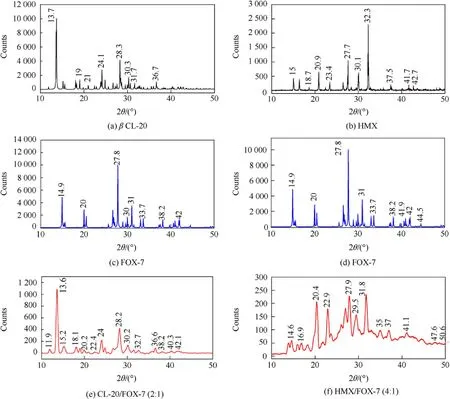
Fig.6.Comparative powder X-ray diffraction pattern of(a)2:1 CL-20/FOX-7 co-crystal and(b)4:1 HMX/FOX-7 co-crystal.
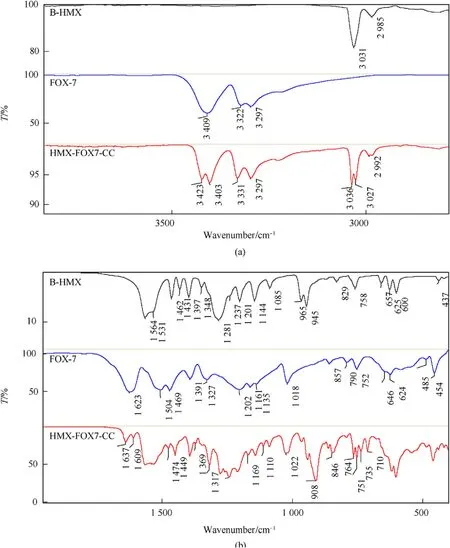
Fig.7.Comparative FTIR spectrum of HMX/FOX co-crystal and pristine materials(a)3800-2800 cm-1,(b)1950-400 cm-1.
3.6.Thermal analysis
3.6.1.Thermally assisted phase transformation
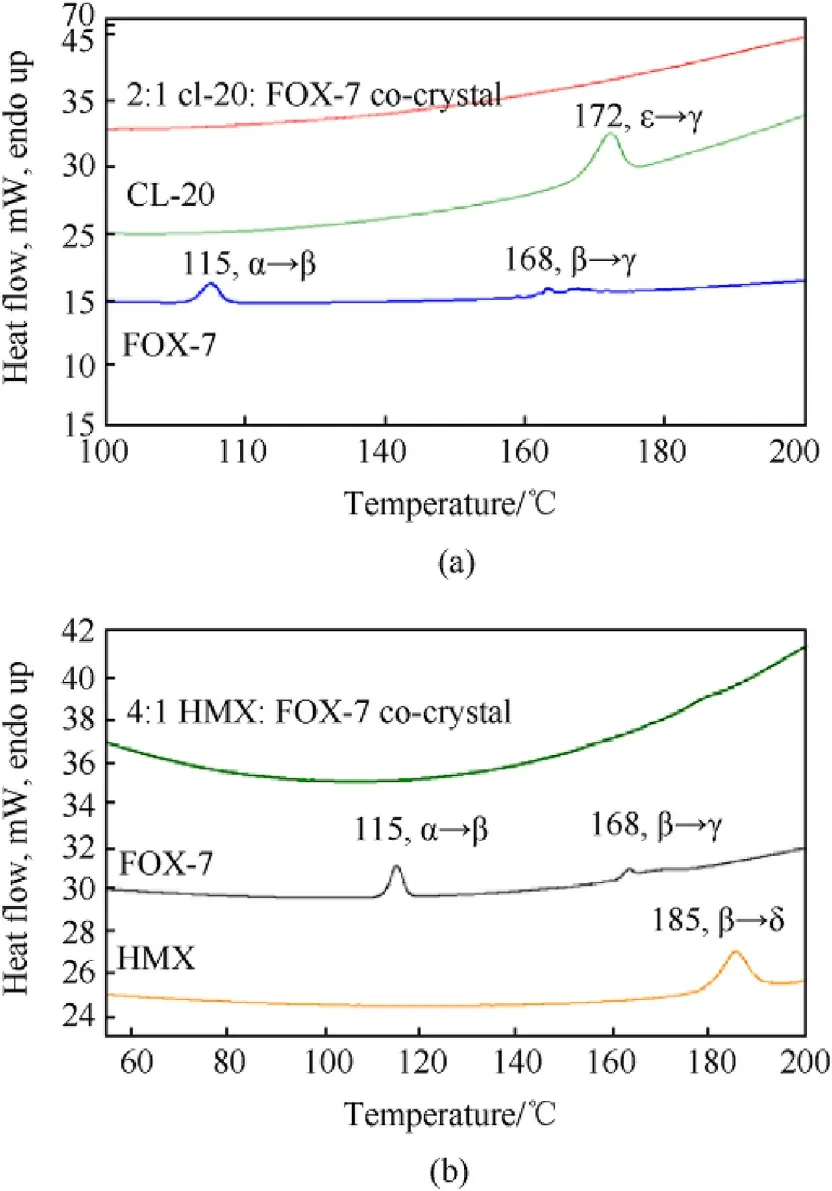
Fig.8.Comparative phase transition profiles collected at heating rate 10°C/min for(a)CL-20/FOX-7 co-crystal,and(b)HMX/FOX-7 co-crystal.
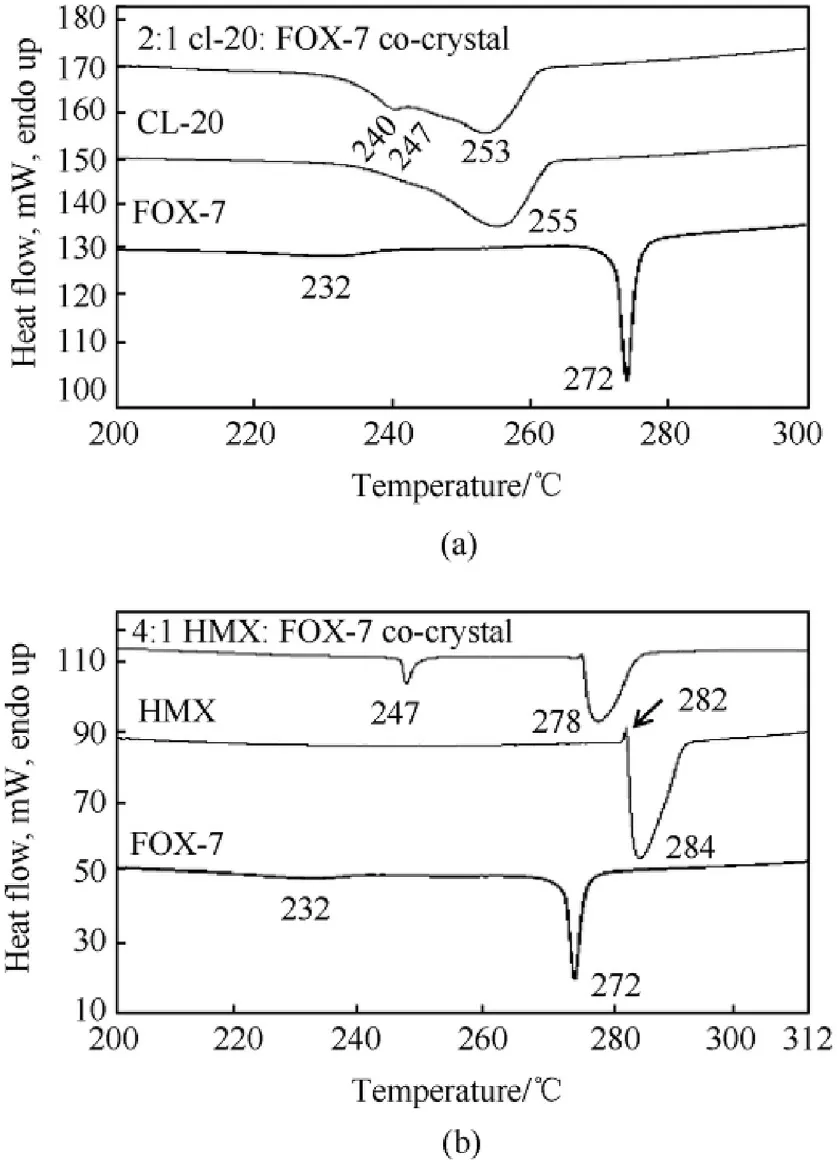
Fig.9.Comparative decomposition profiles recorded at 10°C/min of(a)CL-20/:FOX-7 co-crystal,and(b)HMX/FOX-7 co-crystal.
The thermally assisted endothermic solid-solid phase transformation and decomposition profiles of pristine explosives and their likely co-crystals are presented in Fig.8 and Fig.9,respectively.FOX-7 shows two endothermic transitions at 115°C and 168°C,corresponding to α→β and β→γ solid-solid phase transformations(Fig.8(a)).This is follow ed by two exothermic transitions at 232°C and 272°C,corresponding to two step thermal decomposition of FOX-7(Fig.9(a)).These values are well corroborating with literature reports[34,70].The solid-solid endothermic phase transformation in CL-20 is noted at 172°C representing the ε→γpolymorphic change.Further,in the context of observed CL-20 conformation in spray dried material,as described based on Raman spectroscopic studies,the temperature of thermally assisted β→γCL-20 phase transformation on freshly prepared β-CL-20 was also measured and found to be at 151°C.These values matched well with the literature reports[71,72].In case of the CL-20/FOX-7 2:1 spray dried material,however,no endothermic transition observed up to 200°C(Fig.8(a)).This clearly indicates absence of any isolated lattice structures for both FOX-7 and CL-20,conforming to the conclusion of co-crystal formation.CL-20 shows a wide decomposition exotherm with Tmax=255°C while,an entirely different decomposition pattern was observed for the co-crystal.A closer look into the pattern presented in Fig.9(a)reveals three exothermic peaks overlapping substantially with decomposition maxim as at 240°C,247°C and 253°C.The onset of first step of FOX-7 degradation might have been shifted towards a higher temperature closer to that of CL-20 decomposition.Strong intermolecular interactions,more precisely,the enhanced hydrogen bonding in the co-crystal is believed to be responsible for such alterations.Both inter and intra molecular hydrogen bonding contributes to improved thermal stability in many explosives like TATB,FOX-7 etc.The first maxima thus correspond to first step of FOX-7 decomposition;the second one is possibly due to decomposition of intermediates formed through high temperature reaction of evolved gases from FOX-7 and CL-20,and the last one corresponding to combined phenomena of CL-20 decomposition and 2nd step of FOX-7 degradation.It is important to note that,no separate exotherm corresponding to second step of FOX-7 decomposition was observed.This combined decomposition thus also contributes towards concluding the formation of co-crystal.
β-HMX,show endothermic transition at 185°Crelated to β→δ solid phase change as presented in Fig.8(b).Eisenreich[73]reported this transition to occur at 193°C based on powder X-ray diffraction and Brill et al.reported the same at 185.5°C[74].In the HMX/FOX-7 co-crystal,how ever,no endothermic transitions are observed corresponding to those of FOX-7 and HMX solid-solid phase transformation.Moreover,the first step decomposition of FOX-7 is shifted to 247°C(Fig.9(b)),15°Chigher than that observed in pure FOX-7.The second decomposition is however merged with that of HMX decomposition with maximum temperature of the combined effect at 278°C.The initial decomposition products of FOX-7 instigated HMX to decompose at a lower temperature through secondary reaction in the crystal lattice.The reactive gases from thermolysis of FOX-7 gets into reaction with HMX before could leave the lattice.The thermal profiling is thus supporting our findings saying co-crystal formation in the lattice.
3.6.2.Non-isothermal decomposition kinetics
The kinetic parameters for decomposition of the CL-20/FOX-7 and HMX/FOX-7 co-crystal and the pure ingredients were derived from results of DSC analysis carried out at multiple heating rates following the Kissinger[75]and Ozawa[76,77]models.Fig.8 shows the comparative thermal decomposition profile of both the cocrystals along with their ingredients at heating rate of 10°C/min.
The mathematical forms of Kissinger and Ozawa models are given as follow s-

where,β-linear heating rate(K/min),R-gasconstant 8.314 J/(K·mol),A-pre exponential or frequency factor,E-activation energy,and T-peak decomposition temperature.
These models are based on the fact that,with increasing heating rates during dynamic scanning,the temperature of maximum reaction shifts towards higher temperature[78].Many transitions such as evaporation,crystallization,curing reaction,decomposition,etc.are kinetic events and are function of both time and temperature.This implicates that such transitions(peak maxima)will shift to a higher temperature while heated under an elevatedrate.The fact underlying is that,a particular event would have less time at any specific temperature.Thus,the sensitivity lag to heating rate can be employed to identify kinetic transitions in the sample and thereby deriving their kinetic parameters.Under this study,the decomposition profiles were recorded at four different heating rates 5°C/min,10°C/min,15°C/min&20°C/min.The values of kinetic parameters,the apparent activation energies(E),linear corelation co-efficient(R2),pre exponential factor(A),rate constants(k)calculated using Kissinger and Ozawa methods are listed in Table 3.
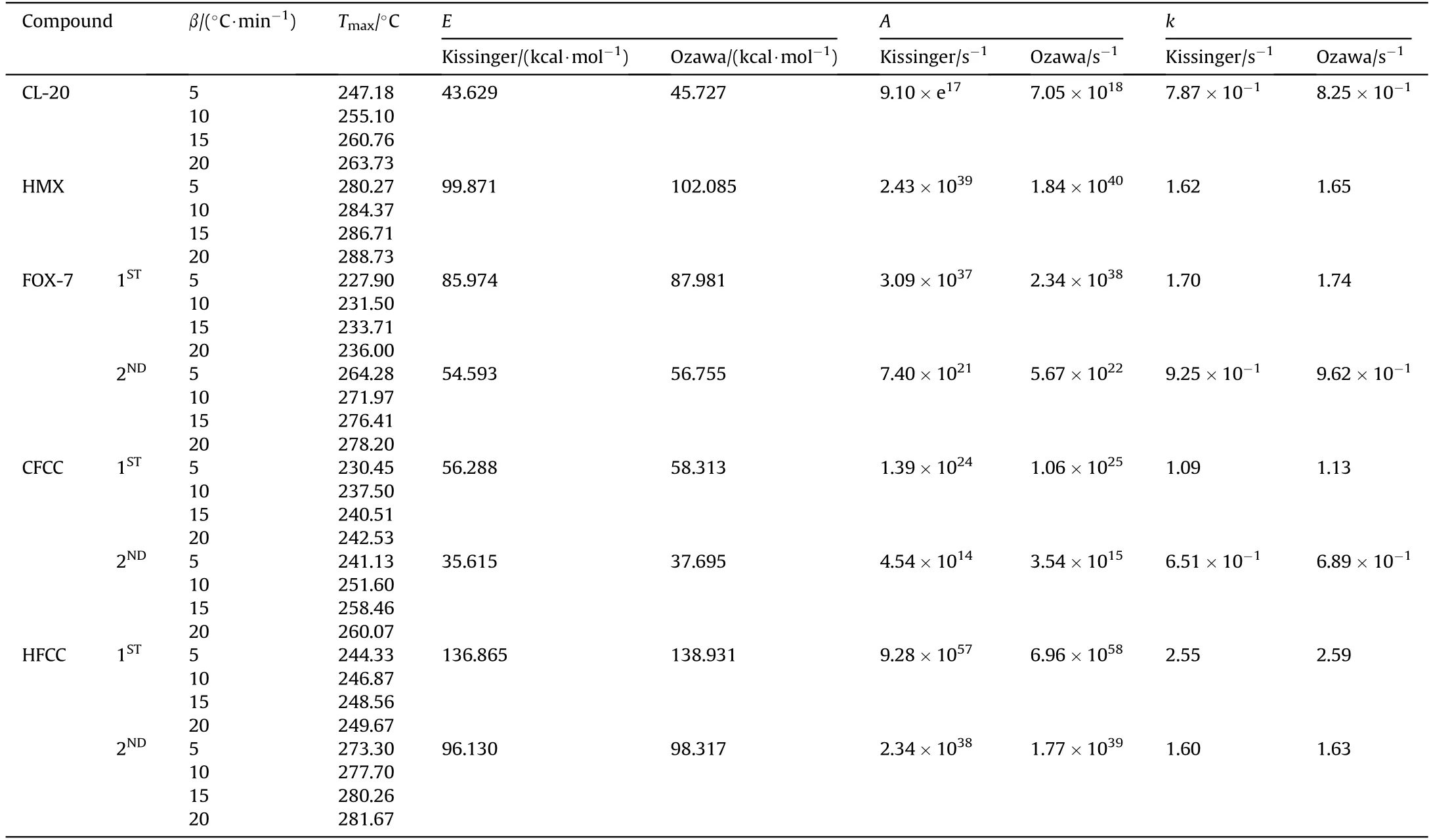
Table 3 Experimental Kinetic parameters for ε-CL-20,β-HMX and the Co-crystals.
The kinetic parameters derived from DSC analysis of the CL-20/FOX-7 co-crystal suggest thermal stability of the co-crystals to be intermediate to that of CL-20 and FOX-7 with energy of activation 56.28 kcal/mol,almost 13 kcal higher than that of CL-20.FOX-7 exhibits a two step decomposition path.The first step decomposition data only has been considered to assess the stability of FOX-7 and its co-crystal and comparison thereof.The decomposition rate constant suggested a faster decomposition rate of CL-20/FOX-7 cocrystal over to that of CL-20.The second decomposition of the cocrystal with E=35.615 kcal/mol,how ever has been more favoured in comparison to both CL-20 and FOX-7.A self catalysis by the decomposition products of CL-20 is expected to be responsible for this low activation barrier.The‘E’of 1st decomposition in HMX/FOX-7 co-crystal has been significantly enhanced compared to that of pristine components with value of E=136.85 kcal/mol.This indicated improved thermal stability of the co-crystal.Such a higher activation barrier can be attributed to enhanced intermolecular interactions as compared to those in neat compounds.A significant rise in decomposition maxima by about 15°C at β=10°C/min is also supportive enhanced intermolecular interaction.A close packing lattice arrangement may have facilitated the heat dissipation within the particle bulk through a non contact energy transfer mechanism thus improving thermal stability.Although,there has been a rise in decomposition maxima,the HMX/FOX-7 co-crystal exhibited much faster decomposition rate.Enhanced surface area owing to spherical particle morphology and possible auto catalytic effects are suspected to be active in this case.From the derived results using Kissinger method,the Arrhenius equation of exothermic decomposition process of the cocrystals may be expressed as-
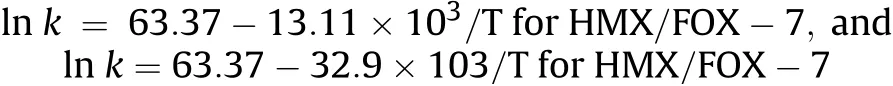
3.7.Impact sensitivity analysis
The sensitivity parameter in terms of fall weight Impact sensitivity was determined using a Fall Hammer apparatus.The measured impact insensitivity values for ε-CL-20,βHMX and FOX-7 were determined as 5.88 J,9.6 J and 18.23 J respectively.Whereas,the co-crystal were found suitably insensitive compared to CL-20 and HMX with impact insensitivity up to 13.13 J and 14.9 J,respectively,for CL-20/FOX-7(2:1)and HMX/FOX-7(4:1)cocrystals.These values indicate the co-crystals to be safer than the pure nitramine explosives.Inclusion of FOX-7 molecules in the cocrystal lattice through preferential hetero intermolecular hydrogen bodings attributed to the improvement of insensitive characteristics of the novel hybrids.
The co-crystals thus generated through spray flash evaporation carry two very important characteristics(i)reduced sensitivity and(ii)spherical particle morphologies.Coexistence of both the qualities in the same material would make them significantly reliable for futuristic insensitive munitions with high explosive yield.
4.Conclusion
Feasibility of spray flash evaporation has been demonstrated for preparation of co-crystals of differently soluble explosive molecules.CL-20/FOX-7(2:1)and HMX/FOX-7(4:1)co-crystals were prepared and characterized for structure and thermal properties.Xray diffraction indicated polycrystalline nature of the particulates with crystallite sizes possibly in the nano domain.Raman and FTIR spectral analysis indicated possible co-crystal formation through alteration in vibrational pattern.Absence of thermally assisted phase transformations in the co-crystals,corresponding to that of pristine ingredients,demonstrated complete co-crystallization.In both the co-crystals,the first decomposition step of FOX-7 has been shifted towards higher temperature making the co-crystals thermally more stable.The sensitivity to impact figures also suggested the co-crystals to be safer than pure nitramine ingredients.Further exploration of this methodology would be helpful to produce cocrystals in continuous manner to support requisites of future Insensitive Munitions.
Author contributions
The manuscript was written through contributions of all authors.All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest.
Acknowledgment
The authors of this manuscript are highly obliged to the Director,HEMRL for his support to carry out the work.The authors also acknowledge Defense Research&Development Organization(DRDO),India for financial support towards this study.
- Defence Technology的其它文章
- A review of dual-spin projectile stability
- A new look on the electric spark sensitivity of nitramines
- Damage assessment of the target area of the island/reef under the attack of missile warhead
- Physical and damping properties of kenaf fibre filled natural rubber/thermoplastic polyurethane composites
- Effect of transverse compression on the residual tensile strength of ultrahigh molecular weight polyethylene(Dyneema®SK-76)yarns
- Design of infrared camouflage cloak for underground silos

