The Effect of Bioturbation Activity of the Ark Clam Scapharca subcrenata on the Fluxes of Nutrient Exchange at the Sediment–Water Interface
ZHANG Shuo, FANG Xin, ZHANG Junbo, 3), 4),*, YIN Fang, ZHANG Hu, WU Lizhen, and KITAZAWA Daisuke
The Effect of Bioturbation Activity of the Ark Clamon the Fluxes of Nutrient Exchange at the Sediment–Water Interface
ZHANG Shuo1), 2), FANG Xin1), ZHANG Junbo1), 3), 4),*, YIN Fang5), ZHANG Hu6), WU Lizhen7), and KITAZAWA Daisuke8)
1)College of Marine Sciences, Shanghai Ocean University, Shanghai 201306, China 2) Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources, Ministry of Education, Shanghai Ocean University, Shanghai 201306, China 3) National Demonstration Center for Experimental Fisheries Science Education (Shanghai Ocean University), Shanghai 201306, China 4) National Engineering Research Center for Oceanic Fisheries, Shanghai Ocean University, Shanghai 201306, China 5)College of Ocean Science and Engineering, Shanghai Maritime University, Shanghai 201306, China 6) Maine Fisheries Research Institution of Jiangsu, Nantong 226000, China 7) Lianyungang City Oceanic and Fishery Administration, Lianyungang 222002, China 8) Institute of Industrial Science, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Japan
Filter-feeding shellfish are common benthos and significantly affect the biogeochemical cycle in the shallow coastal ecosystems. Ark clamis one of the widely cultured bivalve species in many coastal areas owing to its tremendous economic value. However, there is little information regarding the effects of the bioturbation ofon the fluxes of nutrient exchange in the sediment-water interface (SWI). In this regard,was sampled during October 2016 to determine the effects of its bioturbation activity on the nutrient exchange flux of the SWI. The results showed that the biological activity ofcould increase the diffusion depth and the rate of the nutrients exchange in the sediments. The bioturbation ofcould allow the nutrients to permeate into the surface sediments at 6-10cm and increase the release rate of nutrients at the SWI. The releasing fluxes of DIN and PO43−-P in the culture area were found to be around three times higher than that in the non-cultured region. The culture ofhas been proved to be an important contributor to nutrient exchange across the SWI in the farming area of Haizhou Bay. Nutrients exchange in the SWI contributes a part of 86% DIN, 71% PO43−-P and 18% SiO32−-Si for the aquaculture farm.
bioturbation; nutrients; exchange flux; ark clam; sediment-water interface
1 Introduction
The behaviors of benthic organisms, such as feeding, excreting, digging, and creeping species, are capable of changing the physicochemical properties of sediments and the biogeochemical processes at the sediment-water interface (SWI) (Widdows., 1998). The biogeochemical effect of these behaviors, namely, bioturbation, has been recognized for the redistribution of particulate organic matter at the surface of the sediments. The activities of caving benthos increase the porosity of the sediments and its permeability, thereby changing the physical properties of the sediments(Jones and Jago, 1993; Creed., 2010). Bioturbation also significantly affects the nitrification and denitrification of nitrogen in the sediments and promotes coupling between the two processes (Pelegrí and Blackburn,1994; Hulth., 2005). Further, due to bioturbation and biological irrigation of macrobenthos, the nutrient exchange of SWI is affected by the destroyed vertical structure of the sediments and their altered physical and chemical environmental conditions (Volkenborn., 2007).
Filter-feeding shellfish are common benthos in the shallow coastal sea, significantly affecting the biogeochemical cycle of the shallow coastal sea. They ingest nutrients from water columns through their filtration activities and egest the particles into the water. The resuspension rate of the sediment could be increased by four times due to the bioturbation of Baltic clam, and also the culture of oyster could increase the deposition rate of inorganic and organic matter by three times (Mortimer., 1999; Forrest and Creese, 2006). It has been found that the resuspension of the sediment bed could increase the concentrations of dissolved silicon by 125%, nitrate by 67% and phosphate by 66% in the water columns (Couceiro., 2013). Oxygen consumption and exchange flux of nutrients in sediments could be largely increased owing to the activity of the venus clam, which may clearly increase the marine primary productivity (Nicholaus and Zheng, 2014). In addition, the burrowing benthic animals can speed up the flow of water in the cave due to their respiratory or other activities (such as feed, defecate, burrow, and respire); therefore, the overlying water flows through the cave to complete the material diffusion between the pore water of deeper sediments, the process of which is called bioirrigation (Peter and Dirk, 2006). Bioirrigation accelerates the exchange of solutes between the pore water and the overlying water in the sediments. On the one hand, it increases the diffusion rate of the dissolved oxygen into the sediments, and on the another hand, it also promotes the dissipation of biological metabolites into the sediments, thus altering the balance of the biogeochemical process in the sediments (Aller and Aller, 1998).
The ark clam, belonging to the phylum Mollusca, class Bivalvia, is an important species in the benthic system, especially in Haizhou Bay, China. Currently, most of the research on the biological effects ofis based on the monoculture ofor its co-culture with other aquatic organisms (.., sea cucumber) (Niu, 2006). However, the understanding of of the bioturbation activity ofaffecting nutrient exchange fluxes at the SWI is still limited.
To reveal the vertical distribution of nutrients and the mechanisms of its effect on the exchange fluxes of nutrients at the SWI, and to provide the basic information on restoring and building the nutrients exchange model to estimate the exchange flux, this study investigated the effects of bioturbation and the density effects ofon the nutrient exchange at the SWI based on laboratory experiments.
2 Materials and Methods
2.1 Sample Collection and Treatment
The sediment samples were collected from the culture area ofat 34˚49´58´´N and 119˚17´30´´E in the Haizhou bay of the Jiangsu Province of China, by the improved Gray-O’Hara box corer on October 10th, 2016. The samples were collected stochastically from the center of the culture area. The surface area of each sample was about 0.1m2, and the depth was 20-25cmand 0.36m3sediment was sampled in total. Meanwhile, the bottom seawater above the sediments was also collected and filtered by a microfiltration membrane with a pore size of 0.45μm (Φ50×0.45μ, Sinopharm Chemical Reagent Co., Ltd.). The bottom water was collected with the help of a water sampler. The collected sediments and water samples were refrigerated at 0-4℃until laboratory treatment. The clams were directly collected from the culture area using the bottom trawling and brought to the laboratory. The sediments were sieved through a 0.5mm mesh screen to remove macrobenthic organisms, sand and other impurities, after which the clams were transferred into a water tank for acclimation (Deng., 2012; Nicholaus., 2014). Healthywith shell sizes ranging from 28.2 to 28.6mm in length were selected and cultured for seven days in the water tank. About 200 clams were cultured in two 90-liter capacity tanks. During the culture period, 10 liters of seawater was changed daily and the dissolved oxygen was mainatained at 8.5±1mgL−1.
2.2 Experimental Design
An opaque PVC cylinder with a diameter of 50cm and a height of 40cm was designed as the experimental container. The sediments treated as described hereinbefore were added into the device with a depth of 15cm, and the sampled and filtered bottom seawater was slowly added above the sediment with a depth of 20cm (Fig.1). The experiment was segregated into four groups: A0, A5, A10 and A20. Group A0 was the control group, which only contained sediments and the bottom seawater; A5 consisted of the low density bioturbation group with sediments, bottom seawater, and 5 clams (25indm−2). A10 group comprised the bioturbation group with sediments, bottom seawater and 10(51indm−2), whe- rein the biological density was consistent with that in the field; A20 included the highest density bioturbation group with sediments, bottom seawater and 20individuals (102indm−2). Triplicate samples were set for each group, and the culture period lasted for 15 days without feeding. The temperature, dissolved oxygen and pH of the overlying water were measured daily. During the culture period, the stability of dissolved oxygen being maintained by using a pump. The data on the water quality throughout the culture period are shown in Table 1. The overlying water was replaced every 24h to avoid resuspension of the sediment. With the help of a syringe, 50mL seawater was collected at 1-3cm above the sediment before and after the water replacement, then filtered using a cellulose acetate membrane with a pore size of 0.45μm and cryopreserved at −80℃ with chloroform before measuring the nutrients. After 15d of the culture period, the pore water in the sediments was sampled at a 1cm interval by the Rhizon soil moisture sampler (Rhi- zon SMS 10cm porous, male luer-19.01.01, Rhizonsphe- re research products B.V., The Netherlands).
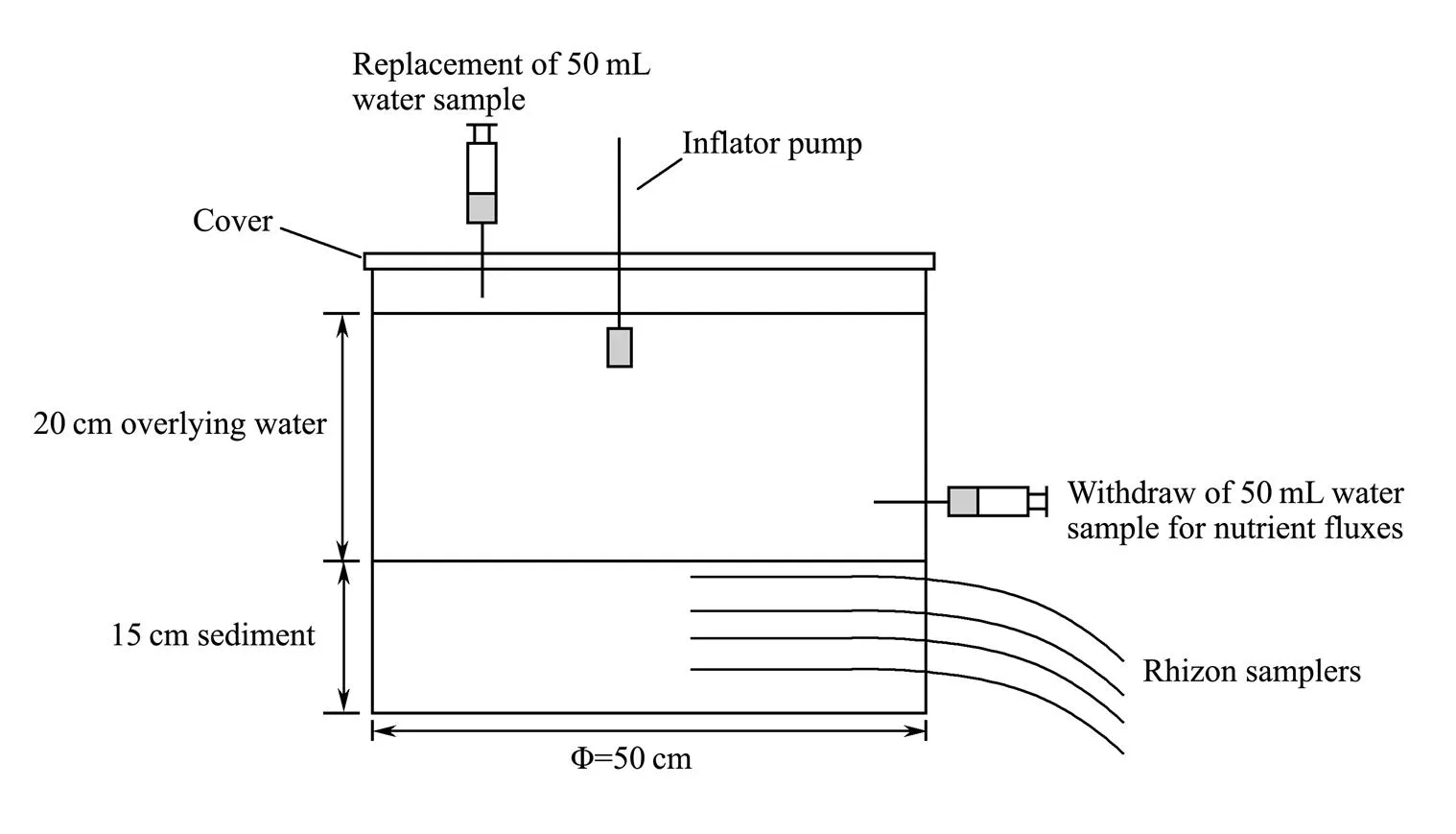
Fig.1 The experimental incubation apparatus specifically designed for this study.
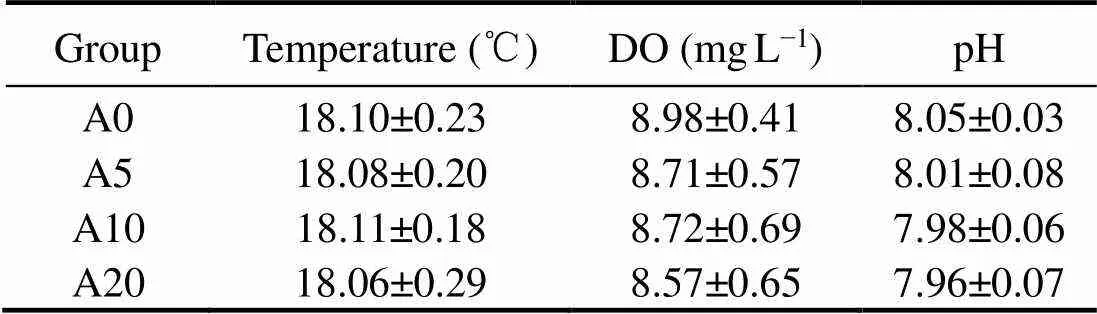
Table 1 Water quality parameters (temperature, dissolved oxygen [DO] and pH) for the mesocosms during the incubation experiment
2.3 Methods of Measurement and Calculation
Measurement of water samples was performed by using the DeChem-Tech CleverChem 380 (DeChem-Tech, Germany) automatic discontinuous analyzer. PO43−, SiO32−, NH4+, NO3−and NO2−were measured by using molybdenum blue spectrophotometry, silicon molybdenum blue method, phenol-hypochlorite colorimetry, cadmium column reduction method and Diazo-azo method, respectively. The SWI nutrient exchange flux was calculated based on the following formula (Michaud., 2006),
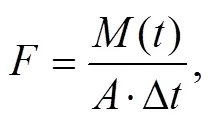
The diffusion rate of the nutrient was calculated based on the Fick’s first law (Boudreau, 1997), which is as follows:
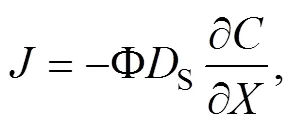
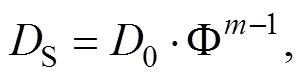
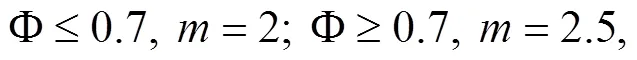
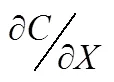
Determination of Porosity: a known volume of a graduated cylinder () is taken and its weight is measured as1. Then the cylinder is filled with the sediment and its total weight was measured as2. Afterward,3is measured after soaking the sediment sample in water for 24h. The porosity is calculated according to the following equation:
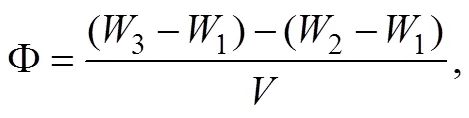
where Φ is the porosity of sediment’s surface,is the weight (g),is the volume (cm3).
2.4 Statistical Analysis
Data analysis was conducted using SPSS (SPSS 20.0. 0). Single sample Kolmogorov-Smirnov (K-S) test was performed to verify whether the data obeyed the normal distribution, and the independent sampletest was performed for the comparison of nutrient diffusion and exchange fluxes.
3 Results
3.1 The Exchange Fluxes of the Nutrients at the SWI
Fig.2 shows the exchange fluxes of the nutrients at the SWI. In the NH4+-N experiment (Fig.2a), the average exchange fluxes of the nutrients at the SWI of groups A0, A5, A10, and A20 were 0.91, 2.18, 3.58 and 4.99mmolm−2d−1, respectively. The average exchange fluxes of nutrients of groups A5, A10 and A20 were 2.39, 3.91 and 5.47 times higher than that of group A0. The changes in the NH4+-N exchange fluxes of groups A10 and A20, which varied greatly, were significantly different from that of group A0 (<0.05).
For NO3-+NO2-N (Fig.2b), the average values of exchange flux of the nutrients at the SWI of groups A0, A5, A10 and A20 were 0.42, 0.97, 1.63 and 2.47mmolm−2d−1, respectively. The average values of exchange flux of nutrients of groups A5, A10, and A20 were 2.28, 3.85 and 5.83 times higher than that of the group A0. The changes in NO3−+NO2−-N exchange flux were more significant for groups A10 and A20 when compared with that in group A0 (<0.05).
The average values of exchange flux of PO43−-P in the SWI of groups A0, A5, A10, and A20 were 0.09, 0.15, 0.27, and 0.37mmolm−2d−1, respectively (Fig.2c). The average values of exchange flux of nutrients of groups A5, A10, and A20 were 1.62, 2.86 and 3.92 times higher than that of group A0. In the first ten days of the experiment, PO43−-P exchange flux was observed at the SWI for groups A10 and A20, which changed faster as compared with that of group A0. As the experiment proceeding, the average exchange flux in groups A5, A10 and A20 decreased was gradually and tended to be stable.
In the experiment of SiO32−-Si (Fig.2d), the average values of exchange flux of the nutrients at the SWI of groups A0, A5, A10 and A20 were 0.42, 0.80, 1.09 and 1.45mmol(m2d)−1, respectively. The average values of exchange flux of nutrients in groups A5, A10, and A20 were 1.56, 2.14 and 2.87 times higher than that of A0 group. The fluxes of the three groups varied from the sediment to the water. In the first ten days of the experiment, the exchange flux in all the groups was found to fluctuate widely. While in the following days, the average value of exchange fluxes in group A10 and A20 decreased gradually and became stable.
In the culture experiment, NH4+-N was the main component of the DIN. In A0 group, the ratios of NH4+-N, NO3−-N and NO2−-N to the DIN were 82%, 13% and 5%, respectively. The DIN constituted 68% and 66% of NH4+- N, followed by NO3−-N (22%, 22%) and NO2−-N (9%, 10%) in groups A5 and A10, respectively. In group A20, NH4+-N accounted for 66% of the DIN, whereas NO3−-N and NO2−-N contributed 21% and 13%, respectively. The mean exchange fluxes of DIN in groups A5, A10, and A20 were, respectively, quantified as 2.36, 3.89 and 5.58 times higher than that in group A0. Similarly, the changes of DIN exchange flux in groups A5, A10, and A20 were larger than that in the A0 group (<0.05).
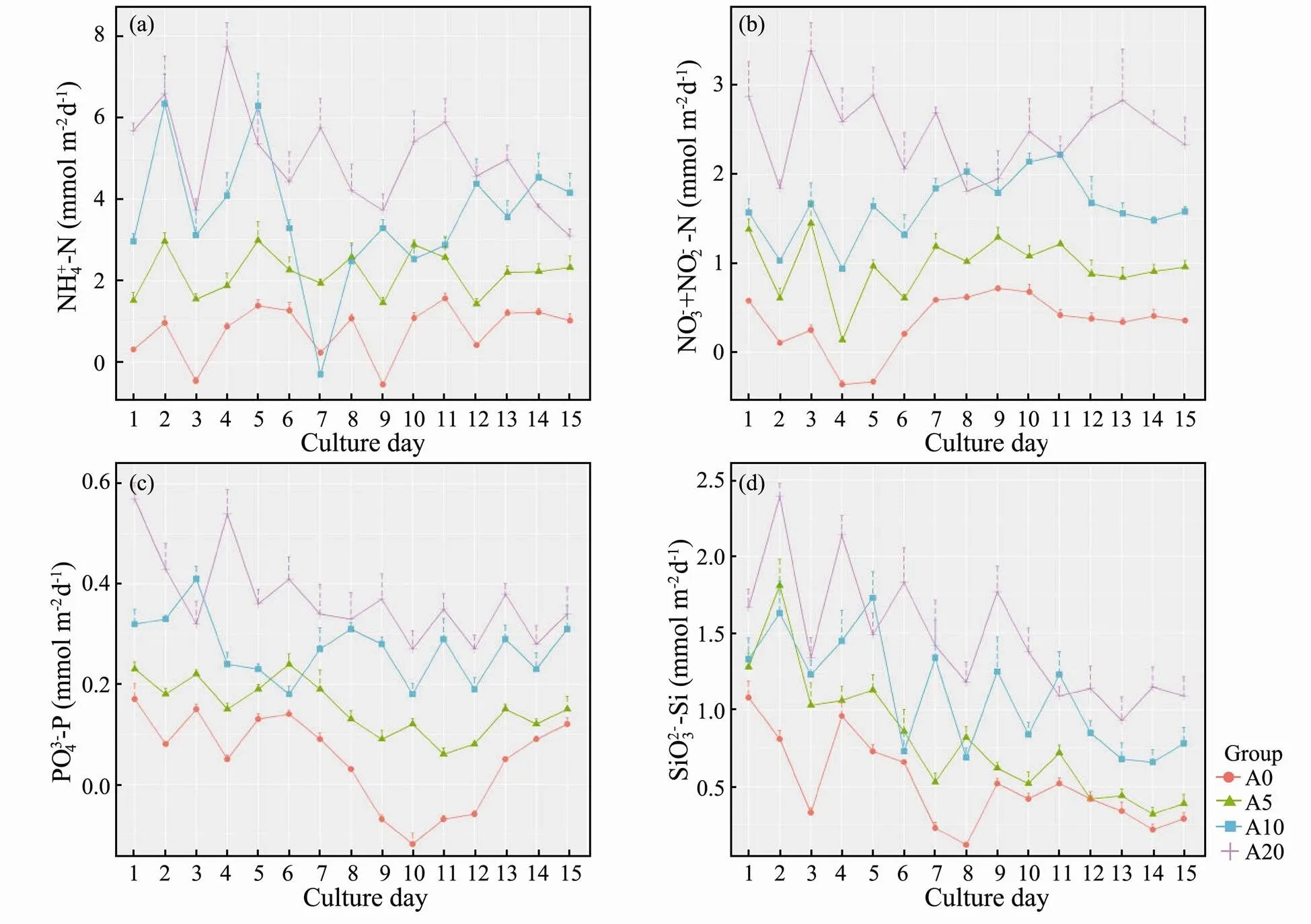
Fig.2 Benthic nutrient fluxes during the experimental period, (a) NH4+-N, (b) NO3−+NO2−-N, (c) PO43−-P, and (d) SiO32−-Si.
3.2 Vertical Distribution Characteristics of Nutrients in the Pore Water
The average concentration of NH4+-N within the pore water of the sediments in the four groups were 1.34, 1.64, 2.13, and 2.95 times higher than that in the overlying water, respectively (Fig.3). A higher concentration of NH4+- N was found with increasing depth. The vertical distribution of NH4+-N in the pore water of A0 group was similar to that in group A5, and the NH4+-N of group A10 was similar to that of group A20. However, a more significant change was found in the vertical distribution of group A20.
With respect to NO3−+NO2−-N, its average concentration in the pore water was lower than that in the overlying water for groups A0 and A5, with average concentration 0.43 and 0.75 times higher than that in the overlying water, respectively (Fig.4). The average concentrations in the pore water of groups A10 and A20 were 1.11 and 1.82 times higher than those in the overlying water, respectively. The NO3−+NO2−-N concentrations in group A0 showed a minor change, whereas those in groups A5, A10, and A20 were found to vary highly at the depth of 1 to 10 cm, and the concentrations in all the four groups tended to increase initially and decrease subsequently. At the depth of 11cm of the sediment, the concentrations of NO3−+ NO2−-N in all the four groups decreased slowly and showed a tendency to be stable.
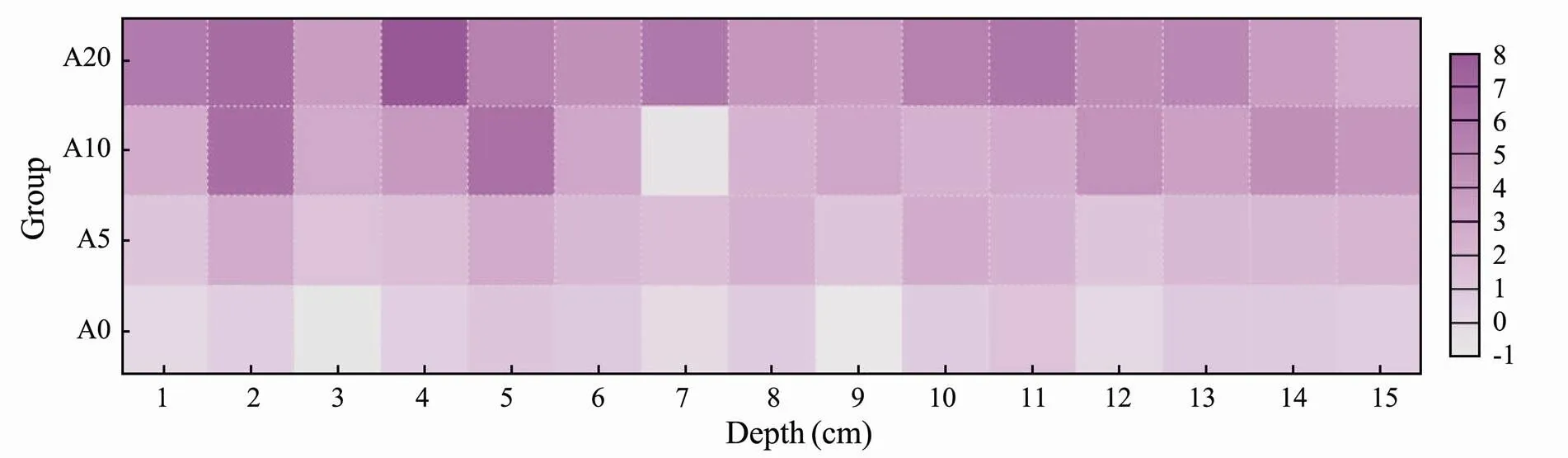
Fig.3 Vertical distribution characteristics of NH4+-N in the pore water (μmolL−1).
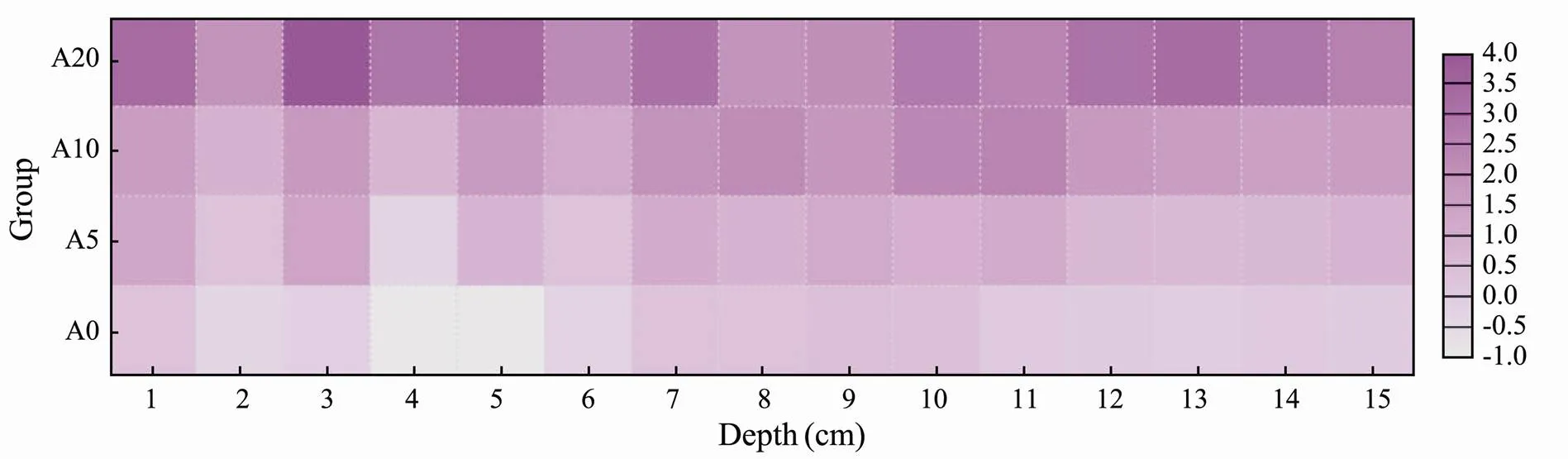
Fig.4 Vertical distribution characteristics of NO3−+NO2−-N in pore water (μmolL−1).
Fig.5 represents that the average concentrations of PO43−-P in the pore water of the sediments were higher than those in the overlying water for groups A0, A5, A10, and A20, the values of which were 1.22, 1.71, 2.45, and 3.29 times higher than that in the overlying water, respectively. The concentrations of PO43−-P in groups A10 and A20 tended to increase significantly at the depth of 1-3 cm. At the depth of 5-10cm, the concentrations of PO43−- P of groups A10 and A20 decreased rapidly. When the depth of the sediment reached 11cm, the concentration of PO43−-P in all the four groups decreased slowly and became stable.
In the SiO32−-Si experiment (Fig.6), the average nutrient concentrations in the pore water of sediments were higher than those in the overlying water for groups A0, A5, A10, and A20, the values of which were 1.08, 1.29, 1.56 and 1.98 times higher than that in the overlying water, respectively. For groups A0, A5, and A10, the data showed similar vertical distribution of SiO32−-Si in the pore water. Stable concentrations of SiO32−-Si were observed in all of four groups at the depth 10cm of the sedi- ment.
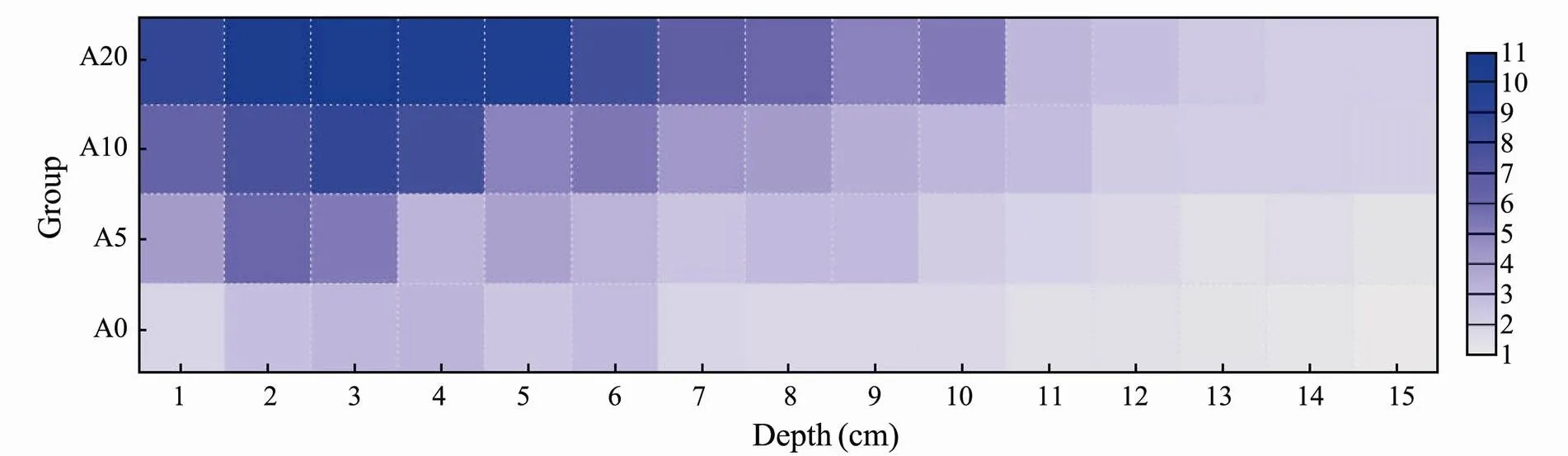
Fig.5 Vertical distribution characteristics of PO43−-P in the pore water (μmolL−1).
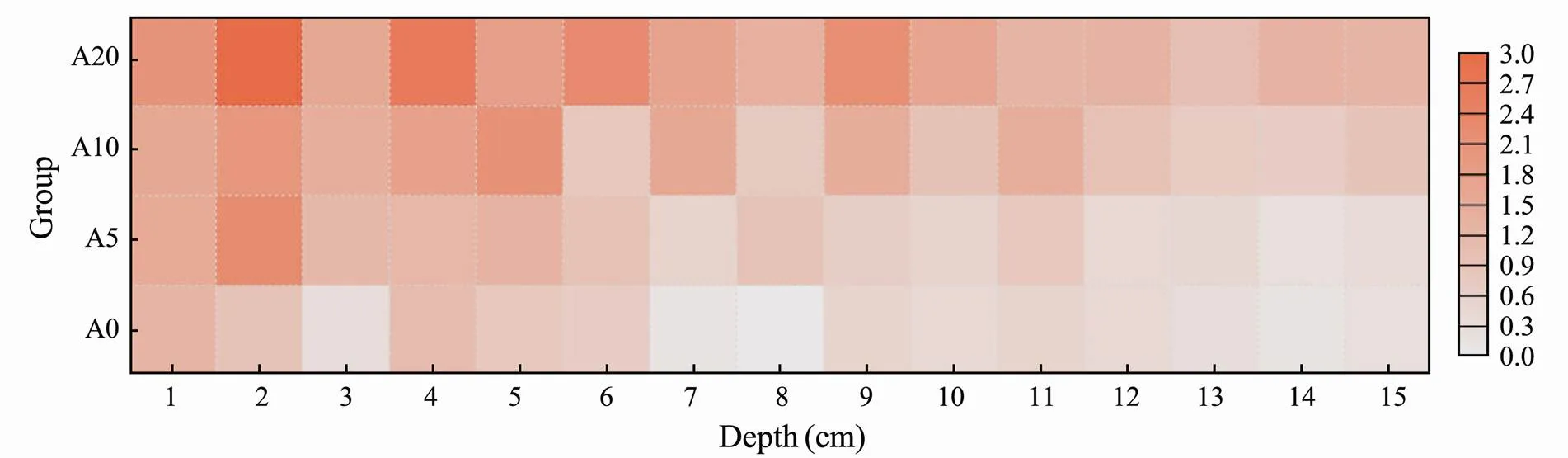
Fig.6 Vertical distribution characteristics of SiO32−-Si in pore water (μmolL−1).
NH4+-N was the main component of DIN in the pore water (Table 2). The mean concentrations of DIN in groups A5, A10, and A20 were 1.37, 1.86 and 2.76 times higher than that in group A0, respectively; there was an apparent difference in the change of DIN concentration between the groups A10 and A0, and a significant difference could also be found between the groups A20 and A0 (<0.05).
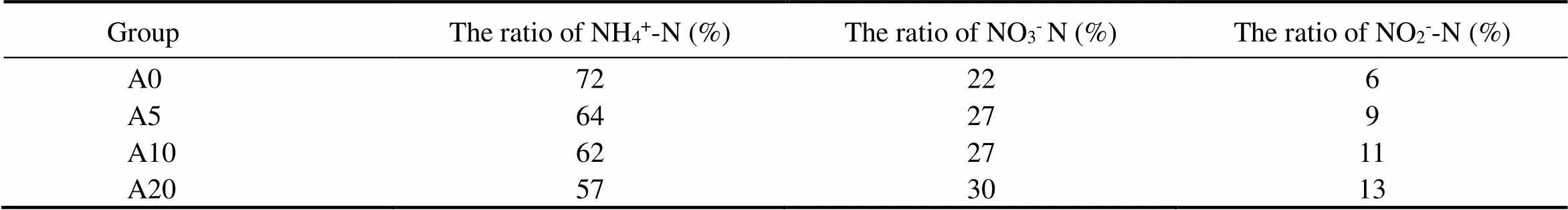
Table 2 The ratio of NH4+-N, NO3−-N and NO2−-N to DIN in the pore water
3.3 Nutrient Diffusive Fluxes Across the SWI
The diffusion rate of nutrients in the SWI was calculated based on Fick’s diffusion law (Table 3). Compared with group A0 (without bioturbation), the nutrients in groups A10 and A20 were found to migrate from the pore water to the overlying water. NO3−+NO2−-N in group A0 were found to migrate from the overlying water to the pore water, while other nutrients were found to be transported in the opposite pathway.
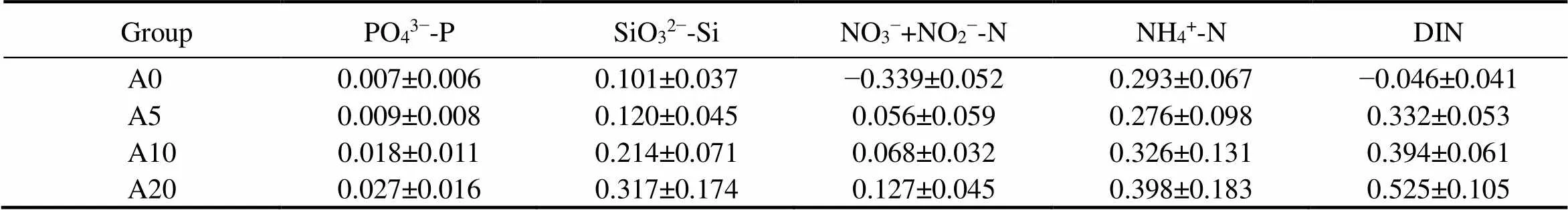
Table 3 Nutrient diffusive flux across the SWI (mmolm−2 d−1)
Note: Positive values denote the movement of nutrients from the sediment to water; Negative values denote the movement of nutrients from water to the sediment.
4 Discussion
4.1 Nutrient Diffusion Across the SWI
plays an important role in the cycling of nutrients in the sediment ecosystem, and has a significant effect on the exchange of nutrients across the SWI. Generally, dissolved oxygen diffuses a few millimeters above the surface of the sediment. NO3−+NO2−-N are dominant in the aerobic layer, so NH4+-N that exists in the anaerobic layer is not released unless the overlying water becomes hypoxic. In this study, the sediment-water interfacial exchange flux of NH4+-N was found to be significantly affected by the bioturbation of(<0.05), and the bioturbation in anoxic sediments also facilitated the release of NH4+-N from the sediments into the water (Bartlett., 2008). Many studies have also shown that bioturbation can significantly alter the exchange flux of nutrients at the SWI (Hewitt., 2006; Nizzoli., 2007; Norling., 2007). This study observed a releasing trend of NH4+-N from the sediments to the overlying water due to the bioturbation of. The exchange flux was much higher than the groups without bioturbation. Similar finding has been reported that NH4+-N was released rapidly when polychaetesettled at the beginning and the releasing rate decreased with culture proceeding (Hansen and Kristensen, 1997). Due to the bioturbation of, the exchange flux of NO3−+NO2−-N increased significantly (<0.05). This could be explained by the fact that more nitrifying bacteria could reach the surface of the sediments and participate in the nitrification reaction, caused by the change of the sediment porosity during the digging process of. Some part of NH4+-N in an anaerobic layer is directly released by bioturbation because the anaerobic layer faces the overlying water with rich dissolved oxygen. The others are nitrified to NO3−+NO2−-N, which are also released from the sediment. Bioturbation not only promoted the diffusion of the dissolved oxygen into the sediments to stimulate the nitrification process in the sediments, it also accelerated the migrating process of NO3−into the SWI (Nizzoli., 2007). With or without the effect ofbioturbation, no apparent exchange flux was found for NO3−+ NO2−-N. Although A20 group showed a decreasing trend within the 6–10 days, other groups presented an increasing trend. The exchange flux of NO3−+NO2−-N increased significantly because of the bioturbation effect. In this study, the exchange flux of PO43−-P in the SWI increased significantly (<0.05), which may be caused by the change in the physical properties of the sediment by. The feeding and digging processes of macrobenthos are often accompanied by the modification of the physical structure of the sediments, which could radically alter the physical and chemical characteristics of the sediments (Rowe, 1974; Gilbert., 2007). In addition, the deposition of organics and dissolved oxygen in the overlying water promotes the release of PO43−-P (Peña., 2010). The decomposition of organics by the bacteria at the surface of the sediment as well as the resuspension effect of bioturbation also facilitates the release of PO43−-P. The exchange flux of PO43−-P was much higher than the group without bioturbation. The exchange flux of SiO32−-Si increased significantly due to bioturbation (< 0.05). This could be explained by the bioturbation that promoted the microbial activity, as the micro-organisms could accelerate the dissolution process of the bio-silicon in the sediments (Bidle and Azam, 2001; Bidle., 2003; Kinoshita., 2003). Therefore, bioturbation might accelerate the regeneration rate of bio-silicon in the sediments. The diatom was fed by the filter feeding shellfish, and the fine debris was discharged into the sediment after digestion, increasing the contact area between the micro-organisms and diatom, further accelerating the dissolution of the bio-silicon.
4.2 Effects of S. subcrenata on Nutrient Diffusion Across the SWI
Activities of benthos are capable of altering the permeability, porosity, and spatial heterogeneity of the sediments (Stockdale., 2009), which promote the diffusion of nutrients into the sediments. In our experiment, NH4+-N in the pore water was the main nitrogen source for the DIN, and the percentage of NH4+-N gradually decreased with more. Some studies have suggested that the concentration of NH4+-N in the pore water is mainly controlled by the redox environment (Canfield., 1993), and its concentration in the pore water is therefore higher than that in the overlying water. Although culturingdecreases the concentration of dissolved oxygen at the surface of the SWI (Shen., 2008), the bioturbation ofaccelerates the diffusion of the dissolved oxygen in the water and the surface sea water with more oxygen is transferred to the bottom consequently. From the daily water quality data, it was found that the dissolved oxygen concentration in thegroup was slightly lower than that in the control group. As denoted in Fig.3, a concentration gradient was formed as the concentration of NH4+-N in the deeper sediments was higher than that in the surface sediments, the result being that NH4+-N in the pore water migrated to the overlying water with a high content of oxygen in the surface sediments and were converted into NO2−-N and NO3−-N by the effect of nitrifying bacteria (Dong., 2009).The respiration and metabolism ofmay also contribute to the increase in the concentration of NO3−-N.
The concentration of NO3−+NO2−-N in the pore water reached the maximum at the depth of 2-6cm, and then decreased with the increasing depth. When NO3−+NO2−-N entered the deeper sediments, the concentration of NO3−+ NO2−-N decreased gradually in the sediments. The oxygen in NO3−+NO2−-N is used for the decomposition of anaerobic bacteria, producing NH4+-N in the deeper regions of the sediment. Although the apparently low concentration of NO3−+NO2−-N was observed when the depth of the sediment was greater than 2cm (Couceiro., 2013), NO3−+NO2−-N could move to the depth of 6cm because of the bioturbation activity of
Generally, phosphorus exists in the sediment with a wide variety of chemical forms such as loosely absorbed phosphorus, iron bound phosphorus, and calcium bound phosphorus. The change in salinity affects the release of PO43−-P from loosely absorbed phosphorus (Froelich, 1988; Suzumura., 2000). Hypoxic or anoxic conditions enhance the release of PO43−-P from the iron bound phosphorus (Bostrom., 1988; Hose and Denison, 2000; Mayer and Jarrell, 2000). The decrease in pH causes the release of PO43−-P from the calcium bound phosphorus (Lijklema, 1994; Gomez., 1999). However, the increase in the release rate of PO43−-P was found to be related to the biological process, and not to the chemical process. The average concentration of PO43−-P in the pore water was 1.22-3.29 times higher than that in the overlying water, and a higher average concentration level could be found with a larger density of. Active organic debris could be found on the surface of the sediments. The organic phosphorus in the organic debris layer maintains a high concentration of PO43−-P during conversion and dissolution, resulting in a higher concentration of PO43−-P in the pore water than that in the overlying water (Huang., 2007). The concentration of PO43−-P changed significantly at the depth of 1-10cm in the sediment and the bioturbation of benthic organisms at the same depth range increased the discharge of phosphorus from the sediments. It was found that the oligochaeteaccelerated the diffusion rate of phosphorus in the sediment (Wu, 2010). In this study, the biological effect ofcaused more PO43−-P to spread to the deeper sediments, which could reach to the depth of 10cm.
The average concentration of SiO32−-Si in the pore water was 1.41-1.83 times higher than that in the overlying water. At the depth of 1-8cm, the average concentration of SiO32−-Si in the bioturbation group was significantly higher than that in the control group (<0.05). Bidle et al. studied the effects of microorganism on the dissolution rate of bio-silicon in the laboratory and found that they can speed up the dissolution of bio-silicon (Bidle and Azam, 2001; Bidle., 2003). The microorganisms in the surface of sediment speed up the dissolution of biogenic silica under aerobic condition, leading to an increase of SiO32−-Si concentration on the surface sediments. Influenced by the bioturbation effect, the dissolved SiO32−-Si diffused into the depth of 8cm in the sediment. The disturbance oflessened as the depth increased, resulting in a decrease in the SiO32−-Si concentration. Karlson. (2005) also believed that SiO32−-Si can mainly migrate through diffusion.
The diffusion rate of nutrient molecules in group A20 was 1.22-1.87 times as high as that of group A10, which indicated that the biological effect of culturingand the increase of biological density could improve the diffusion rate of nutrients at the SWI. Some researchers also believe that the fiddler crabor other benthic polychaetes significantly affect the vertical distribution of the sediments and the concentrations of nutrients (Wolfrath, 1992; Honda and Kikuchi, 2002; Palmer, 2010; Musale and Desai, 2011).
4.3 Contribution of S. subcrenata Culture to the Primary Productivity of Haizhou Bay
The average primary productivity of Haizhou Bay in 2011 was 482.07mgC(m2d)−1(Yang, 2015) and the culture area ofin Haizhou Bay was 352.387 hm2. According to the proportional data of DIN, PO43−-P and SiO32−-Si, the nutrients that were extracted from marine water could be estimated by combining with the Redfield ratio (C:N:P:Si = 106:16:1:16) as 2.99×108mgd−1, 4.14×107mgd−1, and 5.98×108mgd−1, respectively. The average density of the bottom sowing was 50±10indm−2, which is similar to the density of group A10. Referring to the nutrient exchange flux in group A10, the contribution ofculture to the primary productivity in Haizhou Bay could be estimated. It could be concluded that the exchange of nutrients in the SWI would provide a part of 86% DIN, 71% PO43−-P and 18% SiO32−-Si for the culture area ofmeaning thatculture has played a key role in nutrient exchange across the SWI in Haizhou Bay at autumn. It can be found that the contribution ofculture to Si is much lower than that to N and P, which may be explained by two reasons. On one hand, the filter feeding shellfish could not discharge the soluble Si; on the other hand, studies have found that the concentration of SiO32−-Si in the sediments at the depth of 4-5cm is relatively large and the bio-silicon would be buried in the sediments, resulting in inactive Si recycle and decline of the content of Si in the water (Dixit., 2001).
In conclusion, the bioturbation effect ofwas found to significantly improve the diffusion rate of the nutrients at the SWI, which could diffuse nutrients to the surface of the sediment within the depth of 6-10cm. Also, the release of nutrients in the sediments can be improved by the bioturbation of. Referring to group A10 (similar to the actual breeding density), the average nutrient fluxes showed a movement from the pore water to the overlying water during the cultivation. The culture ofplays a greatly important role in nutrient exchange across the SWI in Haizhou Bay. However, the current study is conducted under the condition without feeding, and the excretion of ammonium by thehas not been considered. These potential factors may affect the nutrient exchange at the SWI, which should be considered in the future studies.
Acknowledgements
This study is supported by the Young Orient Scholars Programme of Shanghai, the Doctoral Scientific Research Starting Foundation of Shanghai Ocean University, the Shanghai Special Research Fund for Training College’s Young Teachers, the Fund for Ministry of Agriculture Readjusting the Industrial Structure: Sea Farming Demonstration Project of Haizhou Bay in Jiangsu Province (Nos. D-8006-12-0018, D8006-15-8014), the Special Fund for Agro-Scientific Research in the Public Interest (No. 201303047). The authors would like to thank the Lianyungang City Oceanic and Fishery Administration for assisting with sample collection in Haizhou Bay.
Aller, R. C., and Aller, J. Y., 1998. The effect of biogenic irrigation intensity and solute exchange on diagenetic reaction rates in marine sediments., 56 (4): 905-936.
Bartlett, R., Mortimer, R. J. G., and Morris, K., 2008. Anoxic nitrification: Evidence from humber estuary sediments., 250: 29-39.
Bidle, K. D., and Azam, F., 2001. Bacterial control of silicon regeneration from diatom detritus: Significance of bacterial ectohydrolases and species identity., 46: 1606-1623.
Bidle, K. D., Brzezinski, M. A., Long, R. A., Jones, J. L., and Farooq, A., 2003. Diminished efficiency in the oceanic silica pump caused by bacteria-mediated silica dissolution., 48: 1855-1868.
Bostrom,B., Andersen, J. M., Siegfried, F., and Jansson, M., 1988. Exchange of phosphorus across the sediment water interface.,170: 229-244.
Boudreau, B. P., 1997. Diagenetic models and their implementation., 15 (3): 279.
Canfield, D. E., Jorgensen, B. B., Fossing, H., Glud, R., Gundersen, J., Ramsing, N. B., Thamdrup, B., Hansen, J. W., Nielsen, L. P., and Hall, P. O. J., 1993. Pathways of organic carbon oxidation in three continental margin sediments., 113: 27-40.
Couceiro, F., Fones, G. R., Thompson, C. E. L., Statham, P. J., Sivyer, D. B., Parker, R., Kelly-Gerreyn, B. A., and Amos, C. L., 2013. Impact of resuspension of cohesive sediments at the oyster grounds (North Sea) on nutrient exchange across the sediment-water interface., 113: 37-52.
Creed, R. P., Taylor, A., and Pflaum, J. R., 2010. Bioturbation by a dominant detritivore in a headwater stream: Litter excavation and effects on community structure., 119: 1870- 1876.
Deng, K., Liu, S. M., Zhang, G. L., Lu, X. L., and Zhang, J., 2012. Influence ofaquaculture on benthic fluxes of biogenic elements in Jiaozhou Bay., 33: 782-793 (in Chinese with English abstract).
Dixit, S., Cappellen, P. V., and Bennekom, A. J. V., 2001. Processes controlling solubility of biogenic silica and pore water build-up of silicic acid in marine sediments., 73: 333-352.
Dong, L. F., Smith, C. J., Papaspyrou, S., Stott, A., Osborn, A. M., and Nedwell, D. B., 2009. Changes in benthic denitrification, nitrate ammonification, and anammox process rates and nitrate and nitrite reductase gene abundances along an estuarine nutrient gradient (the Colne Estuary, United Kingdom)., 75: 3171-3179.
Froelich, P. N., 1998. Kinetic control of dissolved phosphate in natural rivers and estuaries: A primer on the phosphate buffer mechanism., 33: 649-668.
Forrest, B. M., and Creese, R. G., 2006. Benthic impacts of intertidal oyster culture, with consideration of taxonomic sufficiency., 112 (1-3): 159-176.
Gilbert, F., Hulth, S., Grossi, V., Poggiale, J., Desrosiers, G., Rosenberg, R., Gérino, M., François-Carcaillet, F., Michaud, E., and Stora, A., 2007. Sediment reworking by marine benthic species from the Gullmar Fjord (Western Sweden): Importance of faunal biovolume., 348: 133-144.
Gomez,E., Durillon, C., Rofes, G., and Pieot, B., 1999. Phosphate adsorption and release from sediments of brackish lagoons: pH, O2and loading influence., 33: 2437-2447.
Hansen, K., and Kristensen, E., 1997. Impact of macrofaunal recolonization on benthic metabolism and nutrient fluxes in a shallow marine sediment previously overgrown with macroalgal mats., 45: 613- 628.
Hewitt, J., Thrush, S., Gibbs, M., Lohrer, D., and Norkko, A., 2006. Indirect effects of Atrina zelandica, on water column nitrogen and oxygen fluxes: The role of benthic macrofauna and microphytes., 330: 261-273.
Honda, H., and Kikuchi, K., 2002. Nitrogen budget of polychaetefed on the feces of Japanese flounder., 68: 1304-1308.
Huang, S., Yang, Y., and Anderson, K., 2007. The complex effects of the invasive polychaetesspp. on benthic nutrient dynamics.,352 (1): 89-102.
Hulth, S., Aller, R. C., Canfield, D. E., Dalsgaard, T., Engström, P., Gilbert, F., Sundbäck, K., and Thamdrup, B., 2005. Nitrogen removal in marine environments: Recent findings and future research challenges., 94 (1-4): 125- 145.
Jones, S. E., and Jago, C. F., 1993.assessment of modification of sediment properties by burrowing invertebrates., 115 (1): 133-142.
Karlson, K., Hulth, S., Ringdahl, K., and Rosenberg, R., 2005. Experimental recolonisation of Baltic Sea reduced sediments: Survival of benthic macrofauna and effects on nutrient cycling., 294: 35-49.
Kinoshita, K., Wada, M., Kogure, K., and Furota, T., 2003. Mud shrimp burrows as dynamic traps and processors of tidal-flat materials., 247: 159-164.
Koretsky, C. M., Meile, C., and Cappellen, P. V., 2002. Quantifying bioirrigation using ecological parameters: A stochastic approach., 3 (1): 17-17.
Lijklema, L., 1994. Nutrient dynamics in shallow lakes: Effects of changesinloadingand role of sediment-water interaction., 94: 335-348.
Mayer, T. D., and Jarrell, W. M., 2000. Phosphorus sorption during iron (II) oxidation in the presence of dissolved silica.,34: 3949-3956.
Michaud, E., Desrosiers, G., Mermillod-Blondin, F., Sundby, B., and Stora, G., 2006. The functional group approach to bioturbation: II. The effects of the, community on fluxes of nutrients and dissolved organic carbon across the sediment–water interface., 337 (2): 178-189.
Mortimer, R. J. G., Davey, J. T., Krom, M. D., Watson, P. G., Frickers, P. E., and Clifton, R. J., 1999. The effect of macrofauna on porewater profiles and nutrient fluxes in the intertidal zone of the Humber Estuary., 48 (6): 683-699.
Musale, A. S., and Desai, D. V., 2011. Distribution and abundance of macrobenthic polychaetes along the South Indian coast., 178: 423- 436.
Nicholaus, R., and Zheng, Z., 2014. The effects of bioturbation by the Venus clamon the fluxes of nutrients across the sediment-water interface in aquaculture ponds., 22: 913-924.
Niu, H. X., 2006. Application study on purification function of,and microbial
products in the shrimp culture. PhD thesis. Ocean University of China (in Chinese with English abstract).
Nizzoli, D., Bartoli, M., Cooper, M., Welsh, D. T., Underwood, G. J. C., and Viaroli, P., 2007. Implications for oxygen, nutrient fluxes and denitrification rates during the early stage of sediment colonisation by the polychaetespp. in four estuaries., 75: 125-134.
Norling, K., Rosenberg, R., Hulth, S., Grémare, A., and Bonsdorff, E., 2007. Importance of functional biodiversity and species-specific traits of benthic fauna for ecosystem functions in marine sediment., 332: 11-23.
Palmer, P. J., 2010. Polychaete-assisted sand filters., 306: 369-377.
Pelegrí, S. P., and Blackburn, T. H., 1994. Bioturbation effects of the amphipod, on microbial nitrogen transformations in marine sediments., 121: 253-258.
Peña, M. A., Katsev, S., Oguz, T., and Gilbert, D., 2010. Modeling dissolved oxygen dynamics and hypoxia., 7: 933-957.
Peter, S., and Dirk, D. B., 2006. Probing the microenvironment of freshwater sediment macrofauna: Implications of deposit- feeding and bioirrigation for nitrogen cycling., 51 (6): 2538-2548.
Rowe, G. T., 1974. The effects of the benthic fauna on the physical properties of deep-sea sediments., 2: 381-400.
Shen, L. W., You, Z. J., and Shi, X. Y., 2008. Study on size and salinity related oxygen consumption and ammonia excretion ofSpat., 29: 53-56.
Stockdale, A., Davison, W., and Hao, Z., 2009. Micro-scale biogeochemical heterogeneity in sediments: A review of available technology and observed evidence., 92: 81-97.
Suzumura, M., Ueda, S., and Sumi, E., 2000. Control of phosphate concentration through adsorption and desorption processes in groundwater and seawater mixing at sandy beaches in Tokyo Bay, Japan., 56 (6): 667- 673.
Volkenborn, N., Hedtkamp, S. I. C., Beusekom, J. E. E. V., and Reise, K., 2007. Effects of bioturbation and bioirrigation by lugworms (Arenicola marina) on physical and chemical sediment properties and implications for intertidal habitat succession., 74: 331- 343.
Widdows, J., Brinsley, M. D., Bowley, N., and Barrett, C., 1998. A benthic annular flume formeasurement of suspension feeding/biodeposition rates and erosion potential of intertidal cohesive sediments., 46: 27-38.
Wolfrath, B., 1992. Burrowing of the fiddler crabin the Ria Formosa in Portugal and its influence on sediment structure., 85: 237-243.
Wu, S. J., 2010. Experimental study on the influence of tubificid Worms’-Bioturbation on pollutions releasing from the sediments of East Dongting Lake. PhD thesis. Changsha University of Science and Technology.
Yang, X. G., 2015. Community structure of plankton in Haizhou Bay and adjacent waters and their relationships with environmental factors. PhD thesis. Ocean University of China (in Chinese with English abstract).
December 29, 2018;
April 2, 2019;
July 11, 2019
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2020
. E-mail: jb_zhang@shou.edu.cn
(Edited by Ji Dechun)
 Journal of Ocean University of China2020年1期
Journal of Ocean University of China2020年1期
- Journal of Ocean University of China的其它文章
- Screening and Characterization of Nitrite-Degrading Bacterial Isolates Using a Novel Culture Medium
- Purification and Characterization of a Novel Lipase from Antarctic Krill
- Quality Assessment of Frozen Solenocera crassicornis Treated with Sodium Metabisulphite by Soaking or Spraying
- Contribution of Mesoscale Eddies to the Subduction and Transport of North Pacific Eastern Subtropical Mode Water
- Semi-Empirical Algorithm for Wind Speed Retrieval from Gaofen-3 Quad-Polarization Strip Mode SAR Data
- An Effective Method of Prompting Juvenile Rainbow Trout (Oncorhynchus mykiss) to Cope with Heat Stress
